Abstract
The green lacewing, Chrysopa pallens Wesmael, is one of the most beneficial and prolific insects found in many horticultural and agricultural cropping system. Here, the effects of low temperature storage on quality of C. pallens were investigated by storing cocoons at 10 °C for different days. Results revealed, after removal from cold storage, emergence rate declined gradually as storage duration increased. After storage of 20 days, the emergence rate in cold-stored group is about 62.8% of that in unstored group. After eclosion, lifetime fecundity, preemergence period, oviposition period and longevity of adults in cold-stored group showed curves similar to emergence rate. However, preoviposition period and egg hatchability were not significantly affected by cold. After being stored for 20 days, the total fecundity of females emerging from cold-stored cocoons was about 64.5% of that of females emerging from unstored cocoons. Six days post emergence, females in cold-stored group showed apparent arrest of ovarian development and significant reductions of protease, lipase and trehalase activities when compared to unstored controls. When bovine insulin was exogenously used, the females emerging from cold-stored cocoons dramatically restored ovarian development and reproductive capacity. These results suggested that C. pallens pupae are suitable for cold storage and insulin hormone can be used as reproduction stimuli in this predatory species after cold storage.
Similar content being viewed by others
Introduction
The green lacewing, Chrysopa pallens Wesmael, is one of the most effective natural predators and has distribution in most agricultural areas of the world1,2. The larval green lacewings are commonly known as “aphid lions” and prey most soft-bodied insects of suitable size, including aphids, caterpillars, and other insect larvae and eggs2,3. C. pallens is different from other green lacewings in that both its adult and larval are predators1,4,5. C. pallens females possess robust fecundity, laying about 1000 eggs in an oviposition period under controlled conditions6,7. Therefore, C. pallens is an excellent predator and has very attractive application potential in management of insect pests.
In recent decades, there has been increasing interest in mass rearing and commercial application of natural enemies, including parasitoids and predators2,8,9,10. One of the main obstacles in efficient biological control using large-scale release is the failure to obtain large enough number of natural enemies during periods of high demand11,12. When pest population changes with the season, natural enemies may be overproduced and underproduced because of fluctuation in demands13,14. So, it is crucial to develop storage techniques for natural enemies because prolonged shelf-life improves flexibility, reliability and efficiency in commercial production8,14,15,16,17. Stinner18 considered storage techniques were critical to the reduction of cost. Many studies revealed that most parasitoids can endure short-period cold storage with minimal impairment in fitness and viability19,20,21,22,23,24. In these studies, important biological characteristics, including survival, longevity, fecundity and behavior, were evaluated17,25,26,27,28,29. Among the different developmental stages, pupal stage is considered to be most suitable for short-term cold storage because pupae have stronger cold tolerance than eggs, larvae and adults30.
Taken together, cold storage of parasitoids has received considerable interest. However, that of predators like green lacewing did not. The larvae of C. pallens feed for 2–3 weeks before pupation. The pupal stage lasts for about 10 days and then emergence take place2,4,31,32. If C. pallens is subjected to cold storage at pupae stage, the poststorage adults will be brought into a reproductive sate rapidly and predictably. So, it is very meaningful to evaluate the effects of cold storage on this species.
Now, cold storage has been accepted as a very practical method for improving production efficiency of natural enemies, but the detrimental effects of cold storage, such as decline of survival and fecundity, have also been extensively recognized17,22,33. Therefore, how to minimize the adverse effects is an inevitable problem and should be soon taken into consideration. Recently, insulin like peptides (ILPs) was found playing crucial roles in reproductive regulation of the green lacewing. Application of bovine insulin promoted ovarian development and elevated reproductive output in lacewing female adults kept at rearing conditions34, implying the application prospect of insulin hormone in production of natural enemies insects.
