Abstract
Endoplasmic reticulum (ER) stress in beta cells is an important pathogenic component of both type 1 and type 2 diabetes mellitus, as well as genetic forms of diabetes, especially Wolfram syndrome. However, there are currently no convenient ways to assess ER stress in beta cells, raising the need for circulating ER stress markers indicative of beta cell health. Here we show that pancreatic stone protein/regenerating protein (PSP/reg) is a potential biomarker for ER stressed beta cells. PSP/reg levels are elevated in cell culture and mouse models of Wolfram syndrome, a prototype of ER stress-induced diabetes. Moreover, PSP/reg expression is induced by the canonical chemical inducers of ER stress, tunicamycin and thapsigargin. Circulating PSP/reg levels are also increased in some patients with Wolfram syndrome. Our results therefore reveal PSP/reg as a potential biomarker for beta cells under chronic ER stress, as is the case in Wolfram syndrome.
Similar content being viewed by others
Introduction
Diabetes mellitus is a global epidemic, affecting an estimated 30.3 million people in the United States1. It causes heavy financial burdens at both the personal and the public health level due to the longitudinal medical care and self-management education required to properly control this disease2. Regardless of its etiology, diabetes is characterized by an absolute or relative deficiency in insulin production by pancreatic beta cells. As the major site of insulin biosynthesis, the endoplasmic reticulum (ER) is particularly important for beta cell function. The ER is responsible for proper protein folding and sorting as well as calcium signaling and storage. Perturbations to ER homeostasis have direct implications for determining between cell life and death3,4. Accordingly, ER dysfunction, or ER stress, is directly involved in the beta cell pathogenesis of both type 1 (T1DM) and type 2 diabetes (T2DM)5,6,7,8,9. In both forms of diabetes, a combination of genetic and metabolic insults to ER homeostasis result in a complex cellular response that drives calcium efflux from the ER and activates the unfolded protein response4. Depending on the severity and duration of the stress, these responses by the ER can culminate in beta cell death4,10,11.
Wolfram syndrome (OMIM 222300) is considered a prototype of human ER stress disease12. As a monogenic, neurodegenerative form of diabetes, stemming from ER dysfunction, Wolfram syndrome is a prime model for studying the pathophysiology of ER stress in beta cells. Most cases of this rare autosomal recessive disorder are caused by mutations in the WFS1 gene, which encodes an ER transmembrane protein13. While the function of this protein is still not clear, accumulating evidence suggests that disease-causing alleles promote chronic, unresolvable ER stress in neural and endocrine tissues. This leads to cellular dysfunction and ultimately cell death, which typically first manifests as juvenile-onset diabetes mellitus, followed by bilateral optic nerve atrophy14. Animal and cell models of Wolfram syndrome are increasingly recapitulating the aspects of ER stress-induced beta cell pathology that lead to disease. More specifically, upregulation of ER stress markers, reduced beta cell mass, and defects in glucose-stimulated insulin secretion are observed in whole body and beta cell-specific WFS1 knockout mice, as well as rodent beta cell models of WFS1 depletion15,16. It is therefore clear that by leveraging our understanding of Wolfram syndrome as a monogenic disorder of ER stress, we can identify novel biomarkers and molecular pathways pertinent to more common diseases resulting from ER dysfunction. Such biomarkers will be very useful as researchers pursue clinical trials for Wolfram syndrome and other metabolic disorders in which beta cell ER stress is an integral component.
This study aimed to identify differentially expressed proteins in rodent models of Wolfram syndrome that could serve as biomarkers of ER stress in beta cells. It then evaluated the potential of one of the candidate proteins, pancreatic stone protein/regenerating protein (PSP/reg), as a clinical biomarker in subjects with Wolfram syndrome. There are several genes in the PSP/reg family, and PSP/reg has various alternative names including: regenerating protein 2, lithostathine-2, pancreatic thread protein, and protein-X17. These studies examine the PSP/reg1 family, where there is closest homology between mouse Reg2 and rat Reg118.
Results
Loss of Wfs1 leads to induction of PSP/reg
Beta cells respond to ER stress through the activation of transcriptional and translational programs aimed at resolving the stress19. We hypothesized that beta cells in Wolfram syndrome would activate signaling pathways that could be utilized as clinical biomarkers of beta cell ER stress. In order to test this hypothesis, we measured differentially expressed proteins in a mouse model of Wolfram syndrome, a genetic model of chronic beta cell ER stress. Two-dimensional gel electrophoresis was used to resolve the proteomes of islets derived from two 17-week-old Wfs1 beta cell-specific male knockout mice and two age-matched littermate control male mice. Due to the relatively small amount of protein that can be isolated from the mouse islets, we chose to combine islets from 2 mice in order to obtain enough protein to peform proteomics via mass spectroscopy. Of the approximately 450 spots analyzed in the molecular mass range of 5–110 kDa, 72 protein spots showed a difference of 1.5-fold or greater between Wfs1 knockout islets and control islets. As only 2 mice were used in each condition, we were unable to calculate statistical significance on these results. To refine our search for potential biomarkers of ER stress, the top 11 most upregulated spots in Wfs1 knockout islets were subjected to mass spectroscopy, resulting in the identification of 7 unique proteins (Supplementary Fig. 1a and Supplementary Table 1). Several of the peptide fragments were predicted to be the same protein, likely representing different post-translational modification states of those proteins.
Notably, many of the proteins identified are digestive enzymes (Supplementary Table 1). This includes chymotrypsinogen B, trypsin 4 precursor, trypsinogen 7 precursor, and chymotrypsin-like elastase. Pancreatic alpha-amylase plays a role in carbohydrate metabolism. In keeping with our hypothesis, we found protein disulfide isomerase to be 3.72-fold higher in Wfs1 knockout islets compared to control islets. Protein disulfide isomerase is an ER resident protein involved in protein folding by catalyzing the formation of disulfide bonds20. The upregulation of protein disulfide isomerase in Wfs1 beta cell-specific knockout islets suggests that ER stress leads to adaptive changes that promote proper protein folding via the formation of disulfide bonds21.
