Abstract
Serum 1,5-anhydroglucitol (1,5-AG) is an emerging biomarker used to monitor glycemic control in persons with diabetes. We performed whole-exome sequencing, examining the association between rare, coding genetic variants and 1,5-AG among European ancestry (N = 6,589) and African ancestry (N = 2,309) participants without diagnosed diabetes in the Atherosclerosis Risk in Communities (ARIC) Study. Five variants representing 3 independent signals on chromosome 17 in SLC5A10, a glucose transporter not previously known to transport 1,5-AG, were associated with 1,5-AG levels up to 10.38 µg/mL lower per allele (1,5-AG range 3.4–32.8 µg/mL) in the European ancestry sample and validated in the African ancestry sample. Together these variants explained 6% of the variance in 1,5-AG. Two of these variants (rs61741107, p = 8.85E-56; rs148178887, p = 1.13E-36) were rare, nonsynonymous, and predicted to be damaging or deleterious by multiple algorithms. Gene-based SKAT-O analysis supported these results (SLC5A10 p = 5.13E-64 in European ancestry, validated in African ancestry, p = 0.006). Interestingly, these novel variants are not associated with other biomarkers of hyperglycemia or diabetes (p > 0.2). The large effect sizes and protein-altering, multiple independent signals suggest SLC5A10 may code for an important transporter of 1,5-AG in the kidney, with a potential nonglucose-related effect on 1,5-AG, impacting its clinical utility as a diabetes biomarker in this subpopulation.
Similar content being viewed by others
Introduction
1,5-andhydroglucitol (1,5-AG) is an emerging biomarker of glycemic control in type 2 diabetes. 1,5-AG is a monosaccharide consumed in food and maintained at high, constant levels in the blood under normoglycemic conditions through filtration by the kidney and reabsorption into the blood. 1,5-AG is the 1-deoxy form of glucose, and during hyperglycemic conditions (i.e., when glucose exceeds the renal threshold), glucose outcompetes 1,5-AG for reabsorption. This causes 1,5-AG excretion in urine and hence lower levels in blood concentrations1. In adults with diabetes, low 1,5-AG concentrations reflect glucose excursions over the previous 2–14 days1,2, and are associated with microvascular and macrovascular disease3,4,5.
In a recent genome-wide association study (GWAS), we identified seven variants at six loci associated with 1,5-AG among persons of European ancestry without diagnosed diabetes6. Two of these variants were also found in a genetic screen of 1,5-AG measured as part of a large non-targeted metabolome panel among Europeans7. These variants map in or near genes which are involved in carbohydrate metabolism (LCT, SI, MGAM, MGAM2) and glucose transport in the gut and kidney (SLC5A10, SLC50A1, SLC5A1)6. Interestingly, the majority of the variants are not associated with traditional measures of hyperglycemia such as fasting glucose and HbA1c8,9.
While array-based analyses such as GWAS often capture common variants in linkage disequilibrium with the putative causal variants, they are not able to assess the impact of rare variants, nor are they designed to identify causal variants. To investigate the association of rare, putatively damaging variants with 1,5-AG, and to further understand the genetic architecture of this biomarker, a whole-exome sequencing association study of 1,5-AG concentrations was performed in the Atherosclerosis Risk In Communities (ARIC) Study.
Materials and Methods
Study population
The ARIC Study is an ongoing, longitudinal cohort study initiated in 1987, when middle-aged adults were recruited from four communities in the U.S.: Forsyth, North Carolina; suburban Minneapolis, Minnesota; Washington County, Maryland and Jackson, Mississippi. A total of 15,792 individuals attended the initial study visit (1987–1989), and subsequent visits occurred in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5), and 2016–2017 (visit 6) with a seventh visit ongoing. The study protocol was approved by the Institutional Review Boards (IRB) of all participating institutions: University of North Carolina at Chapel Hill IRB, Wake Forest University IRB, Johns Hopkins University IRB, University of Minnesota IRB, and University of Mississippi Medical Center IRB. The study was conducted in accordance with the Declaration of Helenski, and all study participants provided written informed consent10. For this study, data from individuals who attended visit 2 (N = 14,348) was used.
