Abstract
Robot-assisted laparoscopic radical prostatectomy (RALRP) is a minimally invasive procedure; however, some amount of surgical trauma that can trigger systemic inflammation remains. Moreover, pneumoperitoneum during RALRP induces ischemia–reperfusion injury (IRI). Propofol, an anesthetic, is known to have anti-inflammatory and antioxidant properties. In the present study, we compared the effects of propofol with those of desflurane on inflammation and IRI during RALRP via measurements of different biomarkers and evaluation of perioperative renal function. Fifty patients were randomized to receive either desflurane (n = 25) or propofol (n = 25) with remifentanil during RALRP. Serum levels of interleukin (IL)-6 (IL-6), tumor necrosis factor alpha, C-reactive protein, and nitric oxide were measured 10 min after anesthesia induction (T1), 100 min after carbon dioxide (CO2) insufflation (T2), and 10 min after CO2 deflation (T3). Perioperative urine outputs and the serum creatinine level at 24 h after surgery were also recorded. We found that IL-6 levels at T2 and T3 were higher than those at T1 in both groups, although the increases were significant attenuated only in the propofol group. The other parameters showed no differences among the three time points in both groups. The intraoperative urine output was significantly higher in the propofol group than in the desflurane group, while the creatinine level showed no significant changes in either group. Our findings suggest that propofol can not only attenuate the inflammatory response during and after pneumoperitoneum in patients undergoing RALRP but also prevent oliguria during pneumoperitoneum.
Similar content being viewed by others
Introduction
Prostate cancer is currently the most common malignancy in men and the second leading cause of cancer-related death in the West. Over the last decade, minimally invasive radical prostatectomy with robot-assisted laparoscopy has gained popularity for the treatment of prostate cancer1,2. Compared with conventional, more invasive procedures, laparoscopic surgery allows for a smaller abdominal incision and causes less tissue trauma along with a reduced stress response. However, such minimally invasive procedures require pneumoperitoneum for adequate visualization and surgical manipulation, and the associated insufflation and deflation procedures often lead to ischemia–reperfusion injury (IRI) and exacerbate inflammation and the oxidative stress response, consequently leading to postoperative complications3,4,5. Although several studies have attempted to minimize inflammation and IRI during laparoscopic surgery, none have reported clinically promising results6,7.
Propofol, a popular intravenous agent for the induction and maintenance of anesthesia, is known to have anti-inflammatory and antioxidant effects8,9,10. Previous studies have demonstrated that propofol reduces lipid peroxidation and proinflammatory cytokine levels after myocardial ischemia reperfusion11. In a previous study involving craniotomy, propofol was associated with significantly higher anti-inflammatory cytokine levels than was the volatile anesthetic12. Furthermore, in an animal model of renal IRI, propofol attenuated oxidative renal damage and accelerated recovery after IRI13. On the other hand, desflurane, a common volatile anesthetic, has shown somewhat conflicting results concerning its anti-inflammatory effects. In a mouse model of ventilator-induced lung injury, desflurane did not prevent inflammatory responses and production of reactive oxygen species14. Another study found that the increase in inflammatory cytokine levels was lesser with desflurane anesthesia than with propofol anesthesia during coronary artery bypass grafting15. However, to our knowledge, no study has evaluated and compared the preventive effects of propofol and desflurane against inflammation and IRI in human patients undergoing robot-assisted laparoscopic surgery.
Therefore, in the present study, we compared the effects of propofol with those of desflurane on inflammation and IRI during robot-assisted laparoscopic radical prostatectomy (RALRP) via measurements of different biomarkers and evaluation of perioperative renal function.
Results
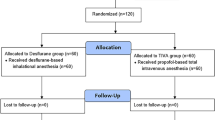
All 50 patients completed the study. There were no significant differences in patient characteristics between the propofol and desflurane groups (Fig. 1). Although the number of patients with diabetes mellitus was higher in the propofol group, the difference was not statistically significant (Table 1). The durations of anesthesia, surgery, and pneumoperitoneum were similar between the two groups.