Pancreatic stone protein (PSP/reg) was the most upregulated protein in WFS1 knockout islets identified by our analysis. Two isoforms of PSP/reg were detected: a higher molecular weight isoform, which was upregulated 3.98-fold compared to wild-type (WT) islets, and a lower molecular weight isoform, which was upregulated 3.55-fold (Supplementary Fig. 1b). This was of particular interest to us because PSP/reg is a small secreted peptide (approximately 16 kDa) that has been studied primarily for its role in islet regeneration, suggesting that PSP/reg may have properties that promote beta cell health and adaptation to ER stress17,22.
Given that PSP/reg was elevated in islets isolated from beta cell-specific Wfs1 knockout mice, we hypothesized that PSP/reg would co-localize with beta cells in the islet. To test this hypothesis, we performed immunohistochemistry on pancreatic sections obtained from 3 whole body Wfs1-knockout mice and 3 wild-type control mice. As demonstrated in previous publications, PSP/reg is strongly expressed in acinar tissue23. Insulin and PSP staining co-localized within both Wfs1+/+ and Wfs1−/− islets. Some of the Wfs1−/− islets demonstrated a subtle increase in PSP/reg staining (Supplementary Fig. 1c).
Given our proteomic data showing that PSP/reg protein levels increase in Wfs1 knockout islets, we hypothesized that Wfs1 depletion would also increase PSP/reg expression at the transcriptional level. To test this hypothesis, we monitored gene expression of PSP/reg in a rat insulinoma (INS-1 832/13) cell line expressing small interfering RNA (siRNA) directed against Wfs1. Consistent with our islet data, cells depleted of Wfs1 expressed a 5-fold increase in Reg1 compared to the control cells (Fig. 1A). This suggests that PSP/reg expression may be negatively regulated by WFS1.
PSP/reg is induced by endoplasmic reticulum stress. (A–D) INS-1 832/13 cells were transfected with siRNA directed against WFS1 (siWFS1) or control scrambled siRNA (siScr). Quantitative real-time PCR was used to measure gene expression of (A) Reg1, (B) Bip (C) Chop, and (D) Txnip. Knockdown of WFS1 increased Reg1 expression 5-fold. Bip expression was increased by ~70%, Chop expression by ~25%, and Txnip expression by ~25%, relative to control. (E–H) INS-1 832/13 cells were treated with two chemical inducers of ER stress, tunicamycin (TM) and thapsigargin (TG), at the doses specified. DMSO was used as a vehicle control. Quantitative real-time PCR was used to measure gene expression of (E) Reg1, (F) Bip (G) Chop, and (H) Txnip. The expression of Reg1 was significantly increased by TM and TG after 8 hours of treatment. As expected, TM and TG treatment led to upregulation of Bip, Chop and Txnip. *p < 0.05, **p < 0.01. Statistical significance was determined by an unpaired two-tailed t-test between a treated condition and its corresponding control condition.
PSP/reg is induced by ER stress
WFS1 has been shown to negatively regulate ER stress24,25. We therefore anticipated that loss of Wfs1 expression would lead to induction of canonical ER stress marker genes such as Bip, Chop and Txnip. To test this hypothesis, we measured expression levels of these ER stress markers by quantitative real-time PCR (qRT-PCR) using siRNA directed against Wfs1 in INS-1 832/13 cells. As expected, BiP expression was increased in Wfs1-knockdown cells compared to control cells (Fig. 1B). Expression of Chop and Txnip was also mildly induced by Wfs1-knockdown, although it did not reach statistical significance (Fig. 1C and D). BiP is an ER stress-inducible molecular chaperone whose upregulation indicates activation of signaling pathways to restore cellular homeostasis26. Conversely, Chop and Txnip are ER stress-inducible molecules whose chronic activation leads to apoptosis27,28. Taken together, our findings suggest that transient knockdown of Wfs1 stimulates a baseline induction of ER stress in beta cells. These results are consistent with previous reports of Wfs1 loss-of-function models in which beta cells exhibit heightened ER stress and thus increased susceptibility to ER stress-mediated cell death12,16.
Since levels of PSP/reg expression were elevated in animal and cell models of Wolfram syndrome, and Wolfram syndrome is itself a model of ER stress disease, we hypothesized that PSP/reg would also be broadly inducible by ER stress. If true, other known endoplasmic reticulum stressors would increase the expression of PSP/reg genes. To test this hypothesis, we treated INS-1 832/13 cells with tunicamycin (TM) and thapsigargin (TG), two well-known chemical inducers of ER stress. TM causes ER stress by inhibiting N-linked glycosylation in the ER, thus disrupting protein folding29. TG elicits ER stress by irreversibly inhibiting the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), thus disrupting calcium homeostasis through ER calcium depletion29,30. To assess the dynamics of PSP/reg induction by ER stress, we performed a time course experiment in which we treated INS-1 832/13 cells with TM (10 µg/mL), TG (100 nM), or DMSO (control). We found that both TM and TG lead to significant upregulation of Reg1 over the span of 8 hours, but not over 4 hours. This suggests that the induction of Reg1 may be part of a late phase response to ER stress. Additionally, TG elicited a much stronger induction of Reg1 expression compared to TM, suggesting that Reg1 may be more sensitive to changes in calcium homeostasis than disruptions in protein folding (Fig. 1E). As expected, both TM and TG treatment increased the expression of the canonical ER stress genes Bip, Chop and Txnip. Notably, the induction of these genes occurred within 4 hours, further indicating that ER stress-induced Reg1 expression is slow in comparison to other canonical, early ER stress response genes (Fig. 1F–H).