1,5-AG measurement
1,5-AG concentrations were measured using a colometric assay where 1,5-AG is oxidized to hydrogen peroxide (GlycoMark, Winston-Salem, NC) by the Roche Modular P800 system. Serum was collected at visit 2 (1990–1992) and analyzed in 2012–2013. The interassay coefficient of variation was 4.8%11 and the reliability coefficient was 0.99, calculated from 610 duplicate pairs of samples. Previous studies have shown high reliability in samples stored for long periods of time12.
Exome sequencing
DNA was extracted from blood primarily collected at visit 1. All sequencing was performed as part of the CHARGE Consortium exome sequencing project at the Baylor College of Medicine Human Genome Sequencing Center (HGSC). Samples were bar-coded, pooled and sequenced using paired-end sequencing, run on the Illumina HiSeq 2000 or 2500 platform (San Diego, CA), and exome capture performed with VCRome 2.1 (NimbleGen, Inc., Madison, WI). Sequence alignment was done using the Burrows-Wheeler alignment13 tool with the Genome Reference Consortium Human Build 37 reference sequence. Aligned reads were then recalibrated using the Genome ANalysis ToolKit (GATK). Variant calling was done with the Mercury pipeline (https://www.hgsc.bcm.edu/content/mercury) in DNAnexus. VCF files were generated using the Atlas2 suite (Atlas-SNP and Atlas-Indel).
Quality Control
Standard quality control exclusion measures were implemented to ensure accurate, reliable results. Single nucleotide variants (SNVs) were excluded if they met any of the following criteria: posterior probability <0.95, variant read count <3, variant read ratio <0.25 or >0.75, strand bias >99% in single direction, total coverage <10 fold for SNVs (<30x for indels), outside exon capture regions, monomorphic variant, missing rate >20%, mappability score <0.8, mean depth coverage >500 fold, Hardy Weinberg Equilibrium p < 5 × 10−6 in ancestry-specific groups. All samples provided consent for use in DNA studies and had adequate DNA for exome sequencing analysis. Samples were excluded if they had >20% missing data or fell less than 6 standard deviations (SD) from mean read depth, more than 6 SD for singleton count, outside of 6 SD for heterozygote to homozygote ratio or transition to transversion (Ti/Tv) ratio. After quality control, 2,556,859 SNVs and 76,133 indels remained, and 7,810 European Ancestry individuals and 3,180 African Ancestry individuals remained. Individuals who did not attend visit 2 (N = 594), were missing diabetes status (N = 2), had diagnosed diabetes (self-reported physician diagnosis or use of diabetes medications; N = 875), or missing 1,5-AG data at visit 2 (N = 621; Supplementary Fig. S1) were also excluded. In total, 6,589 European ancestry samples and 2,309 African ancestry samples were analyzed.
Variant annotation and functional prediction
ANNOVAR8 and dbNSFP v2.0 (https://sites.google.com/site/jpopgen/dbNSFP) were used to annotate variants to genes and functional predictions using the GRCh37 reference sequence and National Center for Biotechnology Information RefSeq. Functional annotation by several metrics predicted if a variant was expected to be damaging (an amino acid change which negatively impacts protein function) or deleterious (a variant which reduces fitness and is subject to purifying selection). SIFT score predicts if an amino acid change is likely to be damaging to protein function based on conservation (i.e., well conserved regions are assumed to be biologically important and thus variants in these regions are more likely to be damaging)14. A SIFT score <0.05 was considered damaging. Polyphen-2 flags amino acid changes that are predicted to be damaging based on the structure and function of a protein. Polyphen-2 score >0.957 was considered damaging, and a score between 0.453 and 0.965 was considered possibly damaging15. GERP predicts substitutions that would have occurred if the region was not under selection and quantifies rate of substitutions that did not occur16. A GERP score >2 was considered deleterious. Finally CADD aggregates annotations of allelic diversity, functionality, pathogenicity, disease severity, regulatory effects, complex trait associations, and known pathogenic variants into a score17. A CADD score >15 was considered deleterious. In addition, the Bravo portal (https://bravo.sph.umich.edu/freeze5/hg38/) was used to obtain TOPMed and 1000 Genomes allele frequencies (Freeze 5, including 463 million variants on 62,784 individuals). For each significant variant, the Genotype-Tissue Expression (GTEx) Project was searched for expression quantitative trait loci (eQTLs; https://www.gtexportal.org/home/).