Moreover, IL-6 levels at 10 min after induction (T1) were similar between the two groups. Within groups, IL-6 levels at T2 (p < 0.001) and T3 (p < 0.001) were significantly higher than the level at T1, while intergroup comparisons showed that IL-6 levels at T2 (1.52 ± 0.96 pg/mL vs. 3.72 ± 2.30 pg/mL; p < 0.001) and T3 (4.68 ± 2.76 pg/mL vs. 8.57 ± 3.72 pg/mL; p < 0.001) were significantly lower in the propofol group than in the desflurane group. There were no significant differences in TNF-α, CRP, and NO levels at any time point between the two groups (Fig. 2). The intraoperative urine output was significantly higher in the propofol group (440 ± 235 ml) than in the desflurane group (299 ± 208 ml; p = 0.031), although there were no differences in the amount of fluid intake and intraoperative bleeding. The remifentanil dose was greater in the propofol group (1161 ± 430 mcg) than in the desflurane group (1104 ± 515 mcg; p = 0.001), with no differences in the duration of infusion (Table 2). The heart rate was consistently lower in the propofol group during surgery. The mean arterial pressure (MAP) was comparable between groups at all time points except 10 min after anesthesia induction (77.8 ± 12.3 vs. 68.5 ± 9.3; p = 0.004; Fig. 3). The frequency of hypotension and the required dose of ephedrine were both higher in the desflurane group than in the propofol group (Table 3).
Perioperative changes in interleukin (IL)-6 (a), tumor necrosis factor alpha (TNF-α) (b), C-reactive protein (CRP) (c), and nitric oxide (NO) (d) levels in patients who received propofol (n = 25) or desflurane (n = 25) anesthesia during robot-assisted laparoscopic radical prostatectomy (RALRP). T1, 10 min after anesthesia induction; T2, 100 min after pneumoperitoneum; T3, 10 min after carbon dioxide deflation. *p < 0.001 for intergroup comparisons. †p < 0.001 vs. T1.
Perioperative hemodynamic changes in the heart rate (a) and mean arterial pressure (b) in patients who received propofol (n = 25) or desflurane (n = 25) anesthesia during robot-assisted laparoscopic radical prostatectomy (RALRP). T1, 10 min after induction; T2, 100 min after pneumoperitoneum; T3, 10 min after carbon dioxide deflation. *p < 0.05 for intergroup comparisons.
The postoperative urine output was similar between the two groups until the third postoperative day. Postoperative serum creatinine levels exhibited a similar and clinically insignificant decrease in both groups (Table 3). No patient experienced any surgical or clinical complication, and all were discharged from the hospital in accordance with the guidelines established for their respective surgical procedures.
Discussion
In the present study, we observed that propofol anesthesia significantly attenuated the increase in IL-6 levels during RALRP, unlike desflurane anesthesia. However, both propofol and desflurane had similar effects on TNF-α, CRP, and NO levels. The intraoperative urine output was significantly greater in the propofol group, although changes in serum creatinine levels were not significantly different between the two groups.
Compared with conventional open surgery, laparoscopic surgery is associated with smaller incisions, reduced intraoperative bleeding, and lesser postoperative pain; however, it may result in increased trauma due to peritoneal insufflation and increased intra-abdominal pressure. In addition, hemodynamic changes and the presence of CO2 may further contribute to inflammation and oxidative stress during pneumoperitoneum, which result in acute-phase responses such as the release of proinflammatory cytokines and acute-phase proteins16,17,18,19,20. Postoperative increases in inflammatory markers, including cytokines and CRP, are associated with tissue damage, postoperative morbidity, and cancer recurrence21,22,23. However, despite the high number of patients undergoing laparoscopic or robotic surgery, few comparative urology-related studies have investigated inflammation induced by surgical tissue damage or pneumoperitoneum-related IRI2,24.
