Abstract
The Dex-CSDH trial is a randomised, double-blind, placebo-controlled trial of dexamethasone for patients with a symptomatic chronic subdural haematoma. The trial commenced with an internal pilot, whose primary objective was to assess the feasibility of multi-centre recruitment. Primary outcome data collection and safety were also assessed, whilst maintaining blinding. We aimed to recruit 100 patients from United Kingdom Neurosurgical Units within 12 months. Trial participants were randomised to a 2-week course of dexamethasone or placebo in addition to receiving standard care (which could include surgery). The primary outcome measure of the trial is the modified Rankin Scale at 6 months. This pilot recruited ahead of target; 100 patients were recruited within nine months of commencement. 47% of screened patients consented to recruitment. The primary outcome measure was collected in 98% of patients. No safety concerns were raised by the independent data monitoring and ethics committee and only five patients were withdrawn from drug treatment. Pilot trial data can inform on the design and resource provision for substantive trials. This internal pilot was successful in determining recruitment feasibility. Excellent follow-up rates were achieved and exploratory outcome measures were added to increase the scientific value of the trial.
Similar content being viewed by others
Introduction
In a chronic subdural haematoma (CSDH), blood and fluid collect in the subdural space overlying the brain. It primarily affects elderly patients, many of whom have experienced a head trauma within the preceding weeks1. Recent literature suggests a critical role for inflammation in causing fluid and blood exudation from neovascularised subdural membranes2. The application of steroids with their potent anti-inflammatory effect is therefore logical, and has shown potential in smaller, non-randomised studies3,4,5,6,7,8,9.
A multi-institutional group of clinicians and academics in the United Kingdom (UK) designed the Dex-CSDH trial to address a gap in evidence. The trial aims to investigate whether dexamethasone can improve the 6-month functional outcome of patients with symptomatic CSDH by reducing the rate of surgical intervention and recurrence. This paper describes the feasibility phase (internal pilot) of the Dex-CSDH trial.
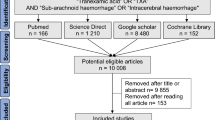
The Consolidated Standards of Reporting Trials (CONSORT) recommendations have recently been extended to include pilot trials, all of which are addressed in this pilot (Fig. 1)10, alongside the standard CONSORT checklist (see supplementary information). The data collected during internal pilots can be used in the final substantive trial analysis, and thus has remained blinded, but is still helpful for informing on the design of future pilot trials11.
relevant methodological considerations in a pilot trial10.
Primary Pilot Trial Objective
The primary objective of the Dex-CSDH pilot Trial is to assess recruitment feasibility across UK neurosurgical units (NSU). Several factors may influence enrolment of patients and the following were considered in design of this trial;
-
1.
The UK has helped take a lead on improving research in neurosurgery, being 3rd in the world for publishing neurosurgery randomised control trials (RCT) between 2000-201412. This has been a challenge due to historically low levels of neurosurgical RCTs which can result in deficient research infrastructure, training and experience13. Of 64 neurosurgical RCTs registered between 2000–2012, 17 (26.6%) were discontinued early, mainly because of insufficient patient recruitment14. To avoid this, focus on engagement of local research teams to maximise patient recruitment and retention is essential.
-
2.
Head trauma confers specific challenges to recruitment. The need for emergency intervention limits the time available for clinicians and patients to consider enrolment into clinical trials. Patients often lack capacity and the non-availability of a legal representative (e.g. next-of-kin (NOK)) can impede recruitment. In 2006, the UK clinical trials directive amended the Medicines and Healthcare Products Regulatory Agency (MHRA) clinical trials guidelines regarding consent of incapacitated adults without an available NOK15. This now permits their enrolment in trials for urgent treatments, provided there is relevant ethics committee approval. The Dex-CSDH pilot trial has this approval in the form of “Independent Healthcare Provider (IHP) consent”.
-
3.
Finally, older patients are under-represented in medical research, even for pathologies or medicines most relevant to their age group16,17,18. As CSDH occurs almost exclusively in older patients, we aimed to demonstrate that the barriers to trial recruitment and follow-up can be overcome. Members from a local public involvement research group (INsPIRE) were involved in the trial design to help ensure it would be acceptable for our target patient group.
Secondary pilot trial objectives
Our secondary objectives were to assess i) the feasibility of outcome measures ii) follow-up rates and iii) any early safety concerns.
Primary outcome data must be accurately and reliably collected to avoid bias from missing data. To optimise follow-up, postal and telephone questionnaires and involvement of the NOK were utilised.