In this paper, C. pallens pupae were maintained at 10 °C for different days and then transferred to rearing conditions. After removal from cold storage, most biological parameters such as emergence rate, lifetime fecundity, preemergence period, oviposition period and longevity of the emerged adults declined gradually with the increase of storage duration. Ovarian development was arrested and digestive enzyme activities were reduced by cold storage. But fortunately, the emergence and reproduction of females emerging from cold-stored cocoons was not seriously damaged after being stored for 20 days. After application of bovine insulin, ovarian scale and reproductive output were dramatically restored. These results suggested that cold storage was a useful technique for improving flexibility and efficiency in mass production of C. pallens and insulin hormone could be applied as reproduction stimuli after chilling treatment.
Materials and Methods
Insect rearing
The C. pallens colony was fed with pea aphid (Acyrthosiphon pisum) and kept at 25 °C and 70% relative humidity (RH) under a 16 h light: 8 h dark photoperiod (D). After eclosion, males and females were reared together for mating.
Cold storage treatment and its effects on biological parameters
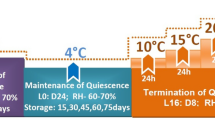
For cold storage, 10 cocoons (24-h old) were contained in a plastic box with a length of 15 cm, width of 20 cm and height of 7.5 cm and kept at 10 °C, 70% RH in darkness for 10, 20, 30, 40, 50 and 60 days. At least 6 boxes were randomly selected for each storage treatment. After storage of different period, the cocoons were transferred to rearing conditions (25 °C, 70% RH, 16 h light: 8 h dark) for emergence. Sixty cocoons (24-h old) consistently maintained at normal rearing conditions were served as control. Following parameters were evaluated: emergence rate (number of emerged adults/number of cocoons); preemergence period after removal of cocoons from cold storage, preoviposition period; oviposition period; female longevity; total fecundity and egg hatching. For hatchability, 50–100 eggs laid by females of the same storage treatment were randomly selected every time on day 12–18th post emergence. Neonates were timely transferred and recorded. The hatching assay was repeated 3–5 times for each storage treatment.
Effects of cold storage on enzyme activity in female adult
After 20 days cold storage, digestive enzyme activity in emerged female adults (5-day old) was examined. The trehalase activity was measured using insect trehalase ELISA kit (SU-B97117, Collodi, Quanzhou, China) according to manufacturer’s manual. Five days after eclosion, female adults were weighted and ground individually in liquid nitrogen. Every 100 mg insect tissue was suspended by 1 ml PBS buffer. The mixture was centrifuged at 10000 g for 20 min at 4 °C. The supernatant (50 μl) and standards (50 μl) were added in duplicate to wells of a microtiter plate. Enzymeconjugate (100 μl) was added to each standard and sample well. The plate was covered with an adhesive strip and incubated at 37 °C for 1 h. The wells were briefly rinsed with washing buffer for 4 times. Substrate A (50 μl) and substrate B (50 μl) were added to each well to develop color. After a dark incubation at 37 °C for 15 minutes, stop solution (50 μl) was added to each well to terminate reaction. The immunoassay plate was analyzed for absorbance with FlexStation 3 (Molecular Devices, California, USA) at 450 nm within 15 minutes.
The protease activity was detected using Pro ELISA kit (SU-B97122, Collodi, Quanzhou, China), and lipase activity was monitored by Lipase ELISA kit (SU-B97006, Collodi, Quanzhou, China) according to procedures described in trehalase activity measurement.
Effects of cold storage on ovarian development
After storage for 20 days, the emerged females (5-day old) were dissected in saline solution and ovaries were microscopically observed at 30-fold magnifications (VHX-2000, Keyence, USA). Three ovarioles were randomly selected from a single ovary and lengths of primary follicles (1 fol) and secondary (2 fol) follicles were measured.
Preparation and injection of bovine insulin
Bovine insulin (Nacalai, Kyoto, Japan) and Bull Serum Albumin (BSA) were solubilized in 25 mM HEPES (pH 8.2) at 10 mg/ml34. After cold storage for 20 days, the emerged females was injected 1 μl insulin solution at the conjunctive between the fourth and fifth abdominal segment on day 2 after emergence. Injection was performed in 0.2 s under a pressure of 400 mP using Eppendorf InjectMan® NI 2 and FemtoJet express Microinjector (Eppendorf, Hamburg, Germany). Females emerging from cold-stored cocoons were injected the same volume of BSA solution and were served as controls. At least 30 females were injected in each treatment. Egg amount was recorded daily after injection.
