Abstract
To date, only three polymorphisms (rs10830962, rs7754840 and rs1470579) are included in the genome-wide association study Catalog (www.ebi.ac.uk/gwas). However, the available evidence is limited in pregnant Chinese women. We aimed to explore the associations of three polymorphisms (rs10830962, rs7754840 and rs1470579) with GDM risk in a Chinese population. We conducted a case-control study (964 GDM cases and 1,021 controls) to evaluate the associations of these polymorphisms with GDM risk. A logistic regression model was used to calculate odds ratios (ORs) and their confidence intervals (CIs). After adjustment for age, prepregnancy BMI, parity, abnormal pregnancy history and family history of diabetes, the minor allele of rs10830962 (C > G) demonstrated a significant association with an increased risk of GDM (OR = 1.16, 95% CI = 1.02–1.31, P = 0.029 in the additive model). However, no significant association was observed between the other two polymorphisms and GDM. Subsequent functional annotation shows that rs10830962 is located in the regulatory elements of pancreatic islets, alters the binding affinity of motifs and regulates SNORA8 expression. Our findings demonstrate that rs10830962 is associated with an increased risk of GDM in the Chinese population. Further functional characterization is warranted to uncover the mechanism of the genotype-phenotype association.
Similar content being viewed by others

Introduction
Gestational diabetes mellitus (GDM) is defined as a glucose intolerance disorder with first onset or recognition in pregnancy that affects an estimated 14% of pregnancies globally1. Recently, GDM has received increasing attention due to its continuous increase in prevalence, especially in developing countries such as China and India2. GDM is associated with an increased risk of adverse pregnancy outcomes for pregnant women and chronic metabolic diseases for both mothers and their offspring3,4,5. It has been reported that family history of diabetes, maternal age, prepregnancy overweight, and obesity are the most common risk factors for GDM6. Because the occurrence of GDM is directed by multiple factors, it is critical to explore novel risk factors to identify high-risk pregnant women for early intervention.
Emerging studies have implicated genetic factors in the etiology of GDM7,8. Insulin secretion defects and insulin resistance are crucial in the development of GDM9. A study on Danish twins demonstrated that genetic components can explain over 75% of insulin secretion dysfunction and at least 53% of peripheral insulin sensitivity10. To date, there has been only one genome-wide association study (GWAS) for GDM7. In the Korean population, two single nucleotide polymorphisms (SNPs), rs10830962 and rs7754840, reached the genome-wide significance (P < 5 × 10−8), and rs1470579 demonstrated near genome-wide significance (P = 2.0 × 10−7). Accordingly, only these three GDM-associated loci are in the GWAS Catalog (www.ebi.ac.uk/gwas), which is a publicly available and manually curated resource of all published GWASs and association results11. Some researchers have performed candidate-gene approaches and examined the associations between the genetic polymorphisms described above and the risk of GDM. For example, Cho et al. confirmed that rs7754840 was associated with insulin secretory capacity and GDM risk in Koreans12. However, significant associations were not observed in the Egyptian and Russian populations13,14. Notably, the available evidence on these associations in the Chinese population is quite limited15,16. Based on a case-control study that included 350 GDM patients and 480 control subjects, Li et al. concluded that rs10830962 was not associated with the development of GDM in a Chinese population17. A relationship between rs7754840 and GDM was also not observed in a Chinese population18. In particular, the effect of rs1470579 on the susceptibility to GDM has not been explored in Chinese pregnant women.
Given the different genetic backgrounds and the inadequate evidence about the effect of these three polymorphisms on GDM risk, we conducted a case-control study to determine whether these polymorphisms contribute to GDM risk in a Chinese population.
Results
Subject characteristics
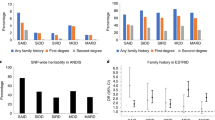
The demographic characteristics of the 964 GDM patients and 1,021 controls are summarized in Table 1. The ages and prepregnancy body mass index (BMI) values were comparable between cases and controls (Page = 0.118, PPrepregnancy BMI = 0.408). The GDM cases had higher rates of multiparity (14.21%), abnormal pregnancy history (12.14%) and family history of diabetes (17.95%) than the controls (all P < 0.05).