An increased mortality in patients treated with steroids in the “Corticosteroid Randomisation After Significant Head injury” (CRASH) trial and the reported complication rate associated with steroid use in older patients, particularly those with co-morbidities, mandated a careful and thorough assessment of drug tolerability in this trial19,20,21. Conversely, successful and safe steroid use in older patients for treatment of polymyalgia rheumatica, temporal arteritis, chronic obstructive pulmonary disease and glioblastoma offers reassurance of beneficence22,23,24,25. Steroid side effects are generally dose and duration dependent23,26. Regimen optimisation is therefore important to minimise serious adverse events (SAEs) and maximise therapeutic benefit.
The internal pilot trial was designed using methodology concordant to the planned substantive trial, with the potential to make amendments if supported by the secondary objectives.
Methods
The Dex-CSDH pilot trial is a randomised, double-blind, placebo-controlled trial of a two-week course of dexamethasone for 100 adult patients with a symptomatic chronic subdural haematoma (see full protocol in supplementary information). Patients were randomly assigned by an interactive web-based system to the intervention (dexamethasone) or control group (placebo). A 1:1 allocation as per a computer-generated randomisation schedule stratified by site using permuted blocks of random sizes is employed.
Recruitment
In this pilot trial, we planned to recruit patients from up to 10 NSUs ranging in catchment population, research experience and resources, to reflect a realistic picture of multi-centre recruitment11. All NSUs had support from a local neurosurgical trainee acting as a co-principal investigator as part of the British Neurosurgical Trainee Collaborative (BNTRC)27. Hospital episode statistics (HES) indicate that a medium sized NSU admits 60–80 CSDH patients per year. Setting a conservative estimate, we predicted a recruitment rate of two patients per month for each NSU. We set an overall target of 100 patients to be recruited to the pilot within 12 months.
Eligibility criteria and overall trial design were planned in a way that would maximise participation in order to support eventual translation of findings to as broad a population as possible (Tables 1 and 2). Informed consent was obtained from the patient, NOK or IHP by an appropriately trained doctor or nurse identified on the delegation log.
Primary outcome measure
The primary outcome measure was determined as the modified Rankin Scale (mRS) at six months’ post-randomisation, as it is a core instrument for measuring the degree of disability or dependence in daily activities of living (Fig. 2)28. It is an ordinal scale but dichotamised values of 0–3 (good outcome) and 4–6 (poor outcome) have been used in previous CSDH research29,30. The final time-point of six months was selected so that most CSDH recurrences had occurred and been treated, and to permit adequate time for recovery and adaptation to disability31. The mRS is assessed by blinded research staff via telephone interviews or by the patient completing a structured postal questionnaire32.
modified Rankin Scale28. Category 6 added to allow for mortality outcomes.
Secondary outcome measures
Secondary outcome measures are listed in Table 3. Amongst others, these included endpoints commonly used in the field of CSDH, such as re-operation, mortality and complications33.
Drug regimen and safety
The Dex-CSDH trial regimen was designed by incorporating a literature review (see Table 4) with the clinical expertise of the protocol development team including neurosurgeons, elderly care specialists and pharmacists. The regimen starts with a high dose (16 mg/day) and tapers down quickly to stop over 14 days, providing an average weekly dose of 62 mg dexamethasone. This is comparable to the average steroid doses reported in previous CSDH studies, and is at the lower end of course duration, to minimise complications from prolonged use. The drug is over-encapsulated so that dexamethasone is indistinguishable from placebo.
The pilot trial could potentially identify early safety concerns, therefore there was close monitoring of SAEs and adverse events of special interest (AESIs). The latter were adverse events we expected in relation to steroid use from our clinical experience in neurosurgery and included; hyperglycaemia requiring treatment or stopping of trial medication, new-onset diabetes, psychosis and gastric symptoms (e.g. dyspepsia, gastric ulcer).
Progression from internal pilot to substantive trial
Progression criteria were determined prior to initiation of the pilot (Fig. 3). These were reviewed alongside the blinded pilot trial data by the trial steering committee (TSC), and the unblinded data by the independent data monitoring and ethics committee (IDMEC). Review of blinded pilot trial data was used to enable minor protocol modifications to assist conduct of the substantive trial (see results).
Sample size calculation and statistical analysis for the substantive trial
The overall sample size of the substantive Dex-CSDH trial is 750 patients. An 8% increase in the rate of favourable outcome (mRS 0–3) at 6 months was considered a clinically important treatment effect. On the basis of the available literature, we estimated that 80–85% of the control group will have a favourable outcome3,29. Using a 2-sided test at the 5% significance level, a sample of 750 patients (allowing 15% loss to follow-up) will detect an absolute difference of 8% with a power of 81–92%. The internal pilot data will be included in the substantive trial results, on an intention-to-treat analysis. Secondary endpoints will also be summarised and an economic analysis performed (see protocol for full details).
