Abstract
Exercise training is a low cost and safe approach for reducing the risk of cardiovascular disease development. Currently, moderate-intensity training (MIT) is the most preferred exercise type. However, high-intensity interval training (HIIT) is gaining interest especially among athletes and healthy individuals. In this study, we examined cardiac remodeling resulting from MIT and HIIT in healthy rats. Healthy male Sprague-Dawley rats were randomly assigned to MIT or HIIT for 13 weeks. Animals kept sedentary (SED) were used as control. Cardiac function was evaluated with echocardiography and hemodynamic measurements. Heart tissue was stained for capillary density and fibrosis. After 13 weeks of training, only HIIT induced beneficial cardiac hypertrophy. Overall global cardiac parameters (such as ejection fraction, cardiac output and volumes) were improved similarly between both training modalities. At tissue level, collagen content was significantly and similarly reduced in both exercise groups. Finally, only HIIT increased significantly capillary density. Our data indicate that even if very different in design, HIIT and MIT appear to be equally effective in improving cardiac function in healthy rats. Furthermore, HIIT provides additional benefits through improved capillary density and should therefore be considered as a preferred training modality for athletes and for patients.
Similar content being viewed by others
Introduction
Exercise training intervention is recognized as an important and low-cost and safe strategy to prevent and treat cardiovascular diseases1,2. Exercising on a regular basis is indeed associated with a decreased risk for coronary artery diseases and heart failure through a reduced blood pressure, increased myocardial perfusion capacity and improved cellular metabolism in the healthy heart3,4. Overall, physiological left ventricular (LV) remodeling, as generated by repeated bouts of endurance exercise, has been shown to improve cardiac performance in healthy subjects and increase tolerance for ischemia and reperfusion injury, resulting in a beneficial effect on disease progression and survival in patients with LV dysfunction5,6,7,8.
In that context, many studies have demonstrated that moderate-intensity endurance training (MIT) is beneficial for cardiac function, both in healthy and pathological situations8,9. Recently, high-intensity interval exercise training (HIIT) has raised much attention, not only to assist coaches in adjusting training programs for elite and recreational athletes10, but also as a new approach to handle heart failure patients11,12. Indeed, HIIT is a widely used and effective training method in various sports, including endurance and sprint events, combining respectively glycolytic and oxidative metabolism13. Recent data indicate that HIIT provides indeed additional benefits and could be even more potent than MIT in improving cardiorespiratory function, metabolic response and cardiac function in healthy adults14,15,16,17,18. A number of studies attribute this potential to a higher cardiomyocyte mitochondrial fatty acid oxidation and metabolism15,16,17,18,19. Data also suggest that HIIT might provide additive benefits compared with the classical MIT modality19. In cardiac rehabilitation in T2DM patients, HIIT provides improved systolic blood pressure (SBP), HbA1C, high-density lipoprotein cholesterol (HDL-C), malondialdehyde (MDA) levels, Nitric Oxide (NO) and von Willebrand factor (vWF). Overall, HIIT seems superior compared with MIT in improving vascular functions20. Importantly, molecular pathways and effects of these different exercise modalities on cardiovascular effects are however still equivocal and remain to be elucidated.
In this study, we hypothesized that HIIT could provide higher cardiovascular gain in physiological remodeling, potentially through improved mitochondrial metabolism and tissue vascularization. The aim of the current study was therefore to perform a thorough comparison on the beneficial effects induced by either MIT or HIIT on cardiac function in healthy rats. This could lead to optimization of exercise programming for healthy individuals, athletes and heart failure patients seeking for strategies to enhance cardiac function.
Results
Different exercise types improve cardiac function to the same extent
At baseline, no difference were observed neither in body weight nor in cardiac function assessed by conventional echocardiography between the three groups (data not shown). Thirteen weeks after the start of exercise intervention, sedentary animals gained significantly more weight than trained animals (body weight SED: 596 ± 16 g; MIT: 483 ± 18 g; HIIT: 472 ± 13 g, p < 0.05). Body weight change was comparable between the two training groups.