Real-time PCR
To detect mRNA levels of C. pallens ILP1 (CpILP1) (GenBank accession number:MH753707), C. pallens ILP2 (CpILP2) (GenBank accession number:MH753709), C. pallens ILP3 (CpILP3) (GenBank accession number: MH753708) in females from cocoons cold-stored for 20 days, total RNA was extracted 3days after emergence using TransZol Up Plus RNA Kit (Transgene, Beijing, China). Elimination of genomic DNA contamination and synthesis of first-strand cDNA were realized simultaneously by using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgene, Beijing, China). Quantitative PCR was performed in a 20 μl reaction volume containing 200 nM each of forward and reverse primers, 8 μl nuclease-free water, 10 μl 2 × iTaq universal SYBR Green supermix (BIO-RAD, California, American), and cDNA produced from 2 μg total RNA. The PCR reaction was carried out by a 7500 real-time system (Applied Biosystems, California, USA). The housekeeping gene actin35 (GenBank accession number: KC505163.1) and ribosomal protein encoding gene S26e36 (GenBank accession number: MH753710) was used as internal control for normalization. Sequences of gene-specific qRT-PCR primer sets are listed in Table 1. Individual animals were randomized into 2 treatment groups, and 3 replications were performed for each treatment. Quantification of relative changes in gene transcripts abundance was performed according to the 2−ΔΔCt method37.
Results
Effects of cold storage on emergence, reproduction and longevity of C. pallens
The emergence of C. pallens after removal from cold storage declined gradually as the storage period elongated. After storage of 20 days, about 51.3% cocoon emerged and the difference between the cold storage group and the control group was significant. The emergence was only about 10.7% when low temperature was kept for 60 days (Fig. 1A).
Effects of cold storage on emergence rate and lifetime fecundity of C. pallens. (A) Emergence rate of C. pallens after cold storage. The emergence of C. pallens after removal from cold storage decreased gradually as the storage duration extended (n = 6–15). (B) Total fecundity after cold storage. Total egg amount per female was significantly lower in cold storage group than that in control group (n = 6–23). Bars (mean ± SE) denoted by the same letter are not significantly different (ANOVA with LSD, P < 0.05).
With the extension of cold storage duration, reproduction of female adults also went down gradually. Lifetime fecundity of females emerging from cocoons cold-stored for 10 and 20 days dropped by about 23.1% and 35.5%, respectively, when compared to that of control group. After storage of 40 days, lifetime fecundity decreased further but was still 62.1% of that in unstored group. When storage lasted for two months, reproduction was seriously damaged with a total egg amount of 226.5 per female (Fig. 1B).
Similarly, the preemergence period also dropped with the increase of cold storage period. After storage for 30 days, lacewings emerged significantly earlier than those from unstored controls. When cocoons were cold stored for 60 days, the preemergence period of adults was 4.8 days and less than half of that in control group (Table 2). The preoviposition period of the emerged adults was not markedly affected by cold storage and spanned from 5.9 to 6.7 days after different storage durations (Table 2). The oviposition period between the cold-stored and unstored groups did not vary significantly until cold storage continued for 50 days. When low temperature was kept for two months, oviposition period of emerged females was about 12 days shorter than that of unstored controls (Table 2). Similar to oviposition period, longevity of emerged adults showed no significant difference between the cold storage group and the control group until storage duration was increased to 50 days (Table 2). Egg hatching was not affected by cold storage. After storage for two months, hatchability of eggs oviposited by females emerging from cold stored cocoons still remained at a high level of 82.4% (Table 2).