IL-6 is one of the most important ILs that is regulated by both surgery and anesthesia. It is considered the most sensitive marker of the inflammatory response to tissue damage2. Among the inflammatory cytokines investigated in our study, IL-6 was significantly increased after surgery in both groups. This finding suggests that IL-6 may represent an appropriate marker of surgical stress in patients undergoing radical prostatectomy for prostate cancer, consistent with the findings of several previous studies2,25,26. Because elevated serum IL-6 levels are associated with the aggressiveness of prostate cancer and may represent a predictive marker of biochemical recurrence after prostatectomy, preoperative evaluation of changes in serum IL-6 levels may be valuable for not only surgical stress detection but also prognosis prediction21. Compared with the increases in IL-6 levels in the desflurane group, those in the propofol group were significantly attenuated during CO2 insufflation and after the termination of pneumoperitoneum in the present study. This result can be attributed to the anti-inflammatory properties of propofol, which have been addressed in numerous studies. Clinically relevant concentrations of propofol inhibit the functions of neutrophils, which include chemotaxis, phagocytosis, and production of reactive oxygen species27. In a model of sepsis involving lipopolysaccharide-activated macrophages, propofol was shown to inhibit the production of IL-6 by macrophages by 83%10. Furthermore, in clinical settings, propofol significantly lowers IL-6 production following reperfusion during cardiopulmonary bypass28,29. Similarly, in a study comparing propofol and sevoflurane anesthesia, the former significantly decreased neutrophil infiltration and systemic inflammation during aortic surgery23.
Although the mechanism underlying these effects remains unclear, several reports have suggested that they may be associated with intracellular calcium signaling. According to a report by Tang et al., propofol promotes the expression of annexin A1 (a membrane calcium protein), which negatively regulates the activation of the p38 signaling pathway in the mitogen-activated protein kinase system and consequently inhibits the release of inflammatory cytokines such as IL-630. Yang et al. further reported that propofol suppresses formyl peptide receptor 1-induced human neutrophil activation via complete blockade of calcium, AKT, and ERK1/2 signaling31.
In the present study, there were no changes in serum TNF-α and CRP levels at any time point in both groups. Narita et al. reported no increases in TNF-α levels during laparoscopic radical prostatectomy, consistent with our findings, although such increases were observed during open radical prostatectomy22. Different inflammatory conditions result in different patterns of alteration in the levels of various stress markers32. Our results suggest that the TNF-α level may not be a useful marker of stress in patients undergoing laparoscopic procedures such as RALRP. Furthermore, no significant changes in CRP levels were observed in either group. Similarly, a previous study reported that CRP levels remain steady during laparoscopic surgery but increase during the postoperative period2. However, further studies are required to determine whether the changes observed in our study persist after RALRP.
There is a strong association between inflammation and oxidative stress, which interact to produce adverse events in several conditions. Free radicals and reactive oxygen species are risk factors for chronic inflammation and exhibit significantly increased expression during the inflammatory response33. Oxidative stress can by quantified by measurement of various biomarkers such as NO and malondialdehyde. Immediately after ischemia, NO levels decrease and vasoconstriction occurs. Subsequently, after reperfusion, NO levels increase because of increased activity of iNOS; this can contribute to the development of problems such as renal injury and dysfunction34,35. However, in the present study, NO levels showed no significant changes at any time point, which suggests that renal ischemia did not occur during pneumoperitoneum, and that reperfusion injury after desufflation may not be strong enough to increase iNOS activity. One possible explanation for this finding is that the CO2 pressure was maintained at <15 mmHg during surgery; this pressure is reported to have a relatively small effect on NO production, although this finding remains debatable36.
Research has demonstrated that pneumoperitoneum may induce transient physiological changes in the kidney, resulting in functional and structural damage associated with transient increases in creatinine values and a decrease in urine output37. The conventionally used CO2 pressure of 15 mmHg has been reported to decrease renal blood flow by 25%38. Oliguria during pneumoperitoneum can be improved by hydration, maintenance of optimal hemodynamic parameters, proper positioning, and administration of protective agents such as N-acetylcysteine or zinc. However, the protective effects of these methods remain controversial, and the mechanisms underlying such effects have not been determined39,40,41. In the present study, the intraoperative urine output was significantly greater in patients who received propofol than in those who received desflurane, although there were no differences in the amount of intraoperative fluid intake and bleeding. These findings can be attributed to the anti-inflammatory and antioxidant effects of propofol. This theory is supported by the findings of a previous study involving patients undergoing mechanical ventilation, where the urine output increased in participants receiving propofol42,43. In an animal model of renal IRI, it was shown that propofol mitigated systemic inflammation and tubular damage in the kidney44. In other studies of valvular heart surgery, the incidence of acute kidney injury was significantly lowered in patients who received propofol than in those who received a volatile anesthetic45,46. Similarly, the anti-inflammatory effects of propofol, which were represented by the decreased