Conventional echocardiographic characteristics of the animals are summarized in Table 1. Ejection fraction, a parameter for global cardiac function, was significantly improved after 13 weeks of exercise training (p < 0.05). Furthermore, animals undergoing HIIT specifically displayed changes in LV morphology with wall hypertrophy (increased of 24% for AWT and 17% for PWT compared to SED, p < 0.05) while MIT did not induce wall hypertrophy. Finally, EDV was not different between groups, while ESV significantly and progressively decreased with MIT and HIIT intervention (p < 0.05). These changes resulted in a significant increase in SV of 27% in both training groups (p < 0.05), while CO was preserved.
Hemodynamic measurements are summarized in Table 2. Both exercise modalities were equally able to decrease LVP as compared to SED animals (p < 0.05). Contractility, as evaluated with dP/dtmax, as well as relaxation parameters, i.e. dP/dtmin and tau, were comparable in all groups (p < 0.05).
Overall, data indicate beneficial cardiac remodeling following exercise training, most global improvements are independent of the exercise modality.
As opposed to MIT, HIIT training is able to increase capillary density
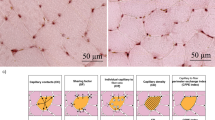
Figure 1a are representative images of interstitial collagen obtained with Sirius red/Fast Green in heart sections after 13 weeks of exercise training. As summarized in Fig. 1b, total interstitial collagen was significantly and equally decreased in animals subjected to exercise training. Citrate synthase activity, a marker of aerobic capacity and mitochondrial mass, improved equally in both groups undergoing a training program (p < 0.05) (Fig. 2a). Additionally, capillary density, evaluated by a CD31 staining in cardiac tissue sections, was unchanged with MIT (p > 0.05) but was significantly increased with HIIT (p < 0.05) (Fig. 2b). Finally, no difference was observed in Endothelin-1 (Fig. 2d), NADPH Oxidase 2 (NOX2) nor oxidative phosphorylation (OXPHOS) protein levels (Fig. 2e). Complex II enzyme activity levels were not altered after exercise, however, a positive trend was observed in HIIT animals (Fig. 2f).
Exercise intervention reduces cardiac collagen content. Representative images of interstitial collagen, as indicated by the arrows, in LV sections obtained with Sirius red/Fast Green in SED, MIT and HIIT groups. (b) Total interstitial collagen quantification in SED (N = 10), MIT (N = 8) and HIIT (N = 8). Data are shown as mean ± SEM. *Denotes p < 0.05 vs. SED.
Exercise intervention increases citrate synthase activity and capillary density. (a) Citrate synthase activity, measured in LV homogenates, in all groups. (b) Capillary density expressed as blood vessels per surface area, in the three groups. (c) Protein level of endothelin-1, normalized to β-actin. (d) Protein level of NADPH Oxidase 2 (NOX2), normalized to β-actin. (e) Protein level of oxidative phosphorylation (OXPHOS) complex IV, normalized to β- actin. (f) Enzyme activity of complex II in cardiac tissue. (g) Results of representative Western blots. Cropped images of Western blot, for full blots see Supplementary Figs 1–3. All blots were performed individually. Data are shown as mean ± SEM from SED (N = 10), MIT (N = 8) and HIIT (N = 8) for graphs, blots represent a partial blot. *Denotes p < 0.05 vs. SED.
Discussion
In this study, we demonstrate that both MIT and HIIT intervention, although very different training types, lead to beneficial effects on cardiac function in healthy animals. Importantly, HIIT might provide additional benefits as opposed to MIT as HIIT is able to increase blood capillary density, an important feature for optimal cardiac muscle metabolism.
Both HIIT and MIT lead to beneficial cardiac remodeling, resulting in an improved cardiac function through reduced afterload
While some studies demonstrate that MIT improves cardiac function, others report very little changes21. The beneficial effects of exercise training are believed to be mainly attributed to improved neurohumoral, inflammatory, metabolic and central hemodynamic responses as well as on endothelial, skeletal and cardiovascular function, leading to an overall cardiac improvement and an improved tolerance for ischemia and reperfusion injury5,6. One major hallmark of LV remodeling related to endurance exercise training, independent of the applied training modality, is the increase in LV mass. Cardiac hypertrophy is commonly accepted to be physiological and not associated with adverse effects, as opposed to pathological hypertrophy which involved substantially different signaling pathways22,23,24,25. Pathological hypertrophy is related with an increase in collagen accumulation and wall thickness, causing reduced cardiac function2 which was not observed here. In our study, we have shown that unlike MIT, HIIT increases AWT and PWT, markers of LV wall hypertrophy, as also confirmed in the study of Hafstad et al.2,26. In the study of Kemi et al. a HIIT modality was more efficient in inducing LV hypertrophy compared when moderate-intensity training was applied27. These data suggest that HIIT might be more efficient than MIT in physiological cardiac remodeling. However, whether this is related to activation of different/specific signaling pathways remains to be elucidated. Endothelin-1 is known to promote hypertrophic remodeling, but no change was observed in the different groups in our study, indicating alternative pathways for remodeling after exercise.