Results
Primary objective: Recruitment
Pilot trial recruitment commenced in August 2015, five months later than the original anticipated start date of March 2015 (Fig. 4). A further six sites opened to recruitment over the subsequent eight months. The 100th patient was recruited within nine months of the August start date, easily surpassing the recruitment target rate and allowing progression onto the substantive trial which has now recruited 653 patients to July 2018 (Table 5).
The time from research and development (R + D) first contact to site opening was an average of 5.7 months in the pilot trial (excluding the sponsor site which required a more rigorous opening procedure), and has increased to nine months for the substantive trial (see Table 5).
Anonymised patient screening logs were available from all pilot sites; 47% of patients screened were enrolled (Table 6). A reason for screening failure was provided in 92 out of the 114 pilot patients screened (81%). The most common reason was patient or NOK declining consent (42%), followed by patient co-morbidities (19%), current steroid use (13%), outside 72 hr recruitment window (9%), NOK unable to attend for consent (8%) and other reasons (9%).
Secondary objectives: outcome measures, follow-up and safety
The outcome measures were completed centrally via postal or telephone questionnaire with few errors. The mRS questionnaire created several queries and the Montreal Cognitive Assessment (MoCA) was poorly completed therefore these two parts were reviewed for amendment (see changes to substantive trial protocol below). The remaining outcome measures were considered appropriate to the trial population and easy to collect.
On completion of the pilot, the first 31 patients had completed their primary outcome at six months and 55 patients had met the secondary 3-month outcome time point (Table 7). Retention in the pilot trial was excellent with primary outcome data received from 100% of the 30 eligible patients (one patient withdrawn). In 5% of patients, data was missing at the secondary time-point of 3-months. At six months, nearly all patients were back at home, or else the General Practitioners (GP) was aware of the patient location, enabling improved follow-up at this later time point.
The outcome data was reviewed again when all 100 pilot patients had met their primary end-point at 6-months and was still collected for 100% of eligible patients (two patients withdrawn). The patterns of change in mRS from data reviewed at the steering committe meeting following completion of the pilot are summarised in Fig. 5. Collection rates of the secondary time-point of 3-months was slightly worse, with 11% of data missing.
An SAE was experienced in 10 (10%) of pilot trial patients, with one patient experiencing two SAEs (Table 8). Twelve patients (12%) experienced an AESI, with hyperglycaemia the most common event (Table 8). As only the IDMEC has access to unblinded data, it is not possible to currently state whether these AESIs occurred in patients receiving dexamethasone or placebo.
Overall, there was a 5% (5/100) withdrawal rate from the trial medication. Two patients withdrew because of hyperglycaemia. One patient withdrew following a stroke, after taking the trial medication for only one day. These three patients remained in the trial with regards to follow-up. Two further patients withdrew completely from the trial and all follow-up; one felt the tablets were too large and the other reported hallucinations after taking one dose (reported as AESI). Unblinded data was reviewed by the IDMEC and the compliance and safety profile of the trial medication was considered acceptable.
The TSC reviewed all pilot data and confirmed that as patient recruitment was feasible and there were no ethical or safety concerns, the substantive trial should take place. Recommendations were made for minor protocol amendments as detailed below. The National Institute for Health Research (NIHR) health technology assessment (HTA) programme subsequently approve progression into the substantive trial.
Changes to substantive trial protocol based upon the pilot
Eligibility changes
One major change occurred to the exclusion criteria following completion of the pilot trial; from “patients who are already on steroids” to “patients who are on (or within one month of) regular oral or IV steroids”. The term “regular” was added because we noted that in some centres patients are given one dose of intra-operative dexamethasone for anti-emesis. This long-standing practice involves a one-off dose too small to have any significant impact on outcome. Nevertheless, it was agreed to collect all data on single-use steroids as a concomitant medication. The route “oral or IV” was added to clarify that patients on inhaled steroids for conditions such as asthma could be included, and a 1-month washout period of recent steroid use was also stated.
Outcome measures and follow-up
The pilot trial identified that remote collection of the mRS led to several data errors due to incorrect completion of this part of the questionnaire by patients or blinded assessors. The complex order of instructions led to essential questions being missed, meaning the final mRS could not be calculated without repeat patient assessment. To rectify this, the questionnaire and instructions were simplified and an adjudication process implemented where all responses were immediately reviewed by a blinded clinician in the study team, to ensure timely calculation of the mRS. Set dates for completion of questionnaires were also amended to “windows” of acceptability to minimise protocol violations and allow patients a wide opportunity to respond (Table 3).