Associations of three SNPs with GDM risk
The genotype distributions of the three SNPs between cases and controls are shown in Supplementary Table S1. The genotype frequencies of the three SNPs were all in Hardy-Weinberg equilibrium among the controls (all P > 0.05). Table 2 summarizes these variant associations under codominant, dominant, recessive and additive models. After adjustment for age, prepregnancy BMI, parity, abnormal pregnancy history and family history of diabetes, the minor allele of rs10830962 (C > G) showed a significant association with an increased risk of GDM [additive model: Odds ratio (OR) 95% confidence interval (CI) = 1.16 (1.02–1.31), P = 0.029, Table 2]. The results were still robust under the recessive model [OR (95% CI) = 1.30 (1.04–1.63), P = 0.023] and a codominant model [GG vs. CC, OR (95% CI) = 1.36 (1.04–1.76), P = 0.022]. However, no significant association was observed between the other two SNPs and GDM risk in any model. To better understand the effect of rs10830962 on the risk of GDM, we performed stratified analyses based on age, prepregnancy BMI, parity, abnormal pregnancy history and family history of diabetes; however, no significant differences were observed among these subgroups (homogeneity test P > 0.05 for all comparisons, Table 3).
Functional annotation of rs10830962
Based on the Encyclopedia of DNA Elements (ENCODE) and Roadmap databases, we found that rs10830962 is located in functional regulatory elements of human pancreatic islets, such as those with high DNaseI hypersensitivity (DNaseI HS) density signals and Formaldehyde-Assisted Isolation of Regulatory Elements (FAIRE) density signals and several histone modification markers including H3K27ac, H3K36me3, and H3K4me1 (Supplementary Fig. S1). Using the HaploReg tool, we found that the rs10830962 G allele could increase the binding affinity of DMRT5, FOXL1, HMBOX 1 and PU.1 while decreasing the binding affinity of HNF1 and MEF2 (Supplementary Table S2). We further consulted PhenoScanner and found that rs10830962 could significantly regulate the expression levels of several genes including SNORA8, SCARNA9, FAT3, TAF1D, SNORA25, SNORA18, SNORA32, C11orf54, SLC36A4 and MED17 in multiple tissues (Supplementary Table S3). These tissues include visceral omental adipose tissue, the anterior cingulate cortex, the cerebellum, the hippocampus, the nucleus accumbens, mammary tissue, transformed fibroblasts, the sigmoid colon, the esophageal mucosa, the tibial nerve, the pancreas, and sun-exposed lower leg skin tissue. Interestingly, rs10830962 and its correlated variants within a linkage disequilibrium (LD) block could significantly regulate the expression levels of SNORA8 in pancreas tissue, as described in Table 4.
Discussion
We examined the associations of 3 SNPs with the risk of GDM in a Chinese population. Our findings suggest that rs10830962 (C > G) confers an increased risk of developing GDM. Further functional annotation indicated that rs10830962 (C > G) alters the binding of multiple motifs and alters the expression levels of SNORA8 in pancreas tissue. These findings indicate that the polymorphism may participate in the pathogenesis of GDM.
Our findings confirmed that the minor allele of rs10830962 increased the GDM risk in a Chinese population, which is consistent with the result in the Korean population7. In a previous study, the minor allele of rs10830962 was associated with decreased fasting insulin concentrations in women with GDM7. It has been reported that this polymorphism also determines glucose-stimulated insulin secretion and plasma glucose concentrations and thus increases the type 2 diabetes mellitus (T2DM) risk in European populations19. As decreased beta-cell insulin secretory function plays a central role in both GDM and type 2 diabetes, it is conceivable that rs10830962 might affect beta-cell function in the pathogenesis of GDM. Rs10830962 is located 4.4 kb upstream of MTNR1B. Bioinformatics analyses revealed that rs10830962 is located in the functional elements of pancreatic islets and alters motif binding. Among the altered motifs, HNF1, a predominant trans-acting factor of hepatic or pancreaticbeta-cells, targets many genes involved in carbohydrate metabolism20. The binding of the HNF1 motif could directly activate beta-cell genes and directly influence glucose-stimulated insulin secretion in pancreatic beta-cells21,22. Considering these findings, we speculate that the C to G base change of rs10830962 may disturb HNF1 binding, regulate beta-cell gene expression, and thus have deleterious effects on beta-cell function. Unfortunately, we did not observe a relationship between rs10830962 and the expression of the nearest gene, MTNR1B. We observed that rs10830962 and its correlated variants were significantly associated with SNORA8 expression. These findings suggested that these SNPs were involved in the regulation of SNORA8 expression and thus contributed to the development of GDM. However, there have been no studies about the role of SNORA8 in beta-cell function or insulin secretion. Therefore, it is worth investigating the functional role of rs10830962 and SNORA8 in the pathogenesis of GDM with functional assays.