Physiological adaptation of cardiac function to exercise training involves structural and metabolic remodeling, resulting in a lower resting HR, improved CO and changed volumes28. In our study, independent of the applied training modality, HR and EDV were not statistically different from sedentary animals. Noteworthy, EF, a marker of global cardiac function, was significantly improved with both exercise training interventions. As also shown by Dawes et al.28, the improved global cardiac function was associated with an increased SV in MIT and HIIT groups. The increase in SV, together with unchanged EDV but reduced ESV, indicates an increased cardiac contractility and/or reduced afterload in trained animals29. The effect of endurance training on cardiac contractility remains however controversial. While some studies demonstrate an increased contractility attributed to an improved oxygen delivery, angiogenesis and nitric oxide (NO) sensitivity after endurance exercise training24,30, others report very little changes21. In our study, dP/dtmax, a parameter reflecting cardiac contractility, was not changed, indicating that the beneficial cardiac changes observed are likely to be attributed to a decreased afterload, rather than a major change in contractility. This is further confirmed by the decreased LVP, which, combined with a CO that tended to be higher, indicates a decrease in vascular resistance, resulting in a reduced afterload. In that context, the effect of exercise training on endothelial function and, as a result, on vasodilatation, was already well described by others in humans and experimental models12,31,32,33. Indeed, exercise training has been shown to lead to vascular adaptation and improved vascular function through increased NO levels34,35. Our in vivo data indicate that an improved cardiac function is likely attributed to vascular adaptations, leading to a reduced afterload. However, the exact mechanisms and further study on endothelial function, examining the effect of the different training interventions on NO levels remains to be performed.
It has been shown in rodents that exercise training is not necessarily accompanied by myocardial collagen change24. In our study, we show that both exercise interventions were able to substantially decrease interstitial collagen content in cardiac muscle. This decrease in fibrosis was however not strongly associated with improvements of diastolic function as previously described36,37,38,39. Indeed, despite the unchanged EDV and unchanged CO, EDP only tended to be decreased in both training modalities (p = 0.07). Whether an improved diastolic function related to a decreased fibrosis content following exercise training can be completely ruled out remains to be further confirmed as it has been shown that diastolic function (i.e. EDP) is directly associated with cardiac fibrosis level40,41. However, most experiments examining fibrosis and diastolic function have been performed under pathological settings39,42. In our study, we examined physiological remodeling in healthy rats. Therefore, the effect of exercise on cardiac fibrosis level and its impact on diastolic function is likely to be substantially more important in pathological situations as levels of fibrosis in pathological settings are at least 2 times larger than what is reported in healthy situations40,43.
What is the added value of HIIT?