PSP/reg levels in human subjects with Wolfram syndrome
Since the expression of PSP/reg was elevated in mouse and cell models of Wolfram syndrome, we were interested in determining whether PSP/reg might be elevated in patients with Wolfram syndrome. Accordingly, we measured circulating PSP/reg levels in the serum of all of the available subjects attending the 2014 Wolfram syndrome research clinic. This included 28 subjects with Wolfram syndrome, and 28 control subjects (parents or siblings of subjects with Wolfram syndrome). The mean PSP/reg level in the Wolfram syndrome group was 23.1 ng/mL (SD 29.9), compared to 15.1 ng/mL (SD 10.2) in the control group. The median PSP/reg level in the Wolfram syndrome group was 12.2 ng/mL (interquartile range 10.4–18.1 ng/mL), compared to 12.9 ng/mL (interquartile range 10.6–17.7 ng/mL) in the control group. (Fig. 2)
Serum Levels of PSP/reg in Human subjects with Wolfram Syndrome. PSP/reg levels were obtained from all available subjects attending the 2014 Wolfram syndrome research clinic. This included 28 subjects with Wolfram syndrome, and 28 control subjects (parents or siblings of subjects with Wolfram syndrome). The mean PSP/reg level in the Wolfram group was 23.1 ng/mL (SD 29.9), compared to 15.1 ng/mL (SD 10.2) in the control group. The median PSP/reg level in the Wolfram group was 12.2 ng/mL (interquartile range 10.4–18.1 ng/mL), compared to 12.9 ng/mL (interquartile range 10.6–17.7 ng/mL). Despite the similarities between the Wolfram and control groups, we noted that 3 subjects with Wolfram syndrome had relatively elevated levels of PSP/reg (WU-WOLF-03, WU-WOLF-11, WU-WOLF-12). From the control group one subject (WU-WOLF-03 (Mother)), also had relatively elevated levels of PSP/reg.
Despite the similarities between both groups, we noted that 3 subjects with Wolfram syndrome had relatively elevated levels of PSP/reg. We carefully evaluated the medical history of these 3 subjects for age, gender, specific WFS1 gene mutations, and the age of onset for the major clinical components of Wolfram syndrome. This included diabetes mellitus (DM), optic atrophy (OA), hearing loss, and diabetes insipidus (DI). We compared the PSP/reg level to their fasting glucose and c-peptide levels (Table 1).
Subject WU.WOLF-03 is male subject who was 22 years old at the time of the study. He carries two deletions in the WFS1 gene. The first WFS1 allele is a 4 base pair deletion, resulting in a frameshift and a premature stop codon, resulting in a truncated protein (c.1230_1233_CTCT; p.V412fs*440_∗). The second WFS1 allele is a 3-base pair deletion. This mutation eliminates a valine at position 415 (c.1243_1245delGTC; p.V415del). WU.WOLF-03 was diagnosed with diabetes mellitus at age 5. He was diagnosed with optic atrophy, hearing loss, and diabetes insipidus at age 6. He had a relatively elevated PSP/reg level, 94.7 ng/mL. His fasting glucose was 279 mg/dL, and fasting c-peptide was 0.65 ng/mL. This subject’s mother, WU.WOLF-03 (mother), was also an outlier amongst the control group. Her PSP/reg level was 60.2 (ng/mL). It is possible that the maternally inherited allele may be particularly deleterious and effective at inducing PSP/reg.
Subject WU.WOLF-11 is a male subject who was 12 years old at the time of the study. He carries a missense mutation and a nonsense mutation of the WFS1 gene. The first WFS1 allele carries a G to A mutation, resulting in an alanine to threonine mutation at position 126 (c.376G>A; p.A126T). The second WFS1 allele carries a G to A mutation, resulting in a premature stop codon at position 613 (c.1838G>A; p.W613X). WU.WOLF-11 was diagnosed with diabetes mellitus and optic atrophy at age 7. He was diagnosed with diabetes insipidus at age 8, and hearing loss at age 9. WU.WOLF-11 had the highest measured PSP/reg level, 126.8 ng/mL. His fasting glucose was 278 mg/dL, and fasting c-peptide was 0.65 ng/mL.
Subject WU.WOLF-12 is a male subject who was 26 years old at the time of the study. He carries two missense mutations in the WFS1 gene. The first WFS1 allele carries a G to A mutation, this results in a glycine to glutamine mutation at position 107 (c.320G>A; p.G107E). The second WFS1 allele carries a C to T mutation, resulting in an arginine to tryptophan mutation at position 629 (c.1882C>T; p.R629W). His PSP/reg level was elevated at 67.3 ng/mL. His fasting glucose was 195 mg/dL, and his fasting c-peptide was 0.14 ng/mL. This subject underwent a mixed-meal tolerance test. At 30 minutes, his glucose was 207 mg/dL, and his c-peptide was 0.3 ng/mL.
We hypothesized that serum PSP/reg levels may be positively correlated to disease severity in Wolfram syndrome. Therefore, we compared serum PSP/reg levels to fasting C-peptide levels obtained from the 28 subjects participating in the 2014 Wolfram syndrome clinic. We noted that the 3 subjects with relatively high serum PSP/reg all had lower levels of fasting C-peptide (0.14–0.65 ng/mL). However, there were several subjects within the same range of fasting C-peptide who had normal PSP/reg levels (Supplementary Fig. 2a). We also compared serum PSP/reg levels to the age of onset for the major clinical components of Wolfram syndrome, including diabetes mellitus, optic nerve atrophy, hearing loss, and diabetes insipidus. We did not identify any clear correlation between the age of onset of these conditions and serum PSP/reg levels (Supplementary Fig. 2b–e). We also compared the serum PSP/reg levels to the Wolfram Unified Rating Scale (WURS)31 obtained from these subjects during the 2014 Wolfram syndrome clinic. We did not identify any clear correlation between serum PSP/reg levels and the total, physical, or behavioral WURS scores (Supplementary Fig. 2f–h). Anecdotally, however the clinical providers at the Wolfram syndrome clinic felt that the subjects with the highest PSP/reg were some of the more severely affected subjects in the cohort.