IL-6 levels in the present study, may have increased the intraoperative urine output. It is also possible that these effects were due to the maintenance of more stable hemodynamic states during surgery under propofol anesthesia. Patients receiving propofol anesthesia in our study required significantly lower doses of inotropic agents, although previous studies have reported that propofol causes a profound decrease in the systemic blood pressure41. Another possible explanation is that remifentanil, which has shown anti-inflammatory and antioxidant effects in in vitro and in vivo studies47,48, may have contributed to the prevention of IRI. The amount of remifentanil infused in the propofol group was significantly greater than that infused in the desflurane group, and this may have increased the intraoperative urine output. Although the renoprotective effects of remifentanil have been investigated at various doses, a dose of 0.1–2 mcg/kg/min was found to exhibit renoprotective effects in previous studies of IRI49,50,51. Accordingly, the doses of remifentanil used in the propofol (0.1 mcg/kg/min) and desflurane (0.07 mcg/kg/min) groups in the present study were somewhat lower than the effective dose affecting stress hormones and inflammatory responses. Further studies should investigate this topic in detail. On the other hand, the postoperative urine output and creatinine levels at 24 h after surgery showed no significant differences between the two groups in the present study. However, this could be critical in cases of limited renal functional reserve, even if the renal blood flow and function return to normal after CO2 deflation41,52. In fact, recent clinical studies of major noncardiac surgeries have reported a close association between intraoperative oliguria and postoperative renal compromise53,54. Further studies are required to clarify the effects of propofol and volatile anesthesia on postoperative clinical outcomes in various surgical cohorts.
This study has several limitations. First, only intraoperative serological markers were analyzed. As previously mentioned, IRI-induced inflammatory responses may persist until 24 h after surgery2,34. In addition, postoperative outcomes except those related to the kidney were not evaluated. Further studies are required to elucidate the effect of propofol on inflammatory responses in later postoperative stages. Second, CRP and NO levels were consistently higher in the propofol group, although the differences were not statistically significant. We believe that an increase in the sample size could lead to statistically significant differences. However, CRP and NO levels in the study were mostly within the reference range, so it can be considered that the influence of pneumoperitoneum and anesthetic type on CRP and NO levels during RALRP are not as drastic as believed.
In conclusion, the findings of this study suggest that propofol anesthesia suppresses the inflammatory response during and after pneumoperitoneum and improves the intraoperative urine output in patients undergoing RALRP.
Methods
Study design
The present study was approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (3-2013-0098) and registered at http://clinicaltrials.gov (NCT02149628, registered on May 29, 2014). Informed consent was obtained from all patients prior to their participation in the study.
Patients (age range: 20–70 years, American Society of Anesthesiologists class I or II) scheduled for RALRP at Yonsei University Gangnam Severance Hospital between July 2014 and July 2015 were included in this study. Patients with renal failure (estimated glomerular filtration rate, <60 ml/min/1.73 m2), obesity (body mass index, >30 kg/m2), allergies to propofol or peanuts, and/or inability to read were excluded55. The enrolled patients were randomly allocated (1:1) to a propofol group or a desflurane group using a randomization table prepared using a random sequence generator (www.random.org). All procedures and measurements were performed in accordance with the relevant guidelines and regulations of our institute.
Before surgery, all patients received intravenous midazolam (0.02 mg/kg) and glycopyrrolate (0.004 mg/kg), following which standard monitoring devices (noninvasive blood pressure, pulse oximetry, electrocardiography, and bispectral spectrometry) were applied. In the propofol group, anesthesia was induced with intravenous propofol [Schnider model with effect site concentration (Ce) of 3–4 mcg/ml] and remifentanil (Minto model with Ce of 3–4 ng/ml) administered via target-controlled infusion (TCI; OrchestraTM BasePrimea, FreseniusVial, France). In the desflurane group, anesthesia was induced with 4 mg/kg of thiopental sodium and remifentanil administered via TCI (Minto model with Ce of 3–4 ng/ml). When the bispectral index (BIS) decreased to <60, rocuronium (0.6 mg/kg) was administered prior to tracheal intubation, following which the patients were mechanically ventilated with a 50% oxygen-in-air mixture. The ventilator was adjusted to maintain a peak airway pressure of <35 cm H2O (tidal volume, 7–8 mL/kg of the ideal body weight for volume-controlled ventilation). The respiratory rate was adjusted to maintain an end-tidal carbon dioxide (ETCO2) pressure of 40 ± 3 mmHg. TCI of remifentanil (Ce of 1–5 ng/ml) was used for anesthesia maintenance in both groups, with BIS maintained between 40 and 60. Following the induction of anesthesia, the radial artery was cannulated for invasive blood pressure monitoring. Atropine (0.5 mg) was injected when the patient’s heart rate decreased to <50 beats/min. Hypotension was defined by a systolic blood pressure of <90 mmHg or a mean blood pressure of <60 mmHg, and it was treated by 4 mg of ephedrine with 200 ml of crystalloid. During the RALRP procedure, the abdominal cavity was insufflated with carbon dioxide (CO2) at a pressure of 15 mmHg, following which the patients were placed in a 30° Trendelenburg position for surgery. When the surgery neared completion, the patients were returned to the supine position, the abdominal cavity was deflated, and an incision measuring approximately 5 cm was placed for specimen removal.