Single-variant tests
Genetic associations with 1,5-AG were analyzed using both single-variant and gene-based tests using the R package SeqMeta. All analyses were run separately by ancestry. 1,5-AG values were winsorized at 1% and 99% to account for long tailed distributions. Single variant analyses were run as linear regressions controlling for age, sex, ARIC study center and significantly associated principal components (p < 0.05; N = 2 for European ancestry, 1 for African ancestry). To ensure our results were not driven by a very small number of individuals, variants with less than 10 copies of the minor allele (minimum minor allele frequency (MAF) = 10/(N*2)) were excluded. For the European ancestry sample MAF <0.008 (10/(6,589*2)) was used and for the African ancestry sample MAF <0.002 (10/(2,309*2)) was used. A Bonferroni correction to calculate a statistical significance threshold as 1.4 × 10−7 (0.05/121,052 variants) was used for European ancestry and 2.8 × 10−7 (0.05/175,583 variants) was used for African ancestry. Variance explained by individual variants was calculated as the difference between the coefficient of determination from the null model (the association between 1,5-AG and covariates) and a model adjusting for effect of the variant controlling for the same covariates.
Gene-based tests
To augment power for situations where multiple rare variants affect association with a phenotype, the SKAT-O test was run, which aggregates variants into genes and tests for association between genes and phenotypes. SKAT-O combines a burden test, which has greater power when variants are associated with the phenotype and in the same direction, with SKAT, a kernel based, variance components test, which has greater power when fewer variants are causal or affect risk in both directions. Genes with ≤1 variant per gene were excluded. Variants were not filtered by MAF in the main analysis. Variants other than nonsynonymous, splicing, stop-gain, stop-loss, or frameshift were excluded from the association analyses. Additionally, genes in which all variants used for the burden test together had a cumulative MAF <0.005 were excluded. A Bonferroni correction was used to calculate a significance threshold: 4.0 × 10−6 (0.05/12,504 genes) for European ancestry and 3.3 × 10−5 (0.05/14,499 genes) for African ancestry. Secondary analyses of SKAT and a T1 burden test (where all variants with MAF <0.01 were collapsed into a score for each gene) were also done.
Conditional analyses
To determine if the single-variant results represent independent signals, the top variant (defined as most significant and most deleterious or damaging by GERP, SIFT, Polyphen2 and CADD) was conditioned on for each locus with multiple significant variants. Secondary conditioning analyses were also performed on the most significant variant and the previous GWAS-identified variant. Regional association plots using LocusZoom (http://locuszoom.org/)18 were created to visualize the region prior to and after conditioning on the top variants.
Variant association with diabetes
To determine if variants significantly associated with 1,5-AG also impact diabetes, the association between these variants and prevalent diabetes status was evaluated. This analysis was performed for both diagnosed diabetes (self-reported physician diagnosis or use of diabetes medications) and the combination of diagnosed diabetes and undiagnosed diabetes (defined as fasting glucose ≥126 mg/dL if fasting for ≥8 hours or non-fasting glucose ≥200 mg/dL).
Results
Study population
There were 6,589 individuals in the European ancestry sample and 2,309 individuals in the African ancestry sample. In both groups, over half were female, and mean age was 56 to 57 years old. 1,5-AG levels were lower, and fructosamine, glycated albumin, fasting glucose and HbA1c were higher in the African ancestry sample as compared to the European ancestry sample. Study population characteristics are detailed in Supplementary Table S1.
Single variant and gene-based analyses
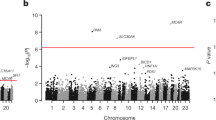
In the European ancestry sample, 15 variants reached exome-wide significance for association with 1,5-AG in single variant testing (Table 1). These variants are located in 6 loci on chromosomes 1, 2, 3, 7, 17, and 22, all of which were also identified in our previous GWAS of 1,5-AG concentrations6. None of the African ancestry single variant or gene-based results were statistically significant.