Discussion
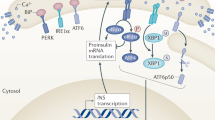
ER stress is increasingly recognized as a significant pathologic component of beta cell dysfunction and beta cell death in diabetes5,6,7,8. Yet there are currently no effective therapies for mitigating beta cell pathology that target the ER. Furthermore, there are no convenient ways to monitor ER health in-vivo. Wolfram syndrome is a rare, monogenic form of diabetes that stems from beta cell ER dysfunction. As such, animal and cell models of Wolfram syndrome serve as unique tools for identifying biomarkers of ER dysfunction. Our results demonstrate the proteomic alterations that occur in islets undergoing ER stress. More specifically, our data identify PSP/reg as a novel secreted protein that may serve as a biomarker for beta cells experiencing ER dysfunction, as in Wolfram syndrome. We propose that ER stress, whether genetic or environmental, results in homeostatic alterations to the ER that result in increased expression, translation, and secretion of PSP/reg. PSP/reg likely exerts its downstream effects in both an autocrine and a paracrine fashion to counterbalance the effects of ER stress by promoting beta cell proliferation and pro-survival pathways (Fig. 3).
Proposed Model of PSP/reg in the ER stress response. ER stress either via genetic (loss of WFS1) or environmental (treatment with tunicamycin or thapsigargin), results in homeostatic alterations in the ER. In turn, this results in increased expression, translation, and secretion of PSP/reg. PSP/reg likely exerts its downstream effects in both an autocrine and paracrine manner. We hypothesize that PSP/reg likely counterbalances the effects of ER stress by simultaneously promoting beta cell proliferation and pro-survival pathways.
In this study, we applied a proteomic profiling technique involving two-dimensional gel electrophoresis followed by protein identification with mass spectrometry to islets derived from Wfs1 beta cell-specific knockout mice. This approach allowed us to identify islet-specific proteins upregulated under chronic ER stress through an unbiased approach. Of the differentially expressed proteins identified, special attention was placed on secreted molecules due to their potential to serve as biomarkers of beta cell ER stress.
This proteomic screen identified PSP/reg as a highly upregulated secretory molecule in Wfs1 knockout islets. PSP/reg is a small (16 kDa) secreted protein that is part of the regenerating protein superfamily and is primarily expressed in the pancreas, and to a lesser extent, in the gastric mucosa and the kidney32. It functions as a C-type lectin and thus contains a carbohydrate-binding protein domain that requires calcium for its activity33. While the exact function and mechanisms of PSP/reg activity in beta cells remains unclear, previous reports have demonstrated that PSP/reg is abundant in islets regenerating after partial pancreatectomy22. Intriguingly, more recent studies of diabetic murine models are also beginning to highlight PSP/reg as a molecule of interest in the pancreas under diabetic states.
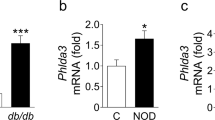
Pérez-Vázquez et al. recently published a proteomics study of db/db mouse pancreata, where they found increased expression of PSP/reg, chymotrypsinogen B, and pancreatic alpha-amylase34. In this report, PSP/reg was increased by 2.5-fold in db/db mice. Qiu et al. published a similar study examining the pancreata of diet-induced diabetic mice and found PSP/reg to be upregulated by 1.2- to 4.6-fold in diabetic animals35. In our proteomic study of Wolfram syndrome, PSP/reg was increased by 3.5- to 4-fold in WFS1 beta cell specific knockout islets compared to controls. Collectively, these proteomic studies suggest that there are common pathways activated in islets under diabetogenic conditions that converge on increased PSP/reg expression. ER stress is the likely culprit. The fact that PSP/reg is upregulated across three different diabetic mouse models in which ER stress is strongly implicated indicates that PSP/reg may serve as a signal of ER-stressed beta cells in diabetes36,37. Indeed, induction and secretion of PSP/reg may be part of a common pathway activated by islet perturbations associated with ER dysfunction.
We hypothesize that PSP/reg is of broader significance to diseases involving ER stress beyond Wolfram syndrome. Accordingly, our data demonstrate that PSP/reg is induced by two mechanistically distinct ER stress-inducing agents (i.e. tunicamycin and thapsigargin). Although this is consistent with our understanding of Wolfram syndrome as a prototype of ER stress disease, the fact that PSP/reg exhibits slower temporal induction dynamics than canonical ER stress genes such as BiP, Chop and Txnip suggests that PSP/reg may function in a different capacity, and towards a different goal, than these other genes. Given that PSP/reg is a secreted molecule, it is possible that its induction at later stages of prolonged ER stress may serve a dual purpose. In addition to signaling ER dysfunction to other cells, beta cell induction and secretion of PSP/reg may also contribute to a cell autonomous adaptive response. Curiously, we found Reg1 to be induced more robustly by thapsigargin than tunicamycin. This indicates that PSP/reg expression is particularly sensitive to cellular calcium fluctuations, which is consistent with PSP/reg being a C-type lectin with calcium-dependent activity. Further studies are required to determine the targets of PSP/reg’s activity.