Blood samples were collected through the radial arterial line at 10 min after induction (T1), 100 min after pneumoperitoneum (T2), and 10 min after CO2 deflation (T3). Subsequently, the samples were centrifuged (14,000 rpm, 15 min) and the separated serum was stored at −80 °C until analysis56.
Analysis of biomarkers
Biomarkers were assayed in the biochemical laboratory of our institution after study completion. An ELISA kit (Quantikine®, R&D System Inc., Minneapolis, MN, USA) was used to assess the levels of interleukin (IL)-6 (IL-6) and tumor necrosis factor alpha (TNF-α). The samples and reference standards were placed into the wells of microplates coated with monoclonal antibodies for each cytokine. The plates were washed to remove nonadherent materials, following which enzyme-linked polyclonal antibodies specific for each cytokine were added to each well. After washing for the removal of nonadherent antibody–enzyme material, substrate solution and amplifier solution were added for color development. After the stop solution was added, absorbance was measured on a plate reader (SpectraMax 190 ELISA Reader, Molecular Devices, China).
C-reactive protein (CRP)
The latex agglutination method was used for the measurement of CRP levels (N-Assay LA CRP-S D-TYPE, Nittobo, Japan). Latex particles saturated with anti-CRP antibody were added to the sample to induce aggregation of particles via antigen–antibody reactions. The change in absorbance was measured at a wavelength of 572 mm (Hitachi 7600-10, Hitachi, Japan), which was proportional to the concentration of CRP in the sample, which was calculated on the basis of interpolation with the standard curve.
Nitric oxide (NO)
Levels of NO were determined through the measurement of nitrite levels using an ELISA kit (Parameter® Total Nitric Oxide and Nitrate/Nitrite Assay, R&D System Inc.). We first measured the nitrite concentration (X) in the sample. Subsequently, reductase was added for conversion from nitrate to nitrite, and the total nitrite concentration (Y) was measured. The final nitrate concentration was calculated as the difference between X and Y. Absorbance was measured on a plate reader (SpectraMax 190 ELISA Reader, Molecular Devices).
Study end points
The serum levels of IL-6, TNF-α, CRP, and NO were considered the primary endpoints, while the perioperative urine outputs and serum creatinine levels were considered secondary endpoints.
Statistical analysis
Based on a report by Ozmen et al., we determined that 25 patients per group were required for the detection of a 20% decrease in IL-6 levels with a power of 80% and type I error of 0.0557. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Variables are presented as means with standard deviations or numbers with percentages, as appropriate. Continuous variables were evaluated using Student’s t-tests or Mann–Whitney U-tests, while categorical variables were evaluated using chi-square tests or Fisher’s exact tests. The Bonferroni method was used for multiple comparisons. According to this method, a two-sided p-value of <0.0004 (=0.05/12) indicated statistical significance for the primary end points. For the other parameters, a two-sided p-value of <0.05 was considered statistically significant.
Data Availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J Clin 60, 277–300, https://doi.org/10.3322/caac.20073 (2010).
Fracalanza, S. et al. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int 101, 1145–1149, https://doi.org/10.1111/j.1464-410X.2008.07513.x (2008).
Yiannakopoulou, E., Nikiteas, N., Perrea, D. & Tsigris, C. Effect of laparoscopic surgery on oxidative stress response: systematic review. Surgical laparoscopy, endoscopy & percutaneous techniques 23, 101–108, https://doi.org/10.1097/SLE.0b013e3182827b33 (2013).