Our results differ from those of Li et al., who suggested that rs10830962 is not associated with any risk of developing GDM in pregnant Chinese women17. The lack of an association of rs10830962 with GDM in the earlier study may be attributed to the limited adjustment factors, including age and prepregnancy BMI, and a small sample size. In addition, there was no significant association between the other two SNPs (rs7754840 and rs1470579) and GDM risk in our study. Consistent with our findings, another study in pregnant Chinese women found no significant association between rs7754840 and GDM risk18, whereas studies in Korean, Caucasian and South Indian populations showed significant associations between rs7754840 and GDM risk12,16,23. This discrepancy could be due to differences in ethnicities, sample sizes and diagnostic criteria for GDM. Although have been no studies about the effect of rs1470579 on GDM risk, in one recent meta-analysis of 36 studies by Ping et al., researchers demonstrated that rs1470579 was associated with T2DM risk in Asians24. However, it was shown that rs1470579 did not predict the development of postpartum diabetes in women with GDM25.
In our study, we attempted to reduce the potential confounding bias. Cases and controls were matched for age and prepregnancy BMI. We adjusted for other factors, including parity, abnormal pregnancy history and family history of diabetes. Nevertheless, we did not consider other GDM-associated factors, such as food intake and physical activity26,27. Further studies are needed to assess the relationship after adjustment for these factors.
In summary, our study provides evidence that rs10830962 is significantly associated with GDM risk in pregnant Chinese women, highlighting the importance of this potentially functional variant in GDM development. Functional investigations are needed to discover the underlying mechanisms.
Materials and Methods
Study population
This study was carried out according to the guidelines in the Declaration of Helsinki and all procedures were approved by the institutional review board of Nanjing Maternity and Child Health Care Hospital. Based on a study population of over 80,000 women who attended pregnancy complications screening between March 2012 and February 2015 at the Nanjing Maternity and Child Health Care Hospital, a total of 964 GDM cases and 1,021 controls were randomly selected as previously described28. All participants were offered a glucose challenge test (GCT) at 24–28 weeks of gestation and gave written informed consent at recruitment. We excluded women who had diabetes before pregnancy from our study. GDM was defined as fasting glucose ≥5.5 mmol/L or a 2-hour plasma glucose ≥8.0 mmol/L following a 75-g oral glucose tolerance test (OGTT)29. The controls were pregnant women without diabetes or previous metabolic diseases and were frequency-matched to GDM cases on age and prepregnancy BMI. We collected participants’ demographics information, including maternal age, prepregnancy height and weight, parity, abnormal pregnancy history and family history of diabetes, from their medical records and subsequent interviews.
SNP selection and genotyping
Three polymorphisms (rs10830962, rs7754840 and rs1470579) reported in GDM GWASs were included. Based on the 1000 Genomes Project Phase I, all the polymorphisms had a minor allele frequency (MAF) greater than 0.05 and did not have strong LD (R2 > 0.80) in the Chinese Han population. As a result, all three SNPs were genotyped in our study.
We used the proteinase K digestion and phenol/chloroform extraction method to extract genomic DNA and then diluted the DNA to working concentrations (20 ng/μL) for genotyping. SNPs were genotyped with the Sequenom MassARRAY platform (Sequenom, San Diego, CA).
In silico analysis
To further elucidate the function of significant polymorphisms in the pathogenesis of GDM, we used the ENCODE (http://genome.ucsc.edu/encode/) database and the Roadmap Epigenomics Project (http://genomebrowser.wustl.edu/) database to explore whether the SNPs were located in functional elements. Subsequently, HaploReg V4 (http://compbio.mit.edu/HaploReg) was used to examine the significant SNPs and the loci in high LD (R2 > 0.8 in Asian from the 1000 Genomes Project) for functional elements available in ChromHMM software (core 15-state model)30. Moreover, we queried the associated SNPs and their high-LD SNPs (r2 > 0.8 from the 1000 Genomes Project) against the PhenoScanner database to investigate the genotype-phenotype associations and extracted all significant associations for expression quantitative trait loci (eQTL) analysis31.