Initially, PSP/reg was suggested to be a marker of pancreatic injury and recurrent pancreatitis38. Serum PSP/reg is robustly increased in patients with acute or chronic pancreatitis, with some potential for false positives in patients with chronic renal failure under hemodialysis, malignancies of the digestive system or hepatic dysfunction39. Elevated levels of PSP/reg have also been recently reported in ventilator-associated pneumonia, chronic obstructive pulmonary disease exacerbation and post-traumatic sepsis40. At baseline, however, PSP/reg is mostly secreted by pancreatic acinar cells, while islets appear to only produce PSP/reg under pathologic conditions17,41. Bonner et al. recently demonstrated that beta cells undergoing apoptosis induce PSP/reg expression in surviving neighboring beta cells, suggesting that PSP/reg signals in a paracrine fashion to promote survival42. Additionally, administration of recombinant PSP/reg peptide has been shown to be protective against the development of autoimmune diabetes in non-obese diabetic mouse models43. This protective effect may be tied to findings from Okamoto et al., which show that PSP/reg is induced by IL-6 and glucocorticoids, thereby inducing beta cell replication through activation of the cell cycle via Cyclin D144. Our study extends these findings to a new model of monogenic diabetes and demonstrates that ER stress also leads to increased expression of PSP/reg in pancreatic beta cells.
Due to these properties, we proposed that PSP/reg could serve as a clinically useful circulating biomarker of beta cell ER stress, in Wolfram syndrome. Unfortunately, there was no significant difference between subjects with Wolfram syndrome and parental or sibling controls. PSP/reg is secreted by both the pancreatic islets and the surrounding acinar tissues. The islets only comprise 1–2% of the pancreatic mass, and receive about 10–15% of the pancreatic blood flow45. There is no way to discriminate islet derived PSP/reg from its acinar counterpart. Therefore, detecting PSP/reg elevations in the serum of subjects with diabetes or Wolfram syndrome is challenging. With these important caveats in mind, we identified 3 subjects in our cohort with remarkably elevated levels of PSP/reg. These patients had relatively severe symptoms of Wolfram syndrome. It is possible that serum levels of PSP/reg may rise according to disease severity and that longitudinal levels of PSP/reg may reflect disease progression. When performing univariate analysis on the serum PSP/reg data, we observed that subjects with higher PSP/reg levels tended to have lower fasting C-peptide levels. However, we did not find any clear correlations to the age of onset of diabetes mellitus, optic nerve atrophy, hearing loss, diabetes insipidus, or the Wolfram Unified Rating Scale. This is not altogether unexpected, given the variability in the phenotypic presentation that is typical in Wolfram syndrome. However, in simpler genetic models of WFS1 loss of function, PSP/reg is clearly upregulated.
Evaluating the correlation between serum PSP/reg and clinical severity in Wolfram syndrome is unfortunately complicated by the rarity of the disease. Given Wolfram syndrome’s prevalence of 1/500,000 people worldwide, it is difficult to obtain a patient sample size sufficiently large enough to assess the relationship between PSP/reg levels and disease progression. This question has, however, been evaluated in other diabetic cohorts with larger affected populations. Recent studies have demonstrated that serum PSP/reg levels are elevated in type 1 and type 2 diabetes, both of which have well-documented ER stress-mediated pathophysiologic components46,47. In addition, a study by Bacon et al. revealed that PSP/reg levels are elevated in human subjects with HNF1A-MODY, another genetic form of diabetes associated with increased beta cell ER stress48. Altogether, these studies suggest that islet injury secondary to beta cell ER stress may lead to elevated serum levels of PSP/reg. Therefore, continued longitudinal measurement of PSP/reg in patients with Wolfram syndrome will help provide clinical correlations and also help determine which patients would benefit most from this this testing.
Our findings provide new evidence that beta cell ER stress alone is sufficient to induce PSP/reg expression in pancreatic beta cells. As PSP/reg is a small secreted peptide, it holds promise as a biomarker of beta cell ER stress in the pathogenesis of both monogenic and common forms of diabetes.
Materials and Methods
Animal experiments
The WFS1 beta cell specific knockout mouse was generated by crossing floxed WFS1 exon 8 animals with mice expressing Cre recombinase under the control of a rat insulin promoter (RIP2-Cre)16,49. 129S6 whole body Wfs1-knockout mice were a kind gift from Dr. Sulev Kõks. This results in a deletion of amino acids 360–890 of the Wfs1 protein50. The Institutional Animal Care and Use Committee at Washington University School of Medicine (A-3381-01) approved all animal experiments performed in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Isolation of mouse islets
Pancreatic islets were isolated from WFS1 beta cell specific knockout mice and age matched, littermate, Cre-negative, control mice. Mice were anesthetized and pancreata were incubated for 13 min at 37 °C and shaken 30 times. Undigested acinar tissue was removed by using a 70-μm screen and the recovered tissues were washed twice with ice-cold Hanks balanced salt solution followed by centrifugation at 250 × g for 2 min. Islets were handpicked and preincubated in RPMI 1640 medium containing 10% FBS and penicillin streptomycin (Sigma) before experimentation.
Proteomic analysis
Protein extraction and 2-dimensional differential in-gel electrophoresis (2-DIGE) were performed at Applied Biomics (Hayward, CA), as described51. Pancreatic proteins were extracted and concentrations standardized between 3 and 8 mg/mL. Control samples were labeled with Cy2 and WFS1 knockout samples were labeled with Cy5. Image scans were carried out using Typhoon TRIO and analysis performed using ImageQuant-5.0; in-gel and cross-gel analyses were performed using DeCyder-6.0 to obtain the ratio change of the protein differential expression (GE, Schenectady, NY). Proteins of interest were selected based on a cutoff ratio more than or equal to 1.5 in the WFS1 knockout samples. Selected spots were identified by Ettan Spot Picker after DeCyder analysis; protein spots were subjected to in-gel trypsin digestion, peptides extraction, and desalting followed by matrix assisted laser desorption/ionization-time of flight analysis to determine their identity. Proteins of interest were identified using database search (Ingenuity Systems, Redwood City, CA; and PubMed).
