Abstract
TiO2 with different chemical structures are successfully synthesized via a one-step single-mode magnetic microwave (SMMW) assisted process, during where Ti selectively oxidizes in magnetic field under Ar-O2 mixed atmosphere. The chemical state and band structure of the as-prepared TiO2 are well-controlled by changing the volume fraction of O2 (φO2) during SMMW synthesis. Ti3+ self-doped TiO2 (TiO2−x, 0 < x < 2) is synthesized under lower φO2, while TiO2 with specific core/shell structure (TiO2+y core/TiO2−x-TiO2+z shell) is observed under higher φO2. The as-synthesized TiO2 with controlled structures show sufficient light absorption in visible region and quite narrow bandgap (2.05 eV∼), whose value can be also tuned by φO2 during SMMW synthesis. In addition, the synthesized TiO2 exhibits highly efficient photocatalytic performance towards the degradation of Rhodamine B under UV and visible light irradiation. The formation mechanism for different structural TiO2 can be attributed to the specific rapid heating and cooling dynamics induced by SMMW irradiation.
Similar content being viewed by others
Introduction
TiO2 photocatalysis have been widely utilized due to its physical and chemical stability, high photocatalytic activity, and nontoxicity1,2. However, the wide bandgap (3.0–3.2 eV) of TiO2 seriously limits its absorption wavelength and photocatalytic performance only in UV light region. Numerous efforts have been paid for enhancing photocatalytic performance of TiO2 in visible light region by inducing doped level from metals3,4 or nonmetals5,6,7,8. However, the element doping may cause thermal or crystal instability and an increase on carrier trapping, which may decrease the photocatalytic efficiency9. In recent years, Ti3+ self-doped TiO2 has attracted much interest, since surface energy level induced by Ti3+ and oxygen vacancies can improve visible-light absorption and results in high photocatalytic performance10,11,12,13,14,15.
In our previous paper, we initially reported a novel single-mode magnetic microwave (SMMW) assisted one-step synthesis of Ti3+ self-doped TiO216. Upon the one-step irradiation of SMMW in pure oxygen atmosphere, Ti target oxidizes in tens of second reaction by rapid temperature change. Such specific heating process can be attributed to the drastic change of MW absorbing property accompanied with changes of chemical state in obtained material. Here in the present research, TiO2 with well-controlled chemical states and band structures were selectively prepared by altering the oxygen fraction (φO2) in an Ar-O2 mixed atmosphere during SMMW synthesis. Ti3+ self-doped TiO2 (TiO2−x, 0 < x < 2) is synthesized under lower φO2, while TiO2 with specific core/shell (TiO2+y core/TiO2−x-TiO2+z shell) structure is observed under higher φO2. The structure-controlled TiO2 show sufficient light absorption in visible region with narrower bandgap (2.05 eV∼), whose value can be also well tuned by φO2. Meanwhile, the synthesized TiO2 show superior photocatalytic performance to commercial TiO2 in degradation of Rhodamine B (RhB) under both UV and visible light irradiation. The formation mechanism for different structural TiO2 is clarified based on systemically investigation on crystallinity analysis with X-ray diffraction (XRD) patter and Raman spectroscopy, confirmation of chemical state by X-ray photoelectric spectroscopy (XPS), characterization of electrical band structures with UV-visible and photoluminescence (PL) spectroscopy. The SMMW assisted synthesis process described in this paper provides new strategy for the development of functional metal oxides with well-controlled chemical structures and specific properties.