It is known that an increase in cardiac capillary density is important for the development of physiological hypertrophy44,45. In that context, the link between angiogenesis and hypertrophy has been previously emphasized44 where the seen effects were shown to be mediated through paracrine effects and secretion of NO and growth factors (such as VGEF, NRG-1). In our study, only HIIT was able to increase capillary density, indicating a somehow higher potential for HIIT as compared to MIT. Very recently, further confirming our results, the study of Machado et al. demonstrated that exercise training was able to prevent capillary rarefaction in obese rats with the metabolic syndrome46. An adequate capillary density allows for greater oxygen transport to the cardiac muscle and improves ultimately cardiac metabolism. In our study, citrate synthase activity, a measure of mitochondrial mass, was significantly improved after MIT and HIIT. Together with this, complex II enzyme activity seemed to be increased in HIIT compared with sedentary animals, however not significantly. Our data therefore suggest that HIIT might provide additional benefits compared to MIT, through increased capillary density and the resulting improved oxidative metabolism, not limited to the skeletal muscle as shown by Takada et al.47 but also in the heart, as suggested in the current study and by others48. Cardiac metabolism requires aerobic production of ATP. In our study, HIIT provides increased cardiac vascularity to counter high oxygen requirements during exercise, as in HIIT exercise is performed at almost maximal oxygen consumption. Increased mitochondrial activity and mass will result in an increased ATP production, necessary to keep up with the cardiac energy demand49. Since blood supply is essential to improve cardiac function, particularly in pathological situations where blood vessels are dramatically reduced50 or when angiogenesis needs to be restored to optimize cardiac repair through stem-cell therapy for instance51, HIIT might be a great advantage compared to MIT. However, further investigation is required to draw conclusions as underlying changes related to the different training interventions on endothelial function, including mitochondrial morphology and mitochondrial function, are so far unknown. Exercise and oxidative stress have a complicated relationship, as exercise can have both a positive and negative effect on oxidative stress52. NOX-2 plays a role in hypertrophy and interstitial fibrosis, is a super-oxide generating enzyme playing a role in oxidative stress and reactive oxygen species (ROS)53. However, in our study, no effect on oxidative stress levels was observed after exercise. This can be due to multiple causes, as mode, intensity and duration of the exercise are known to play an important role in the observed result.
Finally, because training modalities used in HIIT are short in time, motivation and adherence of patients to HIIT training as compared to MIT, could be potentially higher. This could be a serious advantage in the clinical setting since both trainings offer beneficial cardiac remodeling with an added value to HIIT at the molecular level. Last but not least, not limited to a pathological setting, one could speculate that HIIT could be a way to improve in a more efficient and faster way, cardiovascular function in athletes. However, translation of results from bench to bed-side should be done with caution taking into account the differences between animal and human studies.
Conclusion
Our data indicate that even if very different in design, HIIT and MIT appear to be equally effective in improving cardiac function in healthy rats. Furthermore, HIIT shows some promising results and might provide additional benefits through improved capillary density. Therefore, it could be considered as a preferred training modality for athletes and for patients for whom improvements in cardiac function are aimed at.
Methods
This investigation conforms to the EU directive 2010/63/EU for animal experiments and was approved by a local ethical committee (Ethische Commissie Dierproeven, UHasselt, Diepenbeek, Belgium). All experiments and methods were performed according the relevant guidelines.
Study protocol and exercise intervention
Twenty-six male Sprague-Dawley rats (Charles River Laboratories, L’Arbresle, France), weighing 200–225 g were used throughout the study. All animals were familiarized with the treadmill (IITC, California, United States of America) prior the experiment (for 3 days/week) and were randomly assigned (by randomization program) to one of the three experimental groups after 2 weeks familiarization. Group 1 did not undergo exercise training throughout the study (SED, N = 10). Group 2 was subjected to moderate intense endurance training schedule, consisting in running on a treadmill, 18 m/min, 5° inclination, 1 h/day, 5 days/week (MIT, N = 8). Group 3 consisted in 10 bouts of high-intensity treadmill running (18 m/min, 30° inclination), separated by 1 min of active rest, 5 days/week (HIIT, N = 8). Sample size was calculated by a Power analysis based on previous results.
The intensity of exercise training was assessed by measuring blood lactate levels directly after exercise with an Analox apparatus (Analis, Namur, Belgium). Levels > 4 mmol/l lactate were considered HIIT54. Training modalities were adjusted to lead to an equal energy expenditure between interventions by calculating the net caloric cost (kcal/min) using following calculations:
-
VO2 max = Sa * 0.2 + (S * Gb) * 0.9 (Sa = speed (m/min), S = speed (m/min), Gb = inclination);
-
Net caloric cost (kcal/min) = VO2 max * 3.5 * body mass (kg)/200
All animals were maintained in a controlled environmental condition of temperature and humidity and had water and food (normal rodent diet, ENVIGO, The Netherlands) available ad libidum. Blood samples, hemodynamic measurements and echocardiographic measurements were executed 13 weeks after the start of the training program.