Sammour, T. et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br J Surg 96, 836–850, https://doi.org/10.1002/bjs.6651 (2009).
Bae, H. B. Volatile anesthetics and ischemia-reperfusion injury. Korean J Anesthesiol 68, 211–212, https://doi.org/10.4097/kjae.2015.68.3.211 (2015).
Koivusalo, A. M. et al. Effects of esmolol on haemodynamic response to CO2 pneumoperitoneum for laparoscopic surgery. Acta anaesthesiologica Scandinavica 42, 510–517 (1998).
Zhang, Y., Peng, L. & Yu, X. Protective effect of hydrogen sulfide on rats with myocardial ischemia/reperfusion injury and its mechanism. Xi bao yu fen zi mian yi xue za zhi=Chinese journal of cellular and molecular immunology 31, 316–320 (2015).
Runzer, T. D., Ansley, D. M., Godin, D. V. & Chambers, G. K. Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesthesia and analgesia 94, 89–93, table of contents (2002).
Murphy, P. G., Myers, D. S., Davies, M. J., Webster, N. R. & Jones, J. G. The antioxidant potential of propofol (2,6-diisopropylphenol). British journal of anaesthesia 68, 613–618 (1992).
Chen, R. M. et al. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann N Y Acad Sci 1042, 262–271, https://doi.org/10.1196/annals.1338.030 (2005).
Corcoran, T. B. et al. The effects of propofol on lipid peroxidation and inflammatory response in elective coronary artery bypass grafting. Journal of cardiothoracic and vascular anesthesia 18, 592–604 (2004).
Markovic-Bozic, J. et al. Effect of propofol and sevoflurane on the inflammatory response of patients undergoing craniotomy. BMC anesthesiology 16, 18, https://doi.org/10.1186/s12871-016-0182-5 (2016).
Yang, S., Chou, W. P. & Pei, L. Effects of propofol on renal ischemia/reperfusion injury in rats. Exp Ther Med 6, 1177–1183, https://doi.org/10.3892/etm.2013.1305 (2013).
Strosing, K. M. et al. Inhaled Anesthetics Exert Different Protective Properties in a Mouse Model of Ventilator-Induced Lung Injury. Anesthesia and analgesia 123, 143–151, https://doi.org/10.1213/ANE.0000000000001296 (2016).
Baki, E. D. et al. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100beta protein during coronary artery bypass grafting. Inflammation 36, 1327–1333, https://doi.org/10.1007/s10753-013-9671-6 (2013).
Ben-Haim, M. & Rosenthal, R. J. Causes of arterial hypertension and splachnic ischemia during acute elevations in intra-abdominal pressure with CO2 pneumoperitoneum: a complex central nervous system mediated response. International journal of colorectal disease 14, 227–236 (1999).
Jakimowicz, J., Stultiens, G. & Smulders, F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surgical endoscopy 12, 129–132 (1998).
Wong, Y. T., Shah, P. C., Birkett, D. H. & Brams, D. M. Peritoneal pH during laparoscopy is dependent on ambient gas environment: helium and nitrous oxide do not cause peritoneal acidosis. Surgical endoscopy 19, 60–64, https://doi.org/10.1007/s00464-003-9291-6 (2005).
Schietroma, M. et al. Effects of low and standard intra-abdominal pressure on systemic inflammation and immune response in laparoscopic adrenalectomy: A prospective randomised study. J Minim Access Surg 12, 109–117, https://doi.org/10.4103/0972-9941.178513 (2016).
Shin, S. et al. Effect of Pneumoperitoneum on Oxidative Stress and Inflammation via the Arginase Pathway in Rats. Yonsei medical journal 57, 238–246, https://doi.org/10.3349/ymj.2016.57.1.238 (2016).
Shariat, S. F. et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res 14, 3785–3791, https://doi.org/10.1158/1078-0432.CCR-07-4969 (2008).
Narita, S. et al. Comparison of surgical stress in patients undergoing open versus laparoscopic radical prostatectomy by measuring perioperative serum cytokine levels. Journal of laparoendoscopic & advanced surgical techniques. Part A 23, 33–37, https://doi.org/10.1089/lap.2012.0348 (2013).