Rare, deleterious variants on chromosome 17
Four rare (MAF <0.007) and one low frequency (MAF = 0.04) variants in the region of two overlapping genes on chromosome 17, SLC5A10 and FAM83G (Table 1) were associated with 1,5-AG. Of the four rare variants, two were nonsynonymous to SLC5A10 and were highly significant (rs148178887, p = 1.13 × 10−36 and rs61741107, p = 8.85 × 10−56). In addition, the effect sizes of these variants were large, approximately 10 µg/mL per risk allele, and explained 1.71% and 2.95% of the variance in 1,5-AG concentrations, respectively. The other two rare variants were intronic to SLC5A10 but nonsynonymous to FAM83G (rs200038747, p = 1.69 × 10−13 and rs201046878, p = 1.96 × 10−29). The low frequency variant (rs117355297, p = 3.85 × 10−26) was also found in the GWAS6 and was synonymous to SLC5A10. All four nonsynonymous variants were predicted to be damaging or deleterious by the prediction programs GERP, Polyphen-2, SIFT and CADD. In addition, the nonsynonymous variants resulted in amino acid changes which altered polarity and acidity (for example, rs61741107 resulted in a change from nonpolar glycine to acidic glutamic acid, and rs148178887 resulted in a change from polar asparagine to nonpolar isoleucine). Four variants were also nominally (p < 0.05) associated with 1,5-AG in African ancestry individuals. The gene-based SKAT-O test showed significance for SLC5A10 and FAM83G (Table 2). Secondary analyses of separate SKAT (p = 2.8 × 10−55), T1 burden (p = 2.5 × 10−114) and SKAT-O restricting variants to MAF <0.05 (p = 5.1 × 10−64) tests also showed strong significance for SLC5A10 (Supplementary Tables S2–4).
To determine if the variants in this region were representing one signal in linkage disequilibrium or several independent signals, the nonsynonymous variants were conditioned on (Fig. 1). After conditioning on rs61741107, the variants rs148178887, rs201046878, rs200038747 and rs117355297 remained significant. After additionally conditioning on rs148178887, only rs117355297 remained significant. Further conditioning on the synonymous variant, rs117355297, produced no significant variants in this region. Secondary conditioning on the previously identified GWAS variant (rs117355297) showed the rare variants remained significant (p < 2.7 × 10−14). This suggests that these variants represent three independent, significant loci, which together explain 6% of the variance in 1,5-AG (Table 1). GTEx did not show eQTLs for any of the chromosome 17 variants in diabetes-relevant tissue such as the kidney, liver or pancreas.
Regional association plots for top hits on chromosome 17 in European ancestry sample, unconditioned and conditioned. Unconditioned analysis shows five genome-wide significant hits. Conditioning on rs61741107 results in four remaining genome-wide significant hits. Conditioning on both rs61741107 and rs148178887 results in a single genome-wide significant hit, indicating that three of the previous variants represent a single signal. Conditioning on rs61741107 and rs148178887 and rs117355297 results in no genome-wide significant hits, providing evidence that the five significant variants in this region are represented by three independent signals.
To further explore the rare variants in chromosome 17, they were evaluated for an association with diabetes. Of the variants representing the three significant signals on chromosome 17, none were significantly associated with diagnosed or diagnosed + undiagnosed diabetes status in either European or African ancestry samples (Supplementary Table S5). In addition, mean 1,5-AG levels differed substantially between individuals with and without the chromosome 17 variants, while the mean values of other glycemic biomarkers did not. (Fig. 2, Supplementary Fig. S2). No individuals were homozygous for rs61741107 or rs148178887, but eight people were homozygous for rs117355297 (Supplementary Fig. S3). Four individuals had both rs61741107 and rs117355297 (mean 1,5-AG = 2.8 µg/mL, SD = 1.3 µg/mL), 23 had both rs148178887 and rs117355297 (mean 1,5-AG = 9.5 µg/mL, SD = 3.8 µg/mL), and two people were heterozygous for rs148178887 and homozygous for rs117355297 (mean 1,5-AG = 2.8 µg/mL, SD = 0 µg/mL; Fig. 2).