Tissue culture
Rat insulinoma cells (INS-1 832/13) were a gift from C. Newgard (Duke University Medical Center, Durham, North Carolina). INS-1 832/13 cells were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin, 1% sodium pyruvate and 50 µM β-mercaptoethanol. ER stress induction was achieved through 4- or 8-hour treatments with thapsigargin at 100 nM (Sigma) or tunicamycin at 10 µg/mL (Sigma). DMSO (Sigma) was used as a vehicle control.
siRNA Transfection
The Wfs1 gene was silenced with short interfering RNA (siRNA) using the TransIT-X2 dynamic delivery reagent from Mirus Bio LLC according to the manufacturer’s protocol. The siRNA directed against Wfs1 was predesigned and inventoried by Origene (catalog no. SR504899).
Quantitative real-time PCR
Total RNA was extracted by RNeasy kits (Qiagen, Venlo, Netherlands). The RNA was used to prepare cDNA using random primers, and reverse-transcribed with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). Quantitative RT-PCR was performed by monitoring in real-time the increase in fluorescence of the SYBR green dye (Bio-Rad Laboratories, Hercules, CA) as described using the Viaa™ 7 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA)52,53. Gene expression was calculated using the ΔΔCt method and data are expressed as fold change ± SEM. To calculate gene expression, Ct values were first normalized to rat 18S rRNA to calculate the ΔCt, then normalized to the appropriate control (e.g. siScr for siRNA experiments, or corresponding DMSO timepoint for ER stress induction experiments) for calculation of the ΔΔCt. Fold change was calculated as 2−∆∆Ct. All qPCR reactions were performed in replicates of four. The primers used in this study were: rat Reg1a, 5′-CTGCCAGGATCACTTGTCCA-3′ and 5′-AGCACTGACACCAAGTAGCC-3′; rat Chop, 5′-AGAGTGGTCAGTGCGCAGC-3′ and 5′-CTCATTCTCCTGCTCCTTCTCC-3′; rat BiP, 5′-TGGGTACATTTGATCTGACTGGA-3′ and 5′-CTCAAAGGTGACTTCAATCTGGG-3′; rat Txnip 5′-CAAGTTCGGCTTTGAGCTTC-3′ and 5′-ACGATCGAGAAAAGCCTTCA-3′ rat 18S rRNA, 5′-AGGTTTGTGATGCCCTTAGATGTC-3′ and 5′-CACACGCTGAGCCAGTCAGT-3′.
Immunohistochemistry
After the mice were sacrificed, whole pancreata were fixed overnight in 10% neutral buffered formalin (Fisher Scientific), serially dehydrated in ethanol, and processed into paraffin block. The paraffin blocks were cut into 5 µm sections. Pancreatic sections were subjected to antigen retrieval in 10 mM sodium citrate (pH 6). The sections were permeabilized in 0.1% Triton X, blocked in 2% BSA and 10% normal serum54. Incubation occurred overnight with goat anti-PSP/reg (Gift from Dr. Rolf Graf) and guinea pig anti-insulin (Cell Signaling). The slides were washed with PBS and incubated with CF™ 594 conjugated donkey anti-goat (Sigma-Aldrich) and Alex Fluor 488-conjugated rabbit anti guinea pig (Fisher Scientific) for 1 hour and mounted in prolong gold antifade mounting medium containing DAPI (Thermo Fisher Sicentific). Florescence was observed via fluorescent microscopy (Leica DM4000 B and DFC350 FX).
PSP/reg enzyme-linked immunosorbent assay
Enzyme linked immunosorbent assay (ELISA) to quantify human PSP/reg was performed utilizing anti-sera from rabbits and guinea pigs that were immunized against recombinant human PSP/reg protein42,55. Serum was prepared by centrifugation. IgG was then purified by affinity chromatography on protein A columns. A sandwich ELISA was designed on 96-well plates. Guinea pig antibody was used to coat the bottom of the ELISA plates. Subsequently, the plates were blocked with BSA, and aliquots of serum were incubated for 2 h. After washing, the wells were incubated with rabbit antibody. Then a phosphatase-coupled anti-rabbit IgG was used56. A multiplate reader (Dynatech) was used to monitor the reaction of the phosphatase with the substrate. A relative standard curve using recombinant human PSP/reg protein was used to quantify the subject’s PSP/reg levels57.
Clinical information
Clinical data was collected as part the Tracking Neurodegeneration in Early Wolfram Syndrome (TRACK) study at Washington University (ClinicalTrials.gov # NCT02455414). Wolfram syndrome patients were recruited through the Washington University Wolfram Syndrome International Registry and Clinical Study (https://wolframsyndrome.dom.wustl.edu/). All experimental protocols were approved by the Washington University Human Research Protection Office (IRB ID 201107067 and 201104010). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects or, if subjects are under 18, from a parent and/or legal guardian. Natural history of the symptoms of Wolfram syndrome was collected including age, gender, age of onset of diabetes mellitus, optic atrophy, hearing loss, and diabetes insipidus. WFS1 gene sequencing was also performed58,59. Fasting serum samples were drawn to determine glucose and c-peptide. This serum was also used for purposes of biomarker testing. These samples were obtained during the 2014 Wolfram syndrome research clinic. Parents and siblings were used as unaffected controls.
Statistical analyses
To determine whether any treatment was significantly different from the control, Graphpad Prism was used to conduct two-tailed paired Student’s t-tests on the ∆∆Ct values calculated, prior to log transformation. A p value less than 0.05 was considered statistically significant. Box and whisker plot for human PSP/reg data was generated utilizing BoxPlotR online software (http://shiny.chemgrid.org/boxplotr/)60.
References
Prevention, C. F. D. C. A. In Centers for Disease Control and Prevention, US Department of Health and Human (2017).
Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care 39 Suppl 1, S4–5, https://doi.org/10.2337/dc16-S003 (2016).
Berridge, M. J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32, 235–249 (2002).
Hara, T. et al. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology 155, 758–768, https://doi.org/10.1210/en.2013-1519 (2014).