The increase in missing data at three months (Table 7) was reviewed and identified to relate to the follow-up process performed by a central trial administrator. At the sponsor site only, the local research nurse (RN) performed follow-up and had better follow-up rates due to more timely posting of questionnaires and regular follow-up phone calls to the patient, NOK and/or GP where necessary. Therefore, it was determined that follow-up could be maximised if the sponsor site RN performed follow-up for all sites.
Changes to secondary outcome measures
Review of the secondary outcomes showed a paucity of data from discharge and follow-up MoCA scores . As this assessment must be done face-to-face, it was missed if patients were discharged over the weekend or with little notice. It could also not be performed during remote follow-up and very few patients attended clinic. Therefore, it was deemed that the most pragmatic solution was to remove the MoCA from the substantive trial (Table 3).
Changes to safety processes
As a result of three inadvertent administrations of ward stock dexamethasone rather than blinded trial medication, patient trial bracelets were introduced for the substantive trial. These clearly state that the patient is prescribed a trial medication and must not receive ward stock dexamethasone. As identity wristbands must be checked before giving any medication we expected these to deter inadvertent ward dexamethasone use.
Initially all SAEs were collected throughout the 6-month follow-up period, however all SAEs occurring more than 30 days after randomisation were considered to be unrelated to the study medication and more often related to further falls, which are common in this patient group. Limiting SAE collection to the first 30 days is in-keeping with other CSDH studies and avoids unnecessary additional patient contact, given that the 3-month and 6-month follow-up is done remotely3.
Addition of exploratory outcome measures
The original outcome measures were all clinically relevant, but none addressed the mechanistic actions of dexamethasone. The TSC agreed that exploratory outcome measures would be useful to aid scientific understanding of how dexamethasone works in CSDH, supporting its clinical application and helping direct future studies on alternative CSDH pharmacotherapies.
The first exploratory objective was to assess the biological action of dexamethasone by analysing intra-operative and post-operative blood and CSDH fluid samples; excluding patients with active malignancy or immunosuppressive therapy. A range of inflammatory markers involved in CSDH pathophysiology have already been identified34,35,36,37. We planned to measure a panel of these markers, assessing their response to dexamethasone exposure in trial patients and whether this related to the recurrence rate.
Dexamethasone is also well known to reduce cerebral oedema, a feature which has not previously been investigated in CSDH38,39. Cerebral oedema can occur due to fluid leaking through the blood-brain-barrier and has been linked to blood flow patterns in the brain (cerebral perfusion)40,41,42. There is some evidence that cerebral perfusion is globally reduced in CSDH and improves following surgical treatment43,44,45,46. Therefore, the second exploratory objective was to assess the role of dexamethasone in cerebral perfusion and oedema, utilising transcranial Doppler and magnetic resonance imaging (excluding patients with renal dysfunction or a pacemaker/metal implant).
Discussion
Multicentre trials often do not meet their original target recruitment in time and must be extended47. In fact, our average recruitment of 2.2 patients/month per site during the pilot trial exceeded the target of 2 patients/month per site. The target was far exceeded in two of the sites (8/month in Cambridge and 3.3/month in Southampton), and was below the target in the remaining five sites. Of these five sites, two are small centres (Plymouth and Imperial) with limited populations to recruit from and the remaining three had only been open two months. Overall, we considered that our target recruitment plan from the pilot could be applied to the substantive trial, but that we would attempt to open larger centres first and implement techniques to promote recruitment at the lowest recruiting sites. Three of the seven pilot sites have gone on to be in the top five recruiting sites in terms of monthly recruitment rate for the whole trial (Table 5). This may mean that we were successful in identifying strong sites to open during the pilot period, or that the longer sites are open the better they are at recruiting.
Recruitment patterns from individual centres should be carefully observed when assessing recruitment feasibility with a pilot trial. As despite recruitment curves traditionally being exponential in design, this often does not reflect the realities of trial recruitment, which after an initial take-off can remain constant. Recruitment fatigue can also mean that previously well-recruiting centres may decline over the course of the years it takes to complete a large trial. Indeed, many of the sites we recruited after the pilot phase are recruiting less well than those opened during the pilot phase and recruiting rates in the top centres have remained stable or declined (Table 5). Overall the recruitment has declined from an average of 2.2 patients per site per month to 1.2, resulting in recruitment falling behind target in the substantive trial despite exceeding the pilot target (Fig. 4).