Statistical analysis
Differences in the distribution of demographic characteristics between GDM cases and controls were calculated by the χ2 test (for categorical variables) or Student’s t-test (for continuous variables). Genotype frequencies in controls were tested for Hardy-Weinberg equilibrium (HWE) by the goodness-of-fit χ2 test. ORs and their 95% CIs were calculated using logistic regression analysis to assess the associations between genotypes and GDM risk after adjusting for age, prepregnancy BMI, parity, abnormal pregnancy history and family history of diabetes. The χ2-based Q test was used to evaluate the heterogeneity of associations between subgroups. All statistical analyses were performed using PLINK software (V1.07) and R software (version 3.2.5). A two-sided P < 0.05 was considered statistically significant.
Data Availability
The genotype dataset in the current study has been deposited at figshare (10.6084/m9.figshare.7743326). Due to user privacy, the dataset of baseline information in the current study is available from chenboji@njmu.edu.cn on reasonable request. Data that support the findings of this study are available from PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk).
References
Guariguata, L., Linnenkamp, U., Beagley, J., Whiting, D. R. & Cho, N. H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes research and clinical practice 103, 176–185, https://doi.org/10.1016/j.diabres.2013.11.003 (2014).
Tutino, G. E. et al. Diabetes and pregnancy: perspectives from Asia. Diabetic medicine: a journal of the British Diabetic Association 31, 302–318, https://doi.org/10.1111/dme.12396 (2014).
Tam, W. H. et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes care 40, 679–686, https://doi.org/10.2337/dc16-2397 (2017).
Waters, T. P. et al. Maternal and Neonatal Morbidity for Women Who Would Be Added to the Diagnosis of GDM Using IADPSG Criteria: A Secondary Analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes care 39, 2204–2210, https://doi.org/10.2337/dc16-1194 (2016).
Zhu, Y. et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. The American journal of clinical nutrition 103, 794–800, https://doi.org/10.3945/ajcn.115.121780 (2016).
Zhang, C., Rawal, S. & Chong, Y. S. Risk factors for gestational diabetes: is prevention possible? Diabetologia 59, 1385–1390, https://doi.org/10.1007/s00125-016-3979-3 (2016).
Kwak, S. H. et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 61, 531–541, https://doi.org/10.2337/db11-1034 (2012).
Ding, M. et al. Genetic variants of gestational diabetes mellitus: a study of 112 SNPs among 8722 women in two independent populations. Diabetologia 61, 1758–1768, https://doi.org/10.1007/s00125-018-4637-8 (2018).
Buchanan, T. A. & Xiang, A. H. Gestational diabetes mellitus. The Journal of clinical investigation 115, 485–491, https://doi.org/10.1172/jci24531 (2005).
Poulsen, P. et al. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes 54, 275–283 (2005).
MacArthur, J. et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic acids research 45, D896–D901, https://doi.org/10.1093/nar/gkw1133 (2017).
Cho, Y. M. et al. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 52, 253–261, https://doi.org/10.1007/s00125-008-1196-4 (2009).
Noury, A. E. et al. Variants of CDKAL1 rs7754840 (G/C) and CDKN2A/2B rs10811661 (C/T) with gestational diabetes: insignificant association. BMC research notes 11, 181, https://doi.org/10.1186/s13104-018-3288-7 (2018).
Popova, P. V. et al. Effect of gene-lifestyle interaction on gestational diabetes risk. Oncotarget 8, 112024–112035, https://doi.org/10.18632/oncotarget.22999 (2017).
Zhang, C. et al. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Human reproduction update 19, 376–390, https://doi.org/10.1093/humupd/dmt013 (2013).
Rosta, K. et al. Association Study with 77 SNPs Confirms the Robust Role for the rs10830963/G of MTNR1B Variant and Identifies Two Novel Associations in Gestational Diabetes Mellitus Development. PloS one 12, e0169781, https://doi.org/10.1371/journal.pone.0169781 (2017).
Li, C. et al. Association between genetic variations in MTNR1A and MTNR1B genes and gestational diabetes mellitus in Han Chinese women. Gynecologic and obstetric investigation 76, 221–227, https://doi.org/10.1159/000355521 (2013).
Wang, Y. et al. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PloS one 6, e26953, https://doi.org/10.1371/journal.pone.0026953 (2011).
Staiger, H. et al. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PloS one 3, e3962, https://doi.org/10.1371/journal.pone.0003962 (2008).
Wang, L. et al. Selective deletion of the Hnf1beta (MODY5) gene in beta-cells leads to altered gene expression and defective insulin release. Endocrinology 145, 3941–3949, https://doi.org/10.1210/en.2004-0281 (2004).