Engin, F., Nguyen, T., Yermalovich, A. & Hotamisligil, G. S. Aberrant islet unfolded protein response in type 2 diabetes. Sci Rep 4, 4054, https://doi.org/10.1038/srep04054 (2014).
Urano, F. Diabetes: Targeting endoplasmic reticulum to combat juvenile diabetes. Nat Rev Endocrinol 10, 129–130, https://doi.org/10.1038/nrendo.2013.261 (2014).
Tersey, S. A. et al. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61, 818–827, https://doi.org/10.2337/db11-1293 (2012).
O’Sullivan-Murphy, B. & Urano, F. ER stress as a trigger for beta-cell dysfunction and autoimmunity in type 1 diabetes. Diabetes 61, 780–781, https://doi.org/10.2337/db12-0091 (2012).
Harding, H. P. & Ron, D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51(Suppl 3), S455–461 (2002).
Cardozo, A. K. et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54, 452–461 (2005).
Oyadomari, S., Araki, E. & Mori, M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis: an international journal on programmed cell death 7, 335–345 (2002).
Shang, L. et al. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes 63, 923–933, https://doi.org/10.2337/db13-0717 (2014).
Inoue, H. et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20, 143–148, https://doi.org/10.1038/2441 (1998).
Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Current diabetes reports 16, 6, https://doi.org/10.1007/s11892-015-0702-6 (2016).
Ishihara, H. et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Human molecular genetics 13, 1159–1170, https://doi.org/10.1093/hmg/ddh125 (2004).
Riggs, A. C. et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48, 2313–2321, https://doi.org/10.1007/s00125-005-1947-4 (2005).
Graf, R. et al. Exocrine Meets Endocrine: Pancreatic Stone Protein and Regenerating Protein—Two Sides of the Same Coin. Journal of Surgical Research 133, 113–120, https://doi.org/10.1016/j.jss.2005.09.030.
Liu, J. L., Cui, W., Li, B. & Lu, Y. Possible roles of reg family proteins in pancreatic islet cell growth. Endocr Metab Immune Disord Drug Targets 8, 1–10 (2008).
Eizirik, D. L. & Cnop, M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Science signaling 3, pe7, https://doi.org/10.1126/scisignal.3110pe7 (2010).
Wilkinson, B. & Gilbert, H. F. Protein disulfide isomerase. Biochimica et biophysica acta 1699, 35–44, https://doi.org/10.1016/j.bbapap.2004.02.017 (2004).
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and cellular biology 23, 7448–7459 (2003).
Terazono, K. et al. A novel gene activated in regenerating islets. The Journal of biological chemistry (1988).
Rouquier, S., Verdier, J. M., Iovanna, J., Dagorn, J. C. & Giorgi, D. Rat pancreatic stone protein messenger RNA. Abundant expression in mature exocrine cells, regulation by food content, and sequence identity with the endocrine reg transcript. The Journal of biological chemistry 266, 786–791 (1991).
Fonseca, S. G. et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. The Journal of biological chemistry 280, 39609–39615, https://doi.org/10.1074/jbc.M507426200 (2005).
Fonseca, S. G. et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. The Journal of clinical investigation 120, 744–755, https://doi.org/10.1172/JCI39678 (2010).
Pobre, K. F. R., Poet, G. J. & Hendershot, L. M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. The Journal of biological chemistry, https://doi.org/10.1074/jbc.REV118.002804 (2018).
Li, Y., Guo, Y., Tang, J., Jiang, J. & Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta biochimica et biophysica Sinica 46, 629–640, https://doi.org/10.1093/abbs/gmu048 (2014).
Shalev, A. Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Molecular endocrinology (Baltimore, Md.) 28, 1211–1220, https://doi.org/10.1210/me.2014-1095 (2014).
Foufelle, F. & Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect 4, e00211, https://doi.org/10.1002/prp2.211 (2016).
Kaufman, R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13, 1211–1233 (1999).
Nguyen, C. et al. Reliability and validity of the Wolfram Unified Rating Scale (WURS). Orphanet J Rare Dis 7, 89, https://doi.org/10.1186/1750-1172-7-89 (2012).
Okamoto, H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat Surg 6, 254–262 (1999).
De Reggi, M. & Gharib, B. Protein-X, Pancreatic Stone-, Pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr Protein Pept Sci 2, 19–42 (2001).
Perez-Vazquez, V. et al. Differential proteomic analysis of the pancreas of diabetic db/db mice reveals the proteins involved in the development of complications of diabetes mellitus. International journal of molecular sciences 15, 9579–9593, https://doi.org/10.3390/ijms15069579 (2014).
Qiu, L., List, E. O. & Kopchick, J. J. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics 4, 1311–1318, https://doi.org/10.1074/mcp.M500016-MCP200 (2005).
Chan, J. Y., Luzuriaga, J., Bensellam, M., Biden, T. J. & Laybutt, D. R. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes 62, 1557–1568, https://doi.org/10.2337/db12-0701 (2013).
Gupta, D. et al. Temporal characterization of beta cell-adaptive and -maladaptive mechanisms during chronic high-fat feeding in C57BL/6NTac mice. The Journal of biological chemistry 292, 12449–12459, https://doi.org/10.1074/jbc.M117.781047 (2017).
Satomura, Y. et al. The immunohistochemical evaluation of PSP/reg-protein in normal and diseased human pancreatic tissues. International journal of pancreatology: official journal of the International Association of Pancreatology 13, 59–67, https://doi.org/10.1007/bf02795200 (1993).
Satomura, Y. et al. Measurement of serum PSP/reg-protein concentration in various diseases with a newly developed enzyme-linked immunosorbent assay. Journal of gastroenterology 30, 643–650 (1995).
Boeck, L. et al. Pancreatic stone protein: a marker of organ failure and outcome in ventilator-associated pneumonia. Chest 140, 925–932, https://doi.org/10.1378/chest.11-0018 (2011).