An average R + D set-up time of 5.7 months during the pilot meant that most sites opened later than anticipated and only two sites were open for the first six months of the pilot (Table 5). This led to a bias in the pilot recruitment with 75% of the patients recruited from the lead site (Cambridge) and only 25% from other sites. This could affect the generalisability of the trial. To address this, recruitment is continually encouraged at other sites and the TSC specified that the final trial should not have more than 50% of the patients recruited from a single site. Currently 37% of patients have been recruited from the lead site.
The top two recruiting pilot sites were also the sites screening the largest number of patients (11–14 per site per month), whilst other sites only screened 2–4 patients per month. This may relate to the staffing at these sites, as both have a research fellow and nurse dedicated to trials. This enables them to invest more time and effort in identifying, approaching and discussing the trial with potential patients. Most other centres are reliant on the clinical staff to screen and enrol patients, adding to their daily workload and therefore requiring significantly more motivation. It is also highly dependent on the availability and support from the local RN, who may not have a neuroscience affiliation. It is self-evident that limited infrastructure and research staffing will have an impact on the delivery of RCTs.
A recent review on strategies to improve recruitment to RCTs suggested that only open-label studies and telephone reminders have been shown to increase recruitment47. As neither of these strategies were appropriate for this blinded trial in an acute neurosurgical population, we considered that efforts would be best directed towards promoting site engagement and incentivising investigators at each site to screen and enrol patients. This included setting up a trial website; regular newsletters to all sites highlighting trials news with local and national recruitment rates; trial posters for use in clinical areas to remind clinicians about the trial; additional site visits for training; promotion of the trial at national meetings and finally an annual investigators day. Feedback from this latter initiative has been very positive and is perceived to significantly impact on site engagement.
Screening failures were reviewed to assess recruitment and any potential bias in patient selection. The most common reason for screening failure was patient or NOK declining. Aside from ensuring local teams are well trained in giving correct and detailed trial information, this is an acceptable and expected reason for screening failure. The second most common reason was patient co-morbidity and it became apparent from discussion with sites that there was variation in the assessment of this. For example, some sites were reporting general “frailty” as a common cause of exclusion, whilst other sites would be more inclusive of patients with wide ranging co-morbidities and medications. Clinical judgement is clearly required during the screening process but this does also mean that the clinicians own bias will be introduced. Indeed, it is recognised that elderly patients can be perceived by clinicians as being vulnerable and needing “protection from researchers”, despite their desire to engage in trials17. We specifically tried to be as pragmatic and inclusive as possible when designing the eligibility criteria, so that the trial results will be widely applicable to the elderly population affected by CSDH. We have found regular dissemination of the trial progress and low adverse event rate has helped encourage this.
In 8% of screening failures the patient was deemed to lack capacity and there was no available NOK. Patients lacking capacity are usually those with more severe CSDHs resulting in cognitive deficits. To avoid skewing the recruitment towards the CSDH patients with milder symptoms, IHP consent can be used when patients lack capacity. The pilot highlighted the need to promote this and train sites about appropriate use of IHP consent. There is evidence from a public opinion survey on patient inclusion in severe traumatic brain injury (TBI) clinical trials that 91% of respondents would be happy for an independent doctor to assent48. Despite this, we have found there is persistent institutional reluctance to use such proxy consent processes, which is only likely to be overcome by continued adoption into trials where appropriate.
The reliability of trial outcome measure collection was monitored during the pilot leading to a minor amendment to the mRS questionnaire, which had been incomplete in some cases. Primary outcome data was collected in 97% of patients at the end of pilot recruitment period and was maintained at 98% once all pilot patients had reached six months, with only two patients withdrawn. The internal pilot has provided a realistic estimate of retention and follow-up which has been maintained into the substantive trial. It also reflects effective trial design with regard to strategies to maintain patients in the trial. This includes the techniques discussed in Table 2, particularly ensuring that follow-up was remote, as elderly patients are more likely to participate if follow-up is done from home49. A review of strategies to improve retention in randomised trials also showed that financial incentives improve return of postal and electronic questionnaires, however this was not considered appropriate in an NHS setting50. While postal reminders have been reported to have no significant effect on follow-up, we found telephone follow-up reminders very helpful50. This may be specific to elderly patients who will have a higher rate of cognitive and physical impairments that make filling in and returning a written questionnaire difficult. Some patients reported preferring to answer the questionnaires over the phone.