Distribution of 1,5-AG by chromosome 17 variants. a,b With each additional copy of a significant chromosome 17 risk allele, median 1,5-AG values decrease indicating a dose-response effect. a 1,5-AG is winsorised at 1% and 99%. Legend: 0: no copies of rs61741107, rs148178887 or rs117355297 minor alleles. 1a: 1 copy of rs117355297 minor allele. 1b: 1 copy of rs61741107 or rs148178887 minor alleles. 2a: 2 copies of rs117355297 minor alleles. 2b: 1 copy of rs61741107 or rs148178887 + 1 copy of rs117355297 minor allele. 3: 1 copy of rs61741107 or rs148178887 + 2 copies of rs117355297 minor allele.
Additional regions of interest
One common variant in MUC1 on chromosome 1 in linkage disequilibrium (LD) with a variant identified in our GWAS (rs9330264, r2 = 0.5) was associated with 1,5-AG, but the gene-based test was not significant. Neither the single variant or gene-based test was validated in the African ancestry sample. GTEx indicated possible eQTLs in diabetes-related tissues (liver: GBAP1 p = 1.6 × 10−11, THBS3 p = 2.5 × 10−6; pancreas: GBAP1 p = 3.3 × 10−25, THBS3 p = 5.2 × 10−11, GBA p = 6.3 × 10−8).
Five common variants in genes LCT, RAB3GAP1, R3HDM1, and UBXN4 were associated on chromosome 2 across a large region spanning 0.7 Mb. Three of these variants (rs961360, rs1050115, rs2304371) were in LD with the GWAS index variant, rs182549 (r2 = 0.27 to 0.35 in 1000 genomes phase3v5 European population). Two of the five variants were nonsynonymous, one of which (rs961360) was predicted to be possibly damaging by Polyphen-2. The remaining three variants were synonymous and one was also associated in African ancestry individuals (rs1050115, p = 0.01). Conditional analysis on the top nonsynonymous variants revealed two distinct signals in this region (Supplementary Table S6). The GWAS index variant was not present in this dataset and hence could not be conditioned on. None of the genes in this region were associated with 1,5-AG in the gene-based test.
One common variant on chromosome 3 in SI was associated with 1,5-AG in European ancestry individuals. This variant is in near perfect LD with the GWAS index variant, rs9825346 (r2 = 0.98). It is a nonsynonymous variant, but was not predicted to be damaging or deleterious by any of the prediction programs and was not significant in African ancestry individuals.
One low-frequency (MAF = 0.01) variant on chromosome 7 in MGAM was associated with 1,5-AG. This variant is not in LD with the GWAS index variant. It is nonsynonymous and predicted to be damaging or deleterious by GERP, Polyphen-2, SIFT and CADD, but was not significant in African ancestry individuals. MGAM was associated with 1,5-AG in the gene-based test.
Finally, two common variants in SLC5A1 were associated in this region. Both variants were in near perfect LD with each other and the GWAS index variant, rs117086479 (r2 = 0.98 to 1). One variant was nonsynonymous (rs17683011) and the other was synonymous (rs17683448). Neither had evidence for deleteriousness by any measure, and neither variant was associated with 1,5-AG in African ancestry individuals. SLC5A1 was significant in the gene-based test.
Discussion
In this exome sequencing analysis, 15 variants were significantly associated with 1,5-AG among people of European ancestry without diabetes, and four of these variants in two loci were validated in a sample of African ancestry individuals. In addition, 4 genes were associated with 1,5-AG among individuals of European ancestry, of which one (SLC5A10) validated in the African ancestry sample.