Bimmler, D. et al. Regulation of PSP/reg in rat pancreas: immediate and steady-state adaptation to different diets. Pancreas 19, 255–267 (1999).
Bonner, C. et al. INS-1 cells undergoing caspase-dependent apoptosis enhance the regenerative capacity of neighboring cells. Diabetes 59, 2799–2808, https://doi.org/10.2337/db09-1478 (2010).
Gross, D. J. et al. Amelioration of diabetes in nonobese diabetic mice with advanced disease by linomide-induced immunoregulation combined with Reg protein treatment. Endocrinology 139, 2369–2374, https://doi.org/10.1210/endo.139.5.5997 (1998).
Okamoto, H. & Takasawa, S. Recent advances in the Okamoto model: the CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta-cells. Diabetes 51(Suppl 3), S462–473 (2002).
Jansson, L. et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci 121, 81–95, https://doi.org/10.3109/03009734.2016.1164769 (2016).
Astorri, E. et al. Circulating Reg1alpha proteins and autoantibodies to Reg1alpha proteins as biomarkers of beta-cell regeneration and damage in type 1 diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 42, 955–960, https://doi.org/10.1055/s-0030-1267206 (2010).
Yang, J. et al. Pancreatic stone protein/regenerating protein (PSP/reg): a novel secreted protein up-regulated in type 2 diabetes mellitus. Endocrine 48, 856–862, https://doi.org/10.1007/s12020-014-0427-3 (2015).
Bacon, S. et al. Serum levels of pancreatic stone protein (PSP)/reg1A as an indicator of beta-cell apoptosis suggest an increased apoptosis rate in hepatocyte nuclear factor 1 alpha (HNF1A-MODY) carriers from the third decade of life onward. BMC endocrine disorders 12, 13, https://doi.org/10.1186/1472-6823-12-13 (2012).
Bernal-Mizrachi, E., Wen, W., Stahlhut, S., Welling, C. M. & Permutt, M. A. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. The Journal of clinical investigation 108, 1631–1638, https://doi.org/10.1172/JCI13785 (2001).
Luuk, H. et al. Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J Comp Neurol 509, 642–660, https://doi.org/10.1002/cne.21777 (2008).
Zhu, S., Si, M. L., Wu, H. & Mo, Y. Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). The Journal of biological chemistry 282, 14328–14336, https://doi.org/10.1074/jbc.M611393200 (2007).
Morrison, T. B., Weis, J. J. & Wittwer, C. T. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24, 954–958, 960, 962 (1998).
Wittwer, C. T., Herrmann, M. G., Moss, A. A. & Rasmussen, R. P. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 22, 130-131, 134–138 (1997).
Asghar, Z. A., Cusumano, A., Yan, Z., Remedi, M. S. & Moley, K. H. Reduced islet function contributes to impaired glucose homeostasis in fructose-fed mice. Am J Physiol Endocrinol Metab 312, E109–E116, https://doi.org/10.1152/ajpendo.00279.2016 (2017).
Multigner, L., De Caro, A., Lombardo, D., Campese, D. & Sarles, H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochemical and biophysical research communications 110, 69–74 (1983).
Gukasjan, R., Raptis, D. A., Schulz, H. U., Halangk, W. & Graf, R. Pancreatic stone protein predicts outcome in patients with peritonitis in the ICU. Critical care medicine 41, 1027–1036, https://doi.org/10.1097/CCM.0b013e3182771193 (2013).
Yang, J. et al. Pancreatic stone protein/regenerating protein (PSP/reg): a novel secreted protein up-regulated in type 2 diabetes mellitus. Endocrine, https://doi.org/10.1007/s12020-014-0427-3 (2014).
Bischoff, A. N. et al. Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet J Rare Dis 10, 66, https://doi.org/10.1186/s13023-015-0282-1 (2015).
Marshall, B. A. et al. Phenotypic characteristics of early Wolfram syndrome. Orphanet J Rare Dis 8, 64, https://doi.org/10.1186/1750-1172-8-64 (2013).
Spitzer, M., Wildenhain, J., Rappsilber, J. & Tyers, M. BoxPlotR: a web tool for generation of box plots. Nat Methods 11, 121–122, https://doi.org/10.1038/nmeth.2811 (2014).
Acknowledgements
The authors thank all of the participants in the Wolfram syndrome International Registry and Research Clinic for their time and efforts and the Washington University Wolfram Study Group Members and the study staff for their support (https://wolframsyndrome.dom.wustl.edu/). This work was partly supported by the grants from the National Institutes of Health/NIDDK (DK112921, DK113487, DK020579) and National Institutes of Health/NCATS (TR002065) and philanthropic supports from the Unravel Wolfram Syndrome Funds, the Silberman Funds, the Stowe Funds, the Ellie White Foundation for Rare Genetic Disorders, the Eye Hope Foundation, and the Snow Foundation to F. Urano and National Institutes of Health/NICHD (HD070855) to T. Hershey. D. Abreu was supported by the NIH training grant (F30DK111070). S. Stone was supported by the Pediatric Endocrine Society Research Fellowship Awards and NIH training grant (T32HD043010). The authors would like to acknowledge Kohsuke Kanekura and Cris Brown for their assistance and advice with these studies.
Author information
Authors and Affiliations
Contributions
S.S., D.A. and F.U. contributed to conception and design of the study. S.S., D.A., F.U., J.M., R.G., B.A.M., T.H. and F.U. contributed to data collection and analysis. S.S., D.A., F.U., J.M., R.A., K.K., R.G., B.A.M., T.H. and F.U. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stone, S., Abreu, D., Mahadevan, J. et al. Pancreatic stone protein/regenerating protein is a potential biomarker for endoplasmic reticulum stress in beta cells. Sci Rep 9, 5199 (2019). https://doi.org/10.1038/s41598-019-41604-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41604-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.