Rodriguez-Lopez, J. M. et al. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Canadian journal of anaesthesia=Journal canadien d’anesthesie 53, 701–710, https://doi.org/10.1007/bf03021629 (2006).
Miyake, H. et al. Comparison of surgical stress between laparoscopy and open surgery in the field of urology by measurement of humoral mediators. International journal of urology: official journal of the Japanese Urological Association 9, 329–333 (2002).
Oka, Y. et al. Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine 4, 298–304 (1992).
Kragsbjerg, P., Holmberg, H. & Vikerfors, T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg 161, 17–22 (1995).
Mikawa, K. et al. Propofol inhibits human neutrophil functions. Anesthesia and analgesia 87, 695–700 (1998).
Sayed, S. et al. Effects of propofol and isoflurane on haemodynamics and the inflammatory response in cardiopulmonary bypass surgery. British journal of biomedical science 72, 93–101 (2015).
Corcoran, T. B. et al. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. British journal of anaesthesia 97, 825–831, https://doi.org/10.1093/bja/ael270 (2006).
Tang, J. et al. Propofol inhibits the activation of p38 through up-regulating the expression of annexin A1 to exert its anti-inflammation effect. PloS one 6, e27890, https://doi.org/10.1371/journal.pone.0027890 (2011).
Yang, S. C. et al. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J Immunol 190, 6511–6519, https://doi.org/10.4049/jimmunol.1202215 (2013).
Vittimberga, F. J. Jr., Foley, D. P., Meyers, W. C. & Callery, M. P. Laparoscopic surgery and the systemic immune response. Annals of surgery 227, 326–334 (1998).
Ferguson, L. R. Chronic inflammation and mutagenesis. Mutat Res 690, 3–11, https://doi.org/10.1016/j.mrfmmm.2010.03.007 (2010).
Mark, L. A., Robinson, A. V. & Schulak, J. A. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. The Journal of surgical research 129, 236–241, https://doi.org/10.1016/j.jss.2005.06.019 (2005).
Choi, E. K. et al. Inhibition of Oxidative Stress in Renal Ischemia-Reperfusion Injury. Anesthesia and analgesia, https://doi.org/10.1213/ANE.0000000000001565 (2016).
Ozmen, M. M. et al. The correlation between reactive oxygen species and histopathology of the liver, gut, and kidneys in animals with elevated intra-abdominal pressure. Journal of laparoendoscopic & advanced surgical techniques. Part A 19, 339–343, https://doi.org/10.1089/lap.2008.0293 (2009).
Perez, J. et al. Role of dopamine in renal dysfunction during laparoscopic surgery. Surgical endoscopy 16, 1297–1301, https://doi.org/10.1007/s00464-001-9201-8 (2002).
Chiu, A. W. et al. Effects of intra-abdominal pressure on renal tissue perfusion during laparoscopy. Journal of endourology/Endourological Society 8, 99–103, https://doi.org/10.1089/end.1994.8.99 (1994).
Seguro, A. C., Poli de Figueiredo, L. F., Shimizu, M. H. & N-acetylcysteine, N. A. C. protects against acute kidney injury (AKI) following prolonged pneumoperitoneum in the rat. The Journal of surgical research 175, 312–315, https://doi.org/10.1016/j.jss.2011.05.052 (2012).
Oksuz, H. et al. Re-protective effects of pre- and post-laparoscopy conditioning, zinc, pentoxifylline, and N-acetylcysteine in an animal model of laparoscopy-induced ischemia/reperfusion injury of the kidney. Renal failure 31, 297–302, https://doi.org/10.1080/08860220902780044 (2009).
Demyttenaere, S., Feldman, L. S. & Fried, G. M. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surgical endoscopy 21, 152–160, https://doi.org/10.1007/s00464-006-0250-x (2007).
Leite, T. T., Macedo, E., Martins Ida, S., Neves, F. M. & Liborio, A. B. Renal Outcomes in Critically Ill Patients Receiving Propofol or Midazolam. Clin J Am Soc Nephrol 10, 1937–1945, https://doi.org/10.2215/CJN.02330315 (2015).