Conventional echocardiographic measurements
Prior to sacrifice, transthoracic echocardiography was performed under 2% isoflurane in all animals with a Vivid I ultrasound machine (GE Vingmed Ultrasound) using a 10 MHz linear array transducer. The protocol used is as described previously40,41. Briefly, a standard parasternal long axis image and short axis views at the mid-ventricular level were obtained at a temporal resolution of approximately 200 frames per second. Conventional echocardiographic parameters (e.g. LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), posterior wall thicknesses (PWT) and anterior wall thicknesses (AWT)) were obtained from the B-mode images at midpapillary level in the parasternal short-axis view. End-systolic volumes (ESV) and end-diastolic volumes (EDV) were calculated by π*DM2*B/6, where DM indicates the systolic/diastolic diameter of the ventricle in mid-ventricular short-axis view and B is LV length on parasternal long-axis image. Subsequently, ejection fraction (EF) was measured as (EDV–ESV)/EDV, and expressed in %.
Hemodynamic measurements
Conductance measurements were performed in all animals with the use of an SPR-320 MikroTip high-fidelity pressure transducer (Millar Inc) that was advanced into the left ventricle via the right carotid artery, as described previously41. The pressure catheter (2 F) was connected to a quad-bridge amplifier and PowerLab 26 T module (AD Instruments, United Kingdom) was used to transfer the data to LabChart v7.3.7 software (AD Instruments, United Kingdom).
Citrate synthase activity in cardiac homogenates
Citrate synthase activity was determined in LV cardiac homogenates using a citrate synthase assay kit (CS0720; Sigma-Aldrich, St. Louis, MO)55.
Fibrosis and capillary density measurements in tissue sections
Transversal sections of 7 µm thick were obtained at cardiac midventricular level and stained using the Sirius Red/Fast Green kit (Chondrex). Fibrosis was assessed in all animals in four randomly chosen fields per section. The area of collagen deposition indicated by red staining was outlined and quantified using an automated image analysis program (Carl Zeiss, AxoVision 4.6, Zaventem, Belgium). Blood vessels were excluded. Total collagen deposition to the global cardiac area was calculated and expressed as percent collagen deposit.
Capillary density was quantified from histological sections by immunohistochemical staining for CD31 (SC-1506, Santa Cruz, 1/100). Capillaries were visualized by 3-3-diaminobezedine (DAB) and counterstained using hematoxylin. The amount of blood vessels were counted in 10 different fields per section and averaged. Data are expressed as amount of capillaries per µm2.
Endothelin-1, OXPHOS and NOX2 protein levels
Protein concentrations of the LV tissues were determined by the BCA protein assay kit (Thermo Fisher, Erembodegem, Belgium). Western blot was performed as previously described40. Briefly, equal amounts of proteins (15 µg) were separated on a 12% SDS-PAGE gel with a mini protean 3 electrophoresis system (Bio-rad Laboratories, Temse, Belgium), transferred to a polyvinylidene fluoride (PVDF) membrane and subsequently, blocked for 2 h with 5% milk in Tris-buffered solution containing 0.1% Tween-20 (TBS-T) followed by incubation overnight at 4 °C in the presence of a specific endothelin-1 antibody (1/2500, Abcam, ab117757, Cambridge, United Kingdom), NOX2 antibody (1/2500, Abcam, ab31092, Cambridge, United Kingdom) or an OXPHOS antibody (1/1000, Abcam, ab110411, Cambridge, United Kingdom). Horseradish peroxidase-conjugated secondary antibodies (DAKO, Belgium) at a dilution of 1/2000 were used. Both primary and secondary antibodies were diluted in 5% milk-TBS-T. Visualization was performed with the enhanced chemiluminescence (ECL) technique using the Pierce ECL Plus western Blotting Substrate (Thermo Fisher, Erembodegem, Belgium). Data were normalized to β-actin protein levels.
Complex II enzyme activity
Complex II enzyme activity in cardiac tissue was determined using a Complex II Enzyme Activity Microplate Assay Kit (Abcam, ab109908, Cambridge, United Kingdom). Samples were prepared as described in the protocol, and added to an anti-Complex II monoclonal antibody-coated 96-well plate. Absorbance was measured at OD600 nm for 60 minutes, measuring every 20 seconds.