The follow-up rate has remained excellent into the substantive trial, despite the number of trial sites increasing to 21 and extending from England into Scotland and Wales. The follow-up is now undertaken by the sponsor site RN, who dedicates a lot of time to liaising with local hospitals, GPs, patients and their NOKs to get the outcome data. A 6-month lost-to-follow-up (LTFU) rate of 0% in the pilot is exceptional and can only hope to be maintained throughout the substantive trial, however less than 5% LTFU is suggested to be acceptable in minimising bias to trial results51.
The pooled primary outcome (6-month mRS) was found to be favourable in 83% of pilot patients, which was close to that predicted for the control group for the sample size calculation (80–85%). As the aim of the pilot was not to assess efficacy or calculate sample size, no further analysis was done at this stage. An interim analysis with a sample size calculation once approximately 500 patients have reached their primary outcome is planned. This will permit us to make any sample size adjustments if we are close to seeing a significant treatment effect.
We anticipated a relatively high SAE rate in a trial on elderly patients with a surgical condition. This was 10% in the pilot and has remained at a comparable rate of 13% in the substantive trial to date. None of the SAEs in the pilot were considered related to the trial medication and all were events that might be expected in this cohort. Data was also collected on AESIs which were reported in 12% of pilot patients. Interestingly, the cumulative AESI rate in the most recent safety report from July 2018 is only 7% (including pilot and substantive trial data). This highlights the risks of collecting data in a small portion of patients such as a pilot which can have implications on subsequent trial conduct.
The IDMEC reviewed unblinded data on SAEs, AESIs and outcomes reported during the pilot, and had no safety or ethical concerns, recommending continuation onto the substantive trial with the same protocol.
Conclusion
The Dex-CSDH pilot trial demonstrated feasible recruitment, with an excellent follow-up rate and no safety concerns. This supported transition into an on-going substantive trial with only minor trial amendments aimed at improving data collection, streamlining safety processes and promoting recruitment. These changes, alongside the addition of exploratory outcomes, add value and scope to this important clinical trial.
Pilot trials are useful to assess feasibility and guide conduct of the subsequent substantive trial. Careful analysis of both screening and recruitment patterns permits predictable estimation of multi-centre trial recruitment and sharing data from pilot experiences can help guide future pilot and substantive trial design.
Data Availability
The data reported in this manuscript was analysed for internal reporting to the TSC and IDMEC and is available from the trial management group on reasonable request. On completion of the substantive trial, all data will be deposited in the University of Cambridge repository.
References
Kolias, A. G., Chari, A., Santarius, T. & Hutchinson, P. J. Chronic subdural haematoma: modern management and emerging therapies. Nat. Rev. Neurol. 10, 570–578 (2014).
Edlmann, E., Giorgi-Coll, S., Whitfield, P. C., Carpenter, K. L. H. & Hutchinson, P. J. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 14, 108 (2017).
Sun, T. F., Boet, R. & Poon, W. S. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg. 19, 327–333 (2005).
Berghauser Pont, L. M., Dammers, R., Schouten, J. W., Lingsma, H. F. & Dirven, C. M. Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 70, 873–880 (2012).
Berghauser Pont, L. M., Dirven, C. M., Dippel, D. W., Verweij, B. H. & Dammers, R. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. 19, 1397–1403 (2012).
Qian, Z., Yang, D., Sun, F. & Sun, Z. Risk factors for recurrence of chronic subdural haematoma after burr hole surgery: potential protective role of dexamethasone. Br J Neurosurg. 31, 84–88 (2017).
Thotakura, A. K. & Marabathina, N. R. Nonsurgical treatment of chronic subdural haematoma with steroids. World Neurosurg. 84, 1968–1972 (2015).
Bender, M. B. & Christoff, N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 31, 73–79 (1974).
Delgado-Lopez, P. D. et al. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur). 20, 346–359 (2009).
Eldridge, S. M. et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 355, i5239 (2016).
Avery, K. N. L. et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ open. 7, e013537 (2017).
Mansouri, A., Cooper, B., Shin, S. M. & Kondziolka, D. Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered? J Neurosurg. 124, 558–568 (2016).
Barker, F. G. Editorial: Randomized clinical trials and neurosurgery. J Neurosurg. 124, 552–556 (2016).
Jamjoom, A. A. B., Gane, A. B. & Demetriades, A. K. Randomized controlled trials in neurosurgery: an observational analysis of trial discontinuation and publication outcome. J Neurosurg. 127, 857–866 (2017).
Medicines for Human Use (Clinical Trials) Amendment (No. 2) Regulations, http://www.legislation.gov.uk/uksi/2006/2984/regulation/2/made (2006).
Konrat, C. et al. Underrepresentation of Elderly People in Randomised Controlled Trials. The Example of Trials of 4 Widely Prescribed Drugs. PLoS One. 7, e33559 (2012).