Hsing, C. H., Chou, W., Wang, J. J., Chen, H. W. & Yeh, C. H. Propofol increases bone morphogenetic protein-7 and decreases oxidative stress in sepsis-induced acute kidney injury. Nephrol Dial Transplant 26, 1162–1172, https://doi.org/10.1093/ndt/gfq572 (2011).
Yoo, Y. C. et al. Propofol attenuates renal ischemia-reperfusion injury aggravated by hyperglycemia. The Journal of surgical research 183, 783–791, https://doi.org/10.1016/j.jss.2013.02.017 (2013).
Yoo, Y. C., Shim, J. K., Song, Y., Yang, S. Y. & Kwak, Y. L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int 86, 414–422, https://doi.org/10.1038/ki.2013.532 (2014).
Motayagheni, N., Phan, S., Eshraghi, C., Nozari, A. & Atala, A. A Review of Anesthetic Effects on Renal Function: Potential Organ Protection. Am J Nephrol 46, 380–389, https://doi.org/10.1159/000482014 (2017).
Hyejin, J. et al. Remifentanil attenuates human neutrophils activation induced by lipopolysaccharide. Immunopharmacology and immunotoxicology 35, 264–271, https://doi.org/10.3109/08923973.2013.767346 (2013).
Kim, H. S. et al. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiological research 59, 347–356 (2010).
Erkilic, E. et al. Does remifentanil attenuate renal ischemia-reperfusion injury better than dexmedetomidine in rat kidney? Drug Des Devel Ther 11, 677–683, https://doi.org/10.2147/DDDT.S126701 (2017).
Aceto, P. et al. Relationship between blood remifentanil concentration and stress hormone levels during pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. European review for medical and pharmacological sciences 21, 4419–4422 (2017).
Watanabe, K. et al. High-dose remifentanil suppresses stress response associated with pneumoperitoneum during laparoscopic colectomy. Journal of anesthesia 28, 334–340, https://doi.org/10.1007/s00540-013-1738-x (2014).
Kurian, S. M. et al. Laparoscopic donor nephrectomy gene expression profiling reveals upregulation of stress and ischemia associated genes compared to control kidneys. Transplantation 80, 1067–1071 (2005).
Shiba, A., Uchino, S., Fujii, T., Takinami, M. & Uezono, S. Association Between Intraoperative Oliguria and Acute Kidney Injury After Major Noncardiac Surgery. Anesthesia and analgesia 127, 1229–1235, https://doi.org/10.1213/ANE.0000000000003576 (2018).
Mizota, T. et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. British journal of anaesthesia 119, 1127–1134, https://doi.org/10.1093/bja/aex255 (2017).
Blitz, J. D. et al. Preoperative Renal Insufficiency: Underreporting and Association With Readmission and Major Postoperative Morbidity in an Academic Medical Center. Anesthesia and analgesia 123, 1500–1515, https://doi.org/10.1213/ANE.0000000000001573 (2016).
Hsiao, H. T., Wu, H., Huang, P. C., Tsai, Y. C. & Liu, Y. C. The effect of propofol and sevoflurane on antioxidants and proinflammatory cytokines in a porcine ischemia-reperfusion model. Acta anaesthesiologica Taiwanica: official journal of the Taiwan Society of Anesthesiologists 54, 6–10, https://doi.org/10.1016/j.aat.2015.11.002 (2016).
Ozmen, M. M. et al. Effect of increased abdominal pressure on cytokines (IL1 beta, IL6, TNFalpha), C-reactive protein (CRP), free radicals (NO, MDA), and histology. Surgical laparoscopy, endoscopy & percutaneous techniques 19, 142–147, https://doi.org/10.1097/SLE.0b013e31819cdda7 (2009).
Acknowledgements
Funding information: This study was supported by a faculty research grant for 2013 from Yonsei University College of Medicine (6-2013-0098).
Author information
Authors and Affiliations
Contributions
G.U.R. and D.W.H. wrote the main manuscript text. Y.S. and D.W.H. reviewed the initial manuscript and revised it. J.P. and Y.M.K. contributed to the statistical analyses and table preparation. D.W.H. prepared the figures and is the corresponding author. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roh, G.U., Song, Y., Park, J. et al. Effects of propofol on the inflammatory response during robot-assisted laparoscopic radical prostatectomy: a prospective randomized controlled study. Sci Rep 9, 5242 (2019). https://doi.org/10.1038/s41598-019-41708-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41708-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.