Statistical analysis
Results were tested for normality prior to statistical tests. Subsequently, one-way ANOVA test was performed combined with a post- hoc test, dependent of normality. If data were normally distributed, Tukey’s range test was performed. Elsewise, the Dunn’s test was used. Analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). A value of p < 0.05 was considered statistically significant.
References
Shortreed, S. M., Peeters, A. & Forbes, A. B. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart 99, 649–654, https://doi.org/10.1136/heartjnl-2012-303461 (2013).
Cassidy, S. et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 59, 56–66, https://doi.org/10.1007/s00125-015-3741-2 (2016).
Palacios, G. et al. Biomarkers of physical activity and exercise. Nutr Hosp 31(Suppl 3), 237–244, https://doi.org/10.3305/nh.2015.31.sup3.8771 (2015).
Montero, D., Diaz-Canestro, C. & Lundby, C. Endurance Training and V O2max: Role of Maximal Cardiac Output and Oxygen Extraction. Med Sci Sports Exerc 47, 2024–2033, https://doi.org/10.1249/MSS.0000000000000640 (2015).
Marongiu, E. & Crisafulli, A. Cardioprotection acquired through exercise: the role of ischemic preconditioning. Curr Cardiol Rev 10, 336–348 (2014).
Li, Y. et al. Endurance exercise accelerates myocardial tissue oxygenation recovery and reduces ischemia reperfusion injury in mice. PLoS One 9, e114205, https://doi.org/10.1371/journal.pone.0114205 (2014).
Hordern, M. D. et al. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart 95, 1343–1349, https://doi.org/10.1136/hrt.2009.165571 (2009).
Garza, M. A., Wason, E. A. & Zhang, J. Q. Cardiac remodeling and physical training post myocardial infarction. World J Cardiol 7, 52–64, https://doi.org/10.4330/wjc.v7.i2.52 (2015).
Marwick, T. H. et al. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119, 3244–3262, https://doi.org/10.1161/CIRCULATIONAHA.109.192521 (2009).
Stoggl, T. L. & Bjorklund, G. High Intensity Interval Training Leads to Greater Improvements in Acute Heart Rate Recovery and Anaerobic Power as High Volume Low Intensity Training. Front Physiol 8, 562, https://doi.org/10.3389/fphys.2017.00562 (2017).
Weston, K. S., Wisloff, U. & Coombes, J. S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48, 1227–1234, https://doi.org/10.1136/bjsports-2013-092576 (2014).
Wisloff, U. et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115, 3086–3094, https://doi.org/10.1161/CIRCULATIONAHA.106.675041 (2007).
Abe, T. et al. High-intensity interval training-induced metabolic adaptation coupled with an increase in Hif-1alpha and glycolytic protein expression. J Appl Physiol (1985) 119, 1297–1302, https://doi.org/10.1152/japplphysiol.00499.2015 (2015).
Milanovic, Z., Sporis, G. & Weston, M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med 45, 1469–1481, https://doi.org/10.1007/s40279-015-0365-0 (2015).
Rankin, A. J., Rankin, A. C., MacIntyre, P. & Hillis, W. S. Walk or run? Is high-intensity exercise more effective than moderate-intensity exercise at reducing cardiovascular risk? Scott Med J 57, 99–102, https://doi.org/10.1258/smj.2011.011284 (2012).
Roxburgh, B. H., Nolan, P. B., Weatherwax, R. M. & Dalleck, L. C. Is moderate intensity exercise training combined with high intensity interval training more effective at improving cardiorespiratory fitness than moderate intensity exercise training alone? J Sports Sci Med 13, 702–707 (2014).
Seeger, J. P. et al. Interval exercise, but not endurance exercise, prevents endothelial ischemia-reperfusion injury in healthy subjects. Am J Physiol Heart Circ Physiol 308, H351–357, https://doi.org/10.1152/ajpheart.00647.2014 (2015).
Wen, C. P. et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378, 1244–1253, https://doi.org/10.1016/S0140-6736(11)60749-6 (2011).
Chilibeck, P. D., Bell, G. J., Farrar, R. P. & Martin, T. P. Higher mitochondrial fatty acid oxidation following intermittent versus continuous endurance exercise training. Can J Physiol Pharmacol 76, 891–894 (1998).