Arrest of ovarian development after cold storage
Female adults emerged from cold-stored cocoons were dissected 5 days post eclosion. In females emerged from unstored cocoons, all primary follicles (1 fol) were matured and the secondary follicles (2 fol) have formed (Fig. 2A). However, females from cold storage group showedless matured ovaries with smaller developmental scale and follicle size when compared to control group. In ordinary type of the unmatured ovaries in females emerging from cold-stored cocoons, the ovarioles showed uneven development. A minority of the ovarioles yielded matured primary follicles, but for most others, their primary follicles were obviously arrested (Fig. 2B). In extreme type of the unmatured ovaries in females emerging from cold-stored cocoons, the ovarioles was still in an initial development state and produced no visible follicles (Fig. 2C). The primary and secondary follicles in females from cold-stored cocoons were significantly smaller in size than those in females from control group (Fig. 2D).
Ovarian development in C. pallens after cold storage. (A) Ovaries in control female without low temperature treatment. (B) Ordinary type of unmatured ovaries in females after cold storage showed an uneven development of the ovarioles. Only part of the ovarioles produced follicles of normal size. (C) Extreme type of unmatured ovaries in females after cold storage was still in an initial development state. The ovarioles was apparently smaller and yielded no matured follicles. (D) The primary follicles (1 fol) and secondary follicles (2 fol) in females after cold storage are significantly smaller in length than that in control females. Scale bar is 1 mm. Different letters above the bars indicate a significant difference (Student’s t test, P < 0.01), n = 9.
Reduction of digestive enzymes activities after cold storage
Five days post emergence, activity of trehalase in females was statistically lower in cold-stored group than in unstored group (Fig. 3A). Activities of protease (Fig. 3B) and lipase (Fig. 3C) showed a pattern consistent to that of trehalase with a significant difference between the cold-stored and the unstored groups.
Digestive enzymes activities in females after cold storage. Five days post emergence, activity of trehalase (A) in females after cold storage was statistically weaker than that in control ones. Significant differences in protease (B) and lipase (C) activities were also observed between the cold storage group and control group. Mean ± SE (n = 3) of each bar followed by the same letter are not statistically different (Student’s t test, P < 0.01).
Resumption of ovarian development after insulin treatment
As demonstrated above, cold storage of cocoons inhibited ovarian development in female adults. However, topical application of bovine insulin rescued the development arrest. Five days post emergence, ovaries in control group showed normal scale and yielded matured follicles (Fig. 4A). Ovaries in insulin-treated females (Fig. 4B) restored development and their ovarioles formed matured primary follicles comparable to that in BSA-treated group. However, ovaries in females injected with BSA failed to recover development and remained unmatured (Fig. 4C). Primary and secondary follicle length in females emerging from cold-stored cocoons increased apparently after treatment of insulin hormone. Significant difference in follicle size was still present between the BSA treated group and the wild-type group, but absent between the hormone applied group and the wild-type group (Fig. 4D).
Resumption of ovarian development after injection of bovine insulin. (A) Ovaries in wild-type females. (B) Ovaries in cold storage females injected with insulin recovered development and showed uniform ovarioles with matured follicles comparable to the counter parts in wild-type females. (C) Ovaries in cold storage females treated with BSA remained unmatured. (D) Primary follicle (1 fol) and secondary follicle (2 fol) length in cold storage females increased dramatically after treatment of insulin hormone. Scale bar is 1 mm. Mean ± SE with the same letters are significantly different at P = 0.05, n = 9, ANOVA.
Recovery of fecundity after application of insulin
Because of recovery of ovarian development in females emerging from cold stored cocoons, bovine insulin treated females laid significantly more eggs than BSA treated individuals. However, significant difference in total fecundity was still present between the insulin treated females and the wild-type animals (Fig. 5).
Recovery of fecundity after application of bovine insulin. Hormone treated females produced significantly more eggs that BSA treated ones. But significant difference in total fecundity (n = 30–40) was also observed between the hormone treated females and wild-type animals. Scale bar is 1 mm. Bars (Mean ± SE) with the same letters are significantly different at P = 0.05, ANOVA.