McMurdo, M. E., Witham, M. D. & Gillespie, N. D. Including older people in clinical research. BMJ. 331, 1036–1037 (2005).
Aapro, M. S., Kohne, C. H., Cohen, H. J. & Extermann, M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 10, 198–204 (2005).
Roberts, I. et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 364, 1321–1328 (2004).
Caughey, G. E., Preiss, A. K., Vitry, A. I., Gilbert, A. L. & Roughead, E. E. Comorbid diabetes and COPD: impact of corticosteroid use on diabetes complications. Diabetes Care. 36, 3009–3014 (2013).
Walijee, A. K. et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 357, j1415 (2017).
Buttgereit, F., Dejaco, C., Matteson, E. L. & Dasgupta, B. Polymyalgia Rheumatica and Giant Cell Arteritis: A Systematic Review. JAMA. 315, 2442–2458 (2016).
Vondraeck, S. F. & Hemstreet, B. A. Is There an Optimal Corticosteroid Regimen for the Management of an Acute Exacerbation of Chronic Obstructive Pulmonary Disease? Pharmacotherapy. 26, 522–532 (2006).
Walters, J. A., Tan, D. J., White, C. J. & Wood-Baker, R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 3, CD006897 (2018).
Brodbelt, A. et al. Glioblastoma in England: 2007-2011. Eur J Cancer. 51, 533–542 (2015).
Vecht, C. J., Hovestadt, A., Verbiest, H. B., van Vilet, J. J. & van Putten, W. L. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8 and 16 mg per day. Neurology. 44, 675–680 (1994).
Chari, A. et al. The British Neurosurgical Trainee Research Collaborative: Five years on. Acta Neurochir (Wien). 160, 23–28 (2017).
van Swieten, J. C., Koudstaal, P. J., Visser, M. C., Schouten, H. J. & van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 19, 604–607 (1988).
Santarius, T. et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 374, 1067–1073 (2009).
Manickam, A., Marshman, L. A. & Johnston, R. Long-term survival after chronic subdural haematoma. J Clin Neurosci. 34, 100–104 (2016).
Schmidt, L., Gortz, S., Wohlfahrt, J., Melbye, M. & Munch, T. N. Recurrence of Subdural Haematoma in a Population-Based Cohort – Risks and Predictive Factors. PLoS One. 10, e0140450 (2015).
Bruno, A. et al. Simplified Modified Rankin Scale Questionnaire Reproducibility Over the Telephone and Validation with Quality of Life. Stroke. 42, 2276–2279 (2011).
Chari, A. et al. Core Outcomes and Common Data Elements in Chronic Subdural Hematoma: A Systematic Review of the Literature Focusing on Reported Outcomes. J Neurotrauma. 33, 1212–1219 (2016).
Wada, T. et al. Local elevation of the anti- inflammatory interleukin-10 in the pathogenesis of chronic subdural hematoma. Neurosurg Rev. 29, 242–245 (2006).
Hong, H. J. et al. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol. 71, 161–165 (2009).
Frati, A. et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural haematoma: a prospective study. J. Neurosurgery 100, 24–32 (2004).
Stanisic, M. et al. Chemokines as markers of local inflammation and angiogenesis in patients with chronic subdural haematoma: a prospective study. Acta Neurochir (Wein). 154, 113–120 (2012).
Maxwell, R. E., Long, D. M. & French, L. A. The clinical effects of synthetic gluco-corticoid used for brain edema in the practice of neurosurgery in Steroids and brain edema (ed. Reulen, H. J. & Shurmann, K.) 219–232 (Springer-Verlag 1972).
Neuenfeldt, D., Herrmann, H. D. & Loew, F. The influence of dexamethasone on the blood-brain-barrier and water content in experimental brain oedema. Acta Neurochir (Wien). 30, 51–57 (1974).
Ostergaard, L. et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumours. J Neurosurg. 90, 300–305 (1999).
Anderson, C. & Jensen, F. T. Differences in blood-tumour-barrier leakage of human intracranial tumours: quantitative monitoring of vasogenic oedema and its response to glucocorticoid treatment. Acta Neurochir (Wein). 140, 919–924 (1998).
Anderson, C. et al. Resorption of peritumoural oedema in cerebral gliomas during dexamethasone treatment evaluated by NMR relaxation time imaging. Acta Neurochir (Wien). 122, 218–224 (1993).
Kaplan, M., Erol, F. S., Bozgeyik, Z. & Koparan, M. The effectiveness of simple drainage technique in improvement of cerebral blood flow in patients with chronic subdural hamorrhage. Turk Neurosurg. 17, 202–206 (2007).