Miele, E. M. & Headley, S. A. E. The Effects of Chronic Aerobic Exercise on Cardiovascular Risk Factors in Persons with Diabetes Mellitus. Curr Diab Rep 17, 97, https://doi.org/10.1007/s11892-017-0927-7 (2017).
Palmer, G. S., Borghouts, L. B., Noakes, T. D. & Hawley, J. A. Metabolic and performance responses to constant-load vs. variable-intensity exercise in trained cyclists. J Appl Physiol (1985) 87, 1186–1196, https://doi.org/10.1152/jappl.1999.87.3.1186 (1999).
Scheuer, J., Malhotra, A., Hirsch, C., Capasso, J. & Schaible, T. F. Physiologic cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. J Clin Invest 70, 1300–1305 (1982).
Scheuer, J. Catecholamines in cardiac hypertrophy. Am J Cardiol 83, 70H–74H (1999).
Johnson, E. J., Dieter, B. P. & Marsh, S. A. Evidence for distinct effects of exercise in different cardiac hypertrophic disorders. Life Sci 123, 100–106, https://doi.org/10.1016/j.lfs.2015.01.007 (2015).
Bernardo, B. C., Weeks, K. L., Pretorius, L. & McMullen, J. R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128, 191–227, https://doi.org/10.1016/j.pharmthera.2010.04.005 (2010).
Hafstad, A. D. et al. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes 62, 2287–2294, https://doi.org/10.2337/db12-1580 (2013).
Kemi, O. J. et al. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67, 161–172, https://doi.org/10.1016/j.cardiores.2005.03.010 (2005).
Dawes, T. J. et al. Moderate Physical Activity in Healthy Adults Is Associated With Cardiac Remodeling. Circ Cardiovasc Imaging 9, https://doi.org/10.1161/CIRCIMAGING.116.004712 (2016).
Vincent, J. L. Understanding cardiac output. Crit Care 12, 174, https://doi.org/10.1186/cc6975 (2008).
Moore, R. L. et al. Chronic exercise alters contractility and morphology of isolated rat cardiac myocytes. Am J Physiol 264, C1180–1189, https://doi.org/10.1152/ajpcell.1993.264.5.C1180 (1993).
Haram, P. M. et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81, 723–732, https://doi.org/10.1093/cvr/cvn332 (2009).
Rognmo, O. et al. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res 22, 535–542, https://doi.org/10.1519/JSC.0b013e31816354b1 (2008).
Amundsen, B. H., Wisloff, U. & Slordahl, S. A. Exercise training in cardiovascular diseases. Tidsskr Nor Laegeforen 127, 446–448 (2007).
Kemi, O. J., Haram, P. M., Wisloff, U. & Ellingsen, O. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation 109, 2897–2904, https://doi.org/10.1161/01.CIR.0000129308.04757.72 (2004).
Hoydal, M. A. et al. Nitric oxide synthase type-1 modulates cardiomyocyte contractility and calcium handling: association with low intrinsic aerobic capacity. Eur J Cardiovasc Prev Rehabil 14, 319–325, https://doi.org/10.1097/HJR.0b013e3280128bef (2007).
Buakhamsri, A. et al. Impact of left ventricular volume/mass ratio on diastolic function. Eur Heart J 30, 1213–1221, https://doi.org/10.1093/eurheartj/ehp084 (2009).
Motoyasu, M. et al. Correlation between late gadolinium enhancement and diastolic function in hypertrophic cardiomyopathy assessed by magnetic resonance imaging. Circ J 72, 378–383 (2008).
Wang, Y. et al. Female sex is associated with worse prognosis in patients with hypertrophic cardiomyopathy in China. PLoS One 9, e102969, https://doi.org/10.1371/journal.pone.0102969 (2014).
Akahori, H. et al. Atorvastatin ameliorates cardiac fibrosis and improves left ventricular diastolic function in hypertensive diastolic heart failure model rats. J Hypertens 32, 1534–1541; discussion 1541, https://doi.org/10.1097/HJH.0000000000000184 (2014).
Deluyker, D. et al. Cross-linking versus RAGE: How do high molecular weight advanced glycation products induce cardiac dysfunction? Int J Cardiol 210, 100–108, https://doi.org/10.1016/j.ijcard.2016.02.095 (2016).
Deluyker, D. et al. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci Rep 7, 16010, https://doi.org/10.1038/s41598-017-16255-y (2017).