Decrease of CpILPs transcripts after cold storage
The relative expression of the C. pallens ILPs revealed by using actin and S26e as internal controls was significantly affected by cold storage. The transcripts of C. pallens ILPs were dramatically less abundant in females emerging from cold-stored cocoons than in those emerging from unstored cocoons. Among the three ILPs, CpILP1 showed maximum reduction in mRNA level in females from the cold-stored group (Fig. 6).
Relative expression of C. pallens ILPs in cold-stored lacewing. The mRNA levels of three ILPs were analyzed by qPCR. The relative expression of ILPs was normalized to the expression level of actin. Bars are Mean ± SE from three replicates. Bars with the same letters are significantly different at P = 0.05, Student’s t test.
Discussion
To date, different methods, including diapause induction and cold storage have been tried to increase availability and flexibility in commercial production of natural enemies38,39. Diapause is a programmed arrest of development that is controlled by endogenous physiological factors. Once induced, the diapause state cannot be terminated by favorable environmental conditions until the developmental process is completed40,41,42,43. For example, when prepupae diapause was triggered in the green lacewing C. pallens, the prepupae stage was extended from 6.4 days to 49–82 days, depending on photoperiod and tempterature39. Thus, the extension of developmental stage becomes a great impairment to the flexibility and efficiency in mass production. Unlike diapause, cold storage is just a state of dormancy known as quiescence, which is directly imposed on the organisms by unfavorable and acute change in environmental conditions, such as low temperature43. Typically, development will be immediately restored once the adverse conditions come to an end22,44. In recent decades, the effects of cold storage on the performance of parasitoids have received considerable attention, but that of predators did not. The green lacewings, C. pallens is one of the most important naturally occurring predators and rank as some of the most commonly applied natural enemies44. To our knowledge, there are no reports concerning the chilling treatment of this species to data. So, it is very meaningful to evaluate the effects of cold storage on this predatory species. Now, tolerance to cold has been analyzed in various natural enemy insects under different temperatures from 4 to 13 °C11,17,22,33,38. In view of the serious detrimental effects triggered by low temperatures below 10 °C, we adopted 10 °C in present cold storage regime. Maintaining C. pallens cocoon at 10 °C for 20 days did not seriously damage the performance of the emerged adults, suggesting that pupal stage is suitable for short-term cold storage of this species.
It is noteworthy that the preemergence period of C. pallens adults was gradually shortened by the extension of storage duration. After being stored for 60 days, the period from the end of cold storage to emergence was just 47.5% of that in unstored control (Table 2). This led us to conclude that the pupae still underwent development during storage at 10 °C. As the variation in development period is indicative of the fluctuation of development rate, it is usually served as a basis for determining the temperature threshold for development. For instance, in the parasitoid M. raptor, the developmental period after return to 25 °C showed no obvious changes after storage at 10 °C, but was significantly shortened after storage at 13 °C. So, the lower temperature threshold for development of M. raptor was estimated between 10 °C and 13 °C11. In a mysarid wasp G. ashmeadi, the overall development time after removal from cold storage at an average temperature of 6 °C was significantly extended, when compared to unstored control22. The lower threshold of development of G. ashmeadi was estimated at 8.5 °C, above 6 °C45. By this logic, the low temperature threshold for pupae development in C. pallens should be below 10 °C, rather than above 10 °C.
Decline of fecundity is very common in cold-stored insects, especially in parasitoids17. The damage to the reproductive system caused by chilling conditions was viewed as the reason19,46,47. By measuring digestive enzyme activities after cold storage, we inferred that recession of digestive ability might be an important inducement for the decline of fecundity in cold-stored C. pallens. In lacewings emerging from cold-stored cocoons, lipase activity was significantly lower than that in unstored controls, suggesting a reduction in fat catabolism. As catabolism and anabolism are two opposite processes, a decline in fat catabolism can theoretically lead to a promotion of fat reserves as long as fat anabolism is not weakened. Similarly, the decrease in protease and trehalase activities not only reflected a drop in protein and glucose catabolism, but also indirectly suggested an increase in reserve of these two nutrients. So, the metabolic arrest in C. pallens females in cold-stored group is caused by catabolic suppressions of above three macronutrients.