Inao, S. et al. Relation between brain displacement and local cerebral blood flow in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 71, 741–746 (2001).
Slotty, P. J. et al. Cerebral Perfusion Changes in Chronic Subdural Hematoma. J Neurotrauma. 30, 347–351 (2013).
Lee, E. J., Lee, M. Y. & Hung, Y. C. The application of transcranial Doppler sonography in patients with chronic subdural haematoma. Acta Neurochir (Wien). 141, 835–839 (1999).
Treweek, S. et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2, MR000013 (2018).
Clark, D. J. et al. Community consultation in emergency neurotrauma research: results from a pre-protocol survey. Acta Neurochir (Wien). 155, 1329–1334 (2013).
Watts, G. Why the exclusion of older people from clinical research must stop. BMJ. 344, e3445 (2012).
Brueton, V. C. et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev. 12, MR000032 (2013).
Schulz, K. F. & Grimes, D. A. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 359, 781–785 (2002).
Emich, S. et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural haematoma – the DRESH study: straightforward study protocol for a randomized controlled trial. Trials. 15, 6 (2014).
Chan, D. Y. C., Sun, T. F. D. & Poon, W. S. Steroid for chronic subdural hematoma? A prospective phase IIB pilot randomized controlled trial on the use of dexamethasone with surgical drainage for the reduction of recurrence with reoperation. Chinese Neurosurgical Journal. 1, 2 (2015).
Prud’homme, M., Mathieu, F., Marcotte, N. & Cottin, S. A pilot placebo controlled randomized controlled trial of Dexamethasone for Chronic Subdural Haematoma. Can J Neurol Sci. 43, 284–290 (2016).
Acknowledgements
The following should be cited as pubmed collaborators; We would like to acknowledge other protocol contributors: Carol Brayne, Andrew Gardner, Andrew King, Kate Massey, Thais Minett, Patrick Mitchell, Phyo Myint, Elizabeth Warburton. TSC members: Professor Anthony Bell (Chair), Allison Hirst, Laurence Watkins, Peter McCabe. IDMEC members: Martin Smith (Chair), Joan Grieve, Jonathan Cook. The Dex-CSDH trial is funded by the NIHR HTA programme (project 13/15/02). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, National Institute for Health Research or the Department of Health and Social Care. Ellie Edlmann has been supported by the Royal College of Surgeons, Rosetrees Trust Research Fellowship. Eric Thelin is funded by post-doc stipends from the Swedish Society for Medical Research Angelos Kolias is supported by a Clinical Lectureship, School of Clinical Medicine, University of Cambridge. Peter Hutchinson is supported by a Research Professorship from the National Institute for Health Research (NIHR), the NIHR Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Consortia
Contributions
E.E. wrote the manuscript and was involved in trial design, recruitment and data analysis. E.P.T. edited the manuscript and was involved in trial recruitment and data analysis. K.C. was the lead site R.N. involved in patient recruitment and follow-up data collection on all pilot patients. C.T. edited the manuscript and is involved in trial oversight. P.W. was a site P.I., involved in trial recruitment and edited the manuscript. D.B., N.S., Y.Z.A.-T. and S.T. are trial PIs who have led trial recruitment and local centre oversight. P.H., K.O.-A., D.G., I.A.A., O.R. and M.G. are local site Co-PIs who have led trial recruitment and local centre oversight. E.T., D.N. and P.K. led trial recruitment at pilot sites. B.P. reviewed and managed trial data. C.D.-W. is the trial co-ordinator. S.T. is the current sponsor site trial RN collecting outcome data. G.B. was involved in the economic analysis plan for the trial. H.J.M., A.C. and A.B. were involved in protocol development. S.B., R.G. and S.D. were the trial statisticians for the pilot data. L.W. is the trial lead pharmacist. P.B. was a site P.I., involved in protocol development and edited the manuscript. I.W. oversees the Cambridge Clinical Trials Unit supporting running the trial. A.G.K. was involved in trial design, oversight and manuscript editing. P.J.A.H. was involved in trial design, oversight and manuscript editing. All authors read the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A comprehensive list of consortium members appears at the end of the paper
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Edlmann, E., Thelin, E.P., Caldwell, K. et al. Dex-CSDH randomised, placebo-controlled trial of dexamethasone for chronic subdural haematoma: report of the internal pilot phase. Sci Rep 9, 5885 (2019). https://doi.org/10.1038/s41598-019-42087-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42087-z
This article is cited by
-
Postoperative neutrophil-to-lymphocyte ratio variation is associated with chronic subdural hematoma recurrence
Neurological Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.