Lockwood, C. R., Bingham, C. & Frayling, T. M. In silico searching of human and mouse genome data identifies known and unknown HNF1 binding sites upstream of beta-cell genes. Molecular genetics and metabolism 78, 145–151 (2003).
Han, E. H., Gorman, A. A., Singh, P. & Chi, Y. I. Repression of HNF1alpha-mediated transcription by amino-terminal enhancer of split (AES). Biochemical and biophysical research communications 468, 14–20, https://doi.org/10.1016/j.bbrc.2015.11.007 (2015).
Kanthimathi, S. et al. Identification of Genetic Variants of Gestational Diabetes in South Indians. Diabetes technology & therapeutics 17, 462–467, https://doi.org/10.1089/dia.2014.0349 (2015).
Rao, P. et al. Association between IGF2BP2 Polymorphisms and Type 2 Diabetes Mellitus: A Case-Control Study and Meta-Analysis. International journal of environmental research and public health 13, https://doi.org/10.3390/ijerph13060574 (2016).
Ekelund, M. et al. Genetic prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetes research and clinical practice 97, 394–398, https://doi.org/10.1016/j.diabres.2012.04.020 (2012).
Meinila, J. et al. Association between diet quality measured by the Healthy Food Intake Index and later risk of gestational diabetes-a secondary analysis of the RADIEL trial. European journal of clinical nutrition 71, 555–557, https://doi.org/10.1038/ejcn.2016.275 (2017).
Tobias, D. K., Zhang, C., van Dam, R. M., Bowers, K. & Hu, F. B. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes care 34, 223–229, https://doi.org/10.2337/dc10-1368 (2011).
Chen, T. et al. Genetic variants in PTPRD and risk of gestational diabetes mellitus. Oncotarget 7, 76101–76107, https://doi.org/10.18632/oncotarget.12599 (2016).
Hoffman, L., Nolan, C., Wilson, J. D., Oats, J. J. & Simmons, D. Gestational diabetes mellitus–management guidelines. The Australasian Diabetes in Pregnancy Society. The Medical journal of Australia 169, 93–97 (1998).
Ward, L. D. & Kellis, M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic acids research 44, D877–881, https://doi.org/10.1093/nar/gkv1340 (2016).
Staley, J. R. et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics (Oxford, England) 32, 3207–3209, https://doi.org/10.1093/bioinformatics/btw373 (2016).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81770866, 81670773, 81702569), Jiangsu Provincial Medical Innovation Team (CXTDA2017001), Jiangsu Provincial Medical Youth Talent (QNRC2016108), Jiangsu Province Natural Science Foundation (BK20170151, BK20160141), Jiangsu Provincial Key Research and Development program (BE2016619, BE2018614, BE2018616), 333 high level talents training project of Jiangsu Province, Jiangsu Provincial Women and Children Health Research Project (F201762), Jiangsu Province “six talent peak” personal training project (2016-WSW-086, 2015-WSW-043, YY-081), Nanjing Medical Science and Technique Development Foundation (JQX18009, QRX17162), National Key Laboratory of Reproductive Medicine Foundation (SKLRM-GC201805) and Science and Technology Development Foundation Item of Nanjing Medical University (2016NJMUZD065).
Author information
Authors and Affiliations
Contributions
K.X.: statistical analysis and drafting the manuscript. K.X., T.C.: specimen processing and genotyping assays. Y.Z., J.W., X.C., L.Y., L.Z., B.X.: specimen processing. C.J. and X.G.: principal investigators and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, K., Chen, T., Zhang, Y. et al. Association of rs10830962 polymorphism with gestational diabetes mellitus risk in a Chinese population. Sci Rep 9, 5357 (2019). https://doi.org/10.1038/s41598-019-41605-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41605-3
This article is cited by
-
The influence of CDKAL1 (rs7754840) gene polymorphism on susceptibility to gestational diabetes mellitus in pregnant women: a systematic review and meta-analysis
International Journal of Diabetes in Developing Countries (2023)
-
Update on the genetic and epigenetic etiology of gestational diabetes mellitus: a review
Egyptian Journal of Medical Human Genetics (2020)
-
Genetic determinants of gestational diabetes mellitus: a case–control study in two independent populations
Acta Diabetologica (2020)
-
MTNR1B gene on susceptibility to gestational diabetes mellitus: a two-stage hospital-based study in Southern China
Molecular Genetics and Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.