So far, insulin signaling pathway has been implied in different physiological process such as immune response, development, longevity, metabolism and reproduction48,49. Our prior study also implicated that insulin-like peptides (ILPs) are crucial upstream players in nutritional and reproductive signaling35. Here, we confirmed once more the pivotal roles of C. pallens ILPs in reproductive regulation by demonstrating that application of bovine insulin restored lacewing fecundity after cold storage. Presently, the etiology of chilling injury in insects is still relatively unknown. Gathering of toxic metabolites and reduction of energy reserves were implied as possible reasons50. Insect ILPs are a kind of endocrine hormone secreted by media neurosecretory cells (MNCs)51. In present study, transcripts level of C. pallens ILPs in females emerging from cold-stored cocoons was significantly lowered (Fig. 6). This led us to presume that irreversible damage to the MNCs caused by low temperature may be the reason for chilling injury characterized by reproductive decline in C. pallens. The logic is that the harm to the nerve cells reduced insulin secretion, impaired insulin signaling, inhibited ovarian development and ultimately decreased reproductive output. The green lacewing C. pallens has three ILPs35. Quantitative PCR analysis revealed that the transcripts abundance of CpILP1 in adult lacewings emerging from cold-stored cocoon was about 49% of that in adult lacewings emerging from unstored controls. CpILP2 and CpILP3 showed similar expression patterns with CpILP1. Regretfully, we are still not sure whether all of the three ILPs participate in lacewing reproductive regulation. Further work is needed to elucidate this query.
The total fecundity of females emerging from cold-stored cocoons was only recovered, in part, by topical application of bovine insulin and apparent difference in egg amount was still present between thecold-stored and unstored groups (Fig. 5). We inferred that this is because bovine insulin is not as efficient as ILPs in insect body51. The green lacewing C. pallens has attractive application prospect in management of various insect pests. Here, we demonstrated ILPs have the potential to be used as reproductive stimuli in insect after cold storage. The data will contribute to the commercial production of this predatory species.
References
Boo, K. S. et al. Field trapping of Chrysopa cognata (Neuroptera: Chrysopidae) with aphid sex pheromone components in Korea. J Asia-Pac Entomol. 6, 29–36 (2003).
Tauber, M. J. et al. Commercialization of predators: Recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrosoperla). Amer Entomol. 46(1), 26–39 (2000).
Principi, M. M. & Canard, M. Biology of Chrysopidae. (Junk, the Hague, the Netherlands, 1984).
Zhang, F. et al. Effects of the artificial diets and breeding means on growth and development of Chrysopa septempunctata Wesmael. Plant Prot. 35(1), 287–293 (2004).
Bezerra, C. E. S. et al. Biology and thermal requirements of Chrysoperla genanigra (Neuroptera: Chrysopidae) reared on Sitotroga cerealella (Lepidoptera: Gelechiidae) eggs. Biol Control. 60(2), 113–118 (2012).
Liu, F., Liu, C. & Zeng, F. Effects of an artificial diet on development, reproduction and digestive physiology of Chrysopa septempunctata. Biol Control. 58, 789–795 (2013).
Liu, C. Y., Mao, J. J. & Zeng, F. R. Chrysopa septempunctata (Neuroptera: Chrysopidae) Vitellogenin functions through effects on egg production and hatching. J Econ Entomol. 108(6), 2779–2788 (2015).
Greenberg, S. M., Nordlund, D. A. & King, E. G. Mass production of Trichogramma spp.: experiences in the former Soviet Union, China, the United States and Western Europe. Biocontrol News and Information. 17, 51–60 (1996).
Riddick, E. W. Benefits and limitations of factitious prey and artificial diets on life parameters of predatory beetles, bugs, and lacewings: Amini-review. Biol Control. 54, 325–339 (2009).