Both single variant and gene-based tests identified a region on chromosome 17 in or near SLC5A10 and the overlapping gene, FAM83G. SLC5A10 is a glucose transporter exclusively expressed in the kidney19, and is not known to also transport 1,5-AG. Our results, however, suggest SLC5A10 may be an important transporter of 1,5-AG. Conditional analysis identified multiple distinct signals in this locus. Two of the variants identified (rs61741107 and rs148178887) were also found in a whole genome sequencing analysis of a metabolome panel20, adding further evidence to the importance of this region in influencing 1,5-AG levels. The effect sizes of most of the SLC5A10 variants are large. Given the distribution of 1,5-AG in this sample (in European ancestry: 3.4 to 38.2 winsorized) having just one copy of the rs61741107 or rs148178887 allele would result in a lowering of 1,5-AG by 10 µg/mL on average. Although no individuals in this dataset are homozygous for rs61741107 (1000 genomes European ancestry MAF = 0.002) or rs148178887 (1000 genomes European ancestry MAF = 0.005), the allele frequencies indicate that such individuals do exist in the population, and would have lowering of 1,5-AG levels of over 20 µg/mL on average. In addition, these effect sizes and allele frequencies were similar across ancestries. The smaller p value for the T1 gene-based test as compared to the SKAT test indicates that these variants impact 1,5-AG levels in the same direction. The similar p-value for SKAT-O when restricting variants to MAF <0.05 indicates that the relevant variants are low-frequency and rare.
Many of the variants in this region are predicted to be damaging or deleterious by multiple programs. SLC5A10 partially overlaps with FAM83G, which is expressed in the skin and esophagus (eQTLs; https://www.gtexportal.org/home/)19. It is not likely that variants in this region represent diabetes-related factors; neither gene is known to impact diabetes risk, fasting glucose or HbA1c. In addition, variants near SLC5A10 were not associated with diabetes status, and comparing individuals with and without variants in SLC5A10, multiple other measures of hyperglycemia were similar, while mean 1,5-AG differed substantially (Supplementary Fig. S2). Given this evidence, it is likely that rs61741107 and rs148178887 represent putative causal variants for 1,5-AG in this region.
In addition, significant, common variants on chromosomes 1, 2, 3 and 22, and a low frequency variant on chromosome 7 were associated with 1,5-AG concentrations. These loci were all identified by our previous GWAS in the region near LCT/UBXN4/R3HDM1/RAB3GAP1. While several of the variants in this region are nonsynonymous, they were mainly not predicted to be damaging or deleterious, and the effect sizes are relatively small, indicating a potentially more modest impact on 1,5-AG levels. Other regions which were significantly associated among Europeans but not Africans including MGAM, SLC5A1 and SI. Further studies are needed to confirm the role of rare variants in these regions in 1,5-AG.
There is currently debate about the utility of 1,5-AG as a useful biomarker of hyperglycemia in adults with diabetes. Prior to widespread use of any clinical test, it is important to identify limitations overall or for specific subpopulations. Warren et al. have shown that there is a proportion of individuals for whom 1,5-AG produces “false positive results”, i.e., where 1,5-AG concentrations are low while fasting glucose and 2-hour glucose levels are not elevated21. Our work shows evidence of a strong genetic impact on 1,5-AG unrelated to diabetes, which may explain some of these findings. The likely nonglycemic genetic impact on 1,5-AG identified in this work is similar to previous findings in HbA1c, for which variants have been identified which impact HbA1c levels, but are not important mechanisms of glucose control9,22,23. Our results may have implications for the overall utility of the biomarker independent of genetic characterization.
Exome sequencing has highlighted the role of SLC5A10 influencing 1,5-AG levels. This study has provided insight into the biology of this biomarker. Although these rare variants impact a smaller number of individuals than common variants, the large effect sizes would likely alter 1,5-AG levels in a sufficient manner to substantially impact its usefulness as a biomarker of hyperglycemia for carriers.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the dbGAP repository, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000668.v2.p1.
References
Buse, J. B., Freeman, J. L. R., Edelman, S. V., Jovanovic, L. & McGill, J. B. Serum 1,5-anhydroglucitol (GlycoMark): A short-term glycemic marker. Diabetes Technol Ther. 5, 355–363 (2003).
Dungan, K. M. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 8, 9–19 (2008).
Selvin, E. et al. Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem. 60, 1409–1418 (2014).
Selvin, E. et al. Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes. 65, 201–208 (2016).