Takimoto, E. et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11, 214–222, https://doi.org/10.1038/nm1175 (2005).
Stevens, A. L. et al. Exercise improves cardiac function and attenuates insulin resistance in Dahl salt-sensitive rats. Int J Cardiol 186, 154–160, https://doi.org/10.1016/j.ijcard.2015.03.094 (2015).
Tirziu, D. et al. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest 117, 3188–3197, https://doi.org/10.1172/JCI32024 (2007).
Walsh, K. & Shiojima, I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest 117, 3176–3179, https://doi.org/10.1172/JCI34126 (2007).
Machado, M. V. et al. Exercise training dose differentially alters muscle and heart capillary density and metabolic functions in an obese rat with metabolic syndrome. Exp Physiol 102, 1716–1728, https://doi.org/10.1113/EP086416 (2017).
Takada, S. et al. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc Res 111, 338–347, https://doi.org/10.1093/cvr/cvw182 (2016).
Park, S. Y. et al. Exercise training improves vascular mitochondrial function. Am J Physiol Heart Circ Physiol 310, H821–829, https://doi.org/10.1152/ajpheart.00751.2015 (2016).
Kolwicz, S. C. Jr. An “Exercise” in Cardiac Metabolism. Front Cardiovasc Med 5, 66, https://doi.org/10.3389/fcvm.2018.00066 (2018).
Wadowski, P. P. et al. Sublingual functional capillary rarefaction in chronic heart failure. Eur J Clin Invest, https://doi.org/10.1111/eci.12869 (2017).
Kang, H. J. et al. Intracoronary infusion of the mobilized peripheral blood stem cell by G-CSF is better than mobilization alone by G-CSF for improvement of cardiac function and remodeling: 2-year follow-up results of the Myocardial Regeneration and Angiogenesis in Myocardial Infarction with G-CSF and Intra-Coronary Stem Cell Infusion (MAGIC Cell) 1 trial. Am Heart J 153, 237 e231–238, https://doi.org/10.1016/j.ahj.2006.11.004 (2007).
Pingitore, A. et al. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition 31, 916–922, https://doi.org/10.1016/j.nut.2015.02.005 (2015).
Murdoch, C. E., Zhang, M., Cave, A. C. & Shah, A. M. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 71, 208–215, https://doi.org/10.1016/j.cardiores.2006.03.016 (2006).
Pilis, W. et al. Anaerobic threshold in rats. Comp Biochem Physiol Comp Physiol 106, 285–289 (1993).
Galan, D. T. et al. Reduced mitochondrial respiration in the ischemic as well as in the remote nonischemic region in postmyocardial infarction remodeling. Am J Physiol Heart Circ Physiol 311, H1075–H1090, https://doi.org/10.1152/ajpheart.00945.2015 (2016).
Acknowledgements
We thank Marc Jans for embedding the tissues in paraffin. The authors also thank Petra Bex, Rosette Beenaerts and Dora Colson for their skillful technical assistance. This research was funded by a BOF-scholarship from Hasselt University (15NI06-BOF).
Author information
Authors and Affiliations
Contributions
M.V., A.C. and D.D. performed experiments for this manuscript. M.V. and V.B. wrote the main manuscript text, and M.V. prepared all figures. I.L., B.O.E., D.H. and V.B. were happy to give their knowledge about the subject, and prepared the idea of the experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verboven, M., Cuypers, A., Deluyker, D. et al. High intensity training improves cardiac function in healthy rats. Sci Rep 9, 5612 (2019). https://doi.org/10.1038/s41598-019-42023-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42023-1
This article is cited by
-
High-intensity intermittent training ameliorates methotrexate-induced acute lung injury
BMC Pulmonary Medicine (2024)
-
Exercise preconditioning promotes myocardial GLUT4 translocation and induces autophagy to alleviate exhaustive exercise-induced myocardial injury in rats
Journal of Molecular Histology (2023)
-
Superior cardiometabolic and cellular adaptive responses to multiple versus single daily sessions of high-intensity interval training in Wistar rats
Scientific Reports (2022)
-
Network analysis of the left anterior descending coronary arteries in swim-trained rats by an in situ video microscopic technique
Biology of Sex Differences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.