Results and Discussion
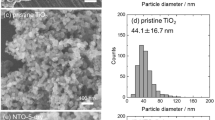
Figure 1a shows XRD pattern of the as-synthesized TiO2 samples. In the case of TiO2 prepared by MW irradiation under lower φO2 (MW-O5/MW-O10/MW-O20), two sets of diffraction peaks can be observed, corresponding to rutile TiO2 and Ti2O. With increasing of φO2, the intensity of rutile diffraction peaks increased while Ti2O peaks was disappeared, clearly demonstrates the progressive oxidation of target Ti. In addition, Figure 1b presents the change of lattice parameter of rutile TiO2 as a function of φO2. Interestingly, the lattice constant of a-axis and cell volume (V) significantly varied with φO2, taking the minimum value in the sample MW-O10. In the case of sample synthesized at the lowest φO2 (MW-O5), the a-axis lattice constant and V show the largest value. For samples synthesized under higher φO2 (MW-O10, MW-O20, MW-O30), the a-axis lattice constant and V are much smaller than MW-O5 and these values are gradually increased as φO2 increased. It has been reported that when oxygen vacancy is induced in TiO2, the Ti-Ti and Ti-O bonds are strongly relaxed and the nearest-neighbor Ti atoms move outward from the vacancy along a-axial, leading to the expanding of lattice constant of a-axis17. Thus, the expended large a-axis constant and V in sample MW-O5 can be attributed to the induce of oxygen defects. On the other hand, in the case of TiO2 possessing excessive oxygen atom, Ti-O bond is slightly relaxed because interstitial O atom repulse against lattice O atom, and coordinate with nearest-neighbor Ti atom, whose phenomenon seems to cause a slightly expanded lattice. It can be suggested that the TiO2 prepared under higher φO2 consists of both TiO2−x and TiO2+y states with regular arrangement of oxygen vacancy and excessive O atom, respectively.
Figure 2 shows Raman spectra of synthesized samples, summarized Raman shift of Eg mode of TiO2 as a function of φO2. As a result, characteristic peaks which are attributed Raman active modes of rutile crystal phase are observed in the spectra18,19. The peak of Eg mode is red-shifted with decrease of φO2. The red-shifting of Eg can be attributed to the inducing of oxygen vacancy defects since it has been reported that Eg mode is sensitive against oxidation state of rutile TiO2 in Raman spectra, which would be significantly red-shifted due to formation of oxygen vacancy defects in TiO220,21.
The chemical structure of TiO2 were furtherly investigated by confirming of surface chemical bonding sates via XPS, as shown in Figure 3. In the spectra of Ti2p orbital (Figure 3a), the peaks varied from 458.8 to 458.9, 458.9, 458.6, and 458.3 eV with increasing of φO2. These peaks are fitted by decomposed peaks at 458.8, 457.1 and 455.5 eV. The peak at 458.8 eV can be assigned as Ti4+ state in TiO2, and the peaks at 457.1 and 455.5 eV are assigned as Ti3+ and Ti2+, whose binding energy is 1.7 and 3.4 eV lower than that of Ti4+, respectively22,23. The peak components of Ti4+, Ti3+ and Ti2+ as a function of φO2 are summarized in Figure 4a. It can be clearly demonstrated that Ti3+ and Ti2+ components decrease as φO2 decreases, while Ti4+ component increases as φO2 increases. As for O1s spectra as shown in Figure 3b, peaks vary as 529.75, 529.9, 530.1, 529.55 and 529.7 eV for different samples and a shoulder peak appears at about 532.0 eV with φO2 increases. These peaks at 530.1 and 531.7 eV are assigned as O2− (Ti-O) and OH−, respectively23,24,25. According to the results summarized in Figure 4b, the concentration of OH group increases with increase of φO2. It has been reported that oxygen vacancy may induce dissociation of H2O, then chemisorption of OH group takes place on the surface of TiO21,26. Thus, the obtained results indicate that the TiO2 synthesized under high φO2 contain high concentration of oxygen vacancy. Furthermore, this oxygen vacancy would induce strong relaxation between Ti-O bonding as being already described, leading to chemical shift of Ti4+ peak center in Ti2p level toward lower binding energy. Figure 4c shows surface chemical composition of as-prepared samples estimated by peak area ratio in O1s to Ti2p. It demonstrates that excessive oxygen exists in TiO2 lattice synthesized under high φO2. Furthermore, the ratio of O/Ti was likely to decrease with decrease of φO2. This result might be not consistent with the fact that TiO2 synthesized under high φO2 contains high concentration of oxygen vacancy. Thus, here we suggest that the obtained TiO2 may possess quite specific chemical structure, which oxygen vacancy and interstitial oxygen atom coexist in TiO2 crystal on top of particle surface.