Ikeda, N., Hara, H., Hiroi, Y. & Nakamura, M. Impact of serum 1,5-anhydro-d-glucitol level on prediction of major adverse cardiac and cerebrovascular events in non-diabetic patients without coronary artery disease. Atherosclerosis. 253, 1–6 (2016).
Li, M. et al. Genome-wide association study of 1,5-anhydroglucitol identifies novel genetic loci linked to glucose metabolism. Sci Rep. 7, 2812 (2017).
Shin, S. Y. et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 46, 543–550 (2014).
Liu, C. T. et al. Trans-ethnic meta-analysis and functional annotation illuminates the genetic architecture of fasting glucose and insulin. Am J Hum Genet. 99, 56–75 (2016).
Wheeler, E. et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS Med. 14, e1002383 (2017).
ARIC Investigators. The atherosclerosis risk in communities (ARIC) study: Design and objectives. Am J Epidemiol. 129, 687–702 (1989).
Juraschek, S. P., Steffes, M. W., Miller, E. R. & Selvin, E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 35, 2265–2270 (2012).
Selvin, E., Rynders, G. P. & Steffes, M. W. Comparison of two assays for serum 1,5-anhydroglucitol. Clin Chim Acta. 412(9–10), 793–5 (2011).
Li, H. & Durbin, R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 26, 589–595 (2010).
Ng, P. C. & Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat Methods. 7, 248–249 (2010).
Cooper, G. M. et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 15, 901–913 (2005).
Kircher, M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nature Genetics. 46, 310–315 (2014).
Pruim, R. J. et al. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics. 26, 2336–2337 (2010).
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 45, 580–585 (2013).
Long, T. et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 49, 568–578 (2017).
Warren, B. et al. Diagnostic Performance of 1,5-Anhydroglucitol Compared to 2-H Glucose in the Atherosclerosis Risk in Communities Study. Clin Chem. 64(10), 1536–1537 (2018).
Chen, P. et al. Multiple nonglycemic genomic loci are newly associated with blood level of glycated hemoglobin in East Asians. Diabetes. 63, 2551–62 (2014).
Soranzo, N. et al. Common Variants at 10 Genomic Loci Influence Hemoglobin A1C Levels via Glycemic and Nonglycemic Pathways. Diabetes. 59, 3229–39 (2010).
Acknowledgements
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I and HHSN268201700005I). The authors thank the staff and participants of the ARIC study for their important contributions. Funding support for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium” was provided by the NIH through the American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419). Sequencing was carried out at the Baylor College of Medicine Human Genome Sequencing Center (U54 HG003273 and R01HL086694). The work of AK was funded by DFG KO 3598/3-1 and KO 3598/4-1. SL was supported by an institutional training grant from the NIH/NHLBI (T32 HL007024). This work was also supported by NIH/NIDDK grants K24DK106414 and R01DK089174 to Dr. Selvin. Reagents for the 1,5-anhydroglucitol assays were donated by GlycoMark, Inc.
Author information
Authors and Affiliations
Contributions
S.J.L. designed the study, performed analyses, interpreted results and was wrote the manuscript. A.K., E.S. and P.D. provided substantial guidance in study design, results interpretation and manuscript revisions. R.G. and D.M. performed the exome sequencing. All authors provided subject area expertise and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loomis, S.J., Köttgen, A., Li, M. et al. Rare variants in SLC5A10 are associated with serum 1,5-anhydroglucitol (1,5-AG) in the Atherosclerosis Risk in Communities (ARIC) Study. Sci Rep 9, 5941 (2019). https://doi.org/10.1038/s41598-019-42202-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42202-0
This article is cited by
-
Serum betaine and dimethylglycine in mid-pregnancy and the risk of gestational diabetes mellitus: a case-control study
Endocrine (2024)
-
SGLT5 is the renal transporter for 1,5-anhydroglucitol, a major player in two rare forms of neutropenia
Cellular and Molecular Life Sciences (2023)
-
Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in AppNL-G-F, AppNL-F, and wild type mice
Scientific Reports (2021)
-
Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application
Pflügers Archiv - European Journal of Physiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.