Nadeem, S. et al. Optimization of short and long term storage duration for Trichogrammachilonis (Ishii) (Hymenoptera: Trichogrammatidae) at low temperatures. Pak J Zool. 42, 63–67 (2010).
Lysyk, T. J. Effects of Cold Storage on Development and Survival of Three Species of Parasitoids (Hymenoptera: Pteromalidae) of House Fly, Musca domestica L. Environ Entomol. 33(4), 823–831 (2004).
Coudron, T. A., Ellersieck, M. R. & Shelby, K. S. Influence of diet on long-termcold storage of the predator Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Biol Control. 42, 186–195 (2007).
Tauber, M. J., Tauber, C. A. & Gardescu, S. Prolonged storage of Chrysopa carnea (Neuroptera: Chrysopidae). Environ Entomol. 22(4), 843–848 (1993).
Glenister, C. S. & Hoffmann, M. P. Mass-reared natural enemies: scientific, technological, and informational needs and considerations. (Entomological society of America, Lanham, 1998).
Leopold, R. A. Cold storage of insects for integrated pest management. (Boulder, Westview Press, 1998).
Tezze, A. A. & Botto, E. N. Effect of cold storage on the quality of Trichogramma nerudai (Hymenoptera: Trichogrammatidae). Biol Control. 30, 11–16 (2004).
Yan, Z. et al. Effects of cold storage on the biological characteristics of Microplitis prodeniae (Hymenoptera: Braconidae). B Entomol Res. 107, 506–512 (2017).
Stinner, R. E. Efficacy of inundative release. Annu Rev Entomol. 22(22), 515–531 (2003).
Hance, T. et al. Impact of extreme temperatures on parasitoids in a climate change perspective. Ann Rev Entomol. 52, 107–126 (2007).
Hawes, T. C. & Bale, J. S. Plasticity in arthropod cryotypes. J Exp Biol. 210(Pt 15), 2585–2592 (2007).
Leopold, R. A. Colony maintenance and mass-rearing: using cold storage technology for extending the shelf-life of insects. (Netherlands, Springer, 2007).
Chen, W. L., Leopold, R. A. & Harris, M. O. Cold storage effects on maternal and progeny quality of Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Biol Control. 46(2), 122–132 (2008).
Colinet, H. & Hance, T. Interspecific variation in the response to low temperature storage in different aphid parasitoids. Ann Appl Biol. 156(1), 147–156 (2010).
Alam, M. S. et al. Effect of storage duration on the stored pupae of parasitoid Braconhebetor (Say) and its impact on parasitoid quality. Bangladesh Journal of Agricultural Research. 41(2), 297–310 (2016).
Tauber, M. J., Albuquerque, G. S. & Tauber, C. A. Storage of nondiapausing Chrysoperla externa adults: influence on survival and reproduction. Biol Control. 10(1), 69–72 (1997).
Al-Tememi, N. K. & Ashfaq, M. Effect of low temperature storage on the fecundity and parasitizing efficacy of Bracon hebetor (Say). J Agric Res. 43(2), 155–160 (2005).
Colinet, H. & Hance, T. Male reproductive potential of Aphidius colemani (Hymenoptera: Aphidiinae) exposed to constant or fluctuating thermal regimens. Environ Entomol. 38(1), 242–249 (2009).
Nadeem, S. et al. Optimization of short and long term storage duration for Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) at low temperatures. Pak J Zool. 42(1), 63–67 (2010).
Bourdais, D. et al. Behavioural consequences of cold exposure on males and females of Aphidius rhopalosiphi De Stephani Perez (Hymenoptera: Braconidae). Biol Control. 57, 349–360 (2012).
Van Lenteren, J. & Tommasini, M. Mass production, storage, shipment and quality control of natural enemies. (Netherlands, Springer, 2002).
Brooks, S. J. & Barnard, P. C. The green lacewings of the world: A generic review (Neuroptera: Chrysopidae). Bull Br Mus Nat Hist Entomol. 59, 117–286 (1990).
Uddin, J., Holliday, N. J. & MacKay, P. A. Rearing Lacewings, Chrysoperla carnea and Chrysopa oculata (Neuroptera: Chrysopidae), on prepupae of Alfalfa Leafcutting Bee, Megachile rotundata (Hymenoptera: Megachilidae). Proc Entomol Soc Manit. 61, 11–19 (2005).
Maryam, A. et al. Effect of Cold Storage on the Survival, Sex Ratio and Longevity of Ectoparasitoid, Bracon hebetor (Say) (Hymenoptera: Braconidae). Pak J Zool. 48(6), 1775–1780 (2016).
Zhang, T. T. et al. Insulin-like peptides regulate vitellogenesis and oviposition in the green lacewing, Chrysopa septempunctata. B Entomol Res. 107, 148–154 (2017).
Han, B. et al. Nutritional and reproductive signaling revealed by comparative gene expression analysis in Chrysopa pallens (Rambur) at different nutritional statuses. PLoS One. 12(7), e0180373 (2017).
Li, Z. Q. et al. First transcriptome and digital gene expression analysis in Neuroptera with an emphasis on chemoreception genes in Chrysopa pallens (Rambur). PLoS One. 8(6), e67151 (2013).
Livak, K. J. & Schmittgen, S. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25, 402–408 (2001).
Osman, M. Z. & Selman, B. J. Storage of Chrysoperla carnea Steph. (Neuroptera, Chrysopidae) eggs and pupae. J Appl Entomol. 115(1-5), 420–424 (1993).
Shi, A. J. et al. Effect of photoperiod on induction of prepupal diapause and larval development in Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Acta Ecologica Sinica. 28(8), 3854–3859 (2008).
Denlinger, D. L. Regulation of diapause. Annu Rev Entomol. 47(1), 93–122 (2001).
Macrae, T. H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci. 67, 2405–2424 (2010).
Hahn, D. A. & Denlinger, D. L. Energetics of insect diapause. Annu Rev Entomol. 56(1), 103–121 (2011).
Hand, S. C. et al. Mechanisms of animal diapauses: recent developments from nematodes, crustaceans, insects, and fish. Am J Physiol Regul Integr Comp Physiol. 310(11), R1193–R1211 (2016).
Tauber, M. J., Tauber, C. A. & Masaki, S. Seasonal Adaptations of Insects. (Oxford University Press, New York, 1986).
Chen, W. L. & Peng, H. Developmental Threshold and Effective Accumulated Temperature of Opius dimidiatus. Guizhou Agricultural Sciences. 34(4), 12–14 (2006).
Lacoume, S., Bressac, C. & Chevrier, C. Sperm production and mating potential of males after a cold shock on pupae of the parasitoid wasp Dinarmus basalis (Hymenoptera: Pteromalidae). J Insect Physiol. 53(10), 1008–1015 (2007).
Renault, D. Long-term after-effects of cold exposure in adult Alphitobius diaperinus (Tenebrionidae): the need to link survival ability with subsequent reproductive success. Ecol Entomol. 36(1), 36–42 (2011).
Ikeya, T. et al. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 12(15), 1293–1300 (2002).
Wu, Q. & Brown, M. R. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 51, 1–24 (2006).
Morris, G. J. et al. Cold shock-a widespread cellular reaction. Cryo Lett. 4(3), 179–192 (1983).
Brown, M. R. et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. PNAS. 105(15), 5716–5721 (2008).
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2572018AA09), the National Key R&D Program of China (2018YFD0201202).
Author information
Authors and Affiliations
Contributions
Jianjun Mao, Guocai Zhang designed the study. Tingting Zhang performed the experiments. Jianjun Mao, Guocai Zhang, Tingting Zhang wrote the main manuscript text and prepared all figures. Lisheng Zhang, Mengqing Wang, Hongyin Chen and Chenxi Liu revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Zhang, G., Zhang, L. et al. Effects of cold storage on quality of Chrysopa pallens and recovery of fecundity by insulin. Sci Rep 9, 5311 (2019). https://doi.org/10.1038/s41598-019-41618-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41618-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.