The electrical absorption spectra of as-prepared sample were characterized as shown in Figure 5a. The as-prepared TiO2 exhibit superior light absorption in visible region to compare with commercial rutile TiO2. In addition, the shoulder peak from 400 to 500 nm gradually appear with increase of φO2 due to formation of donor level between valence band (VB) and conduction band (CB) of TiO2 caused by interstitial oxygen atom in lattice27. Furthermore, the optical bandgaps are calculated by Tauc plot in Figure 5b and the bandgap values are summarized as Figure 5c. As a result, the maximum value of bandgap is observed in sample MW-O20, whose value becomes significantly smaller with lower φO2, while becomes slightly narrower with higher φO2 as shown. As bandgap of sample MW-O10 gives value of 2.07 eV, it is in good agreement with TiO2 where Ti3+ and oxygen vacancy introduce localized states at 0.75–1.18 eV below the CB minimum in the case of TiO2−x13. On the other hand, although interstitial oxygen atom localized the state about 0.2~0.3 eV above the VB maximum in the existence of excessive oxygen in TiO2 in general27, the obtained bandgap of TiO2 synthesized under high φO2 (MW-O20, MW-O30 and MW-O35) were much narrower. According to the CCD photograph of synthesized TiO2 as shown in Figure 6, the color of samples changed from metal gray to black, grey, ash grey and yellowish grey with different φO2. It is known that TiO2+x performs yellowish, on the contrast, the color of TiO2 with oxygen vacancy become grey or black because trapped electron in oxygen vacancy act as color center. Thus, TiO2 synthesized under high φO2, especially MW-O20, MW-O30 and MW-O35 have complex impurity level caused by oxygen vacancy and interstitial oxygen atom, resulting in formation of narrow bandgap.
According to XRD pattern, Raman spectra and electrical absorption spectra results, it can be concluded that TiO2 synthesized under high φO2 exhibit specific TiO2+y/TiO2−x-TiO2+z core/shell structure. Here, the formation mechanism of such core/shell TiO2 during SMMW synthesis is concluded in Figure 7. We suggest that the specific heat history in MW heating is one of key points to form the above specific particle structure. During MW synthesis under high φO2 system, TiO2+y would be firstly formed at high temperature, and rapidly cooled to room temperature immediately after formation of TiO2 due to lowering magnetic MW absorption, accompanying with large shrink of expanded lattice and introduction of high stress on outermost particle surface. Then the formed strain induces high concentration of defect, especially oxygen vacancy. It has been reported that rapid cooling after formation of oxide from metal target generates high concentration of defects on particle surface28,29. Furthermore, there are large densities of oxygen vacancies in TiO2 which have plastic strain induced by two anvils30. Therefore, amorphous phase of TiO2−x-TiO2+z formed on TiO2+y, resulting in formation of the specific core-shell structure. On the other hand, during MW synthesis under low φO2 system, Ti3+ doped TiO2 would be formed by the selective oxidation of Ti target in H-field throughout rapid heating and short reaction time14.
We summarized the structure controlling mechanism for TiO2 during SMMW reaction under different φO2 as Figure 8. In the case of SMMW synthesis under low φO2, Ti3+ self-doping TiO2 is formed from Ti particle due to fast oxidation. TiO2 synthesized under low φO2 contains high Ti3+ concentration due to specific heat history through MW reaction. In this case, thermal non-equilibrium reaction lead to uncompleted oxidation of Ti and crystallization of TiO2, resulting in formation of nonstoichiometric TiO2. Under high φO2, oxygen deficient TiO2 could be formed by rapid cooling due to introduction of high stress on top surface though the formation of oxygen excessive TiO2 at first. In the MW heating process, we control kind of introduced defect, Ti3+ and oxygen vacancy and specific chemical structure.
As an application of the prepared TiO2 with well-controlled structure, the photocatalytic activity towards the degradation of RhB under visible irradiation was examined, whose results are summarized in Figure 9(a). The TiO2 synthesized in low φO2 (MW-10) exhibits excellent photocatalytic activity under visible light, which can be attributed to the increased light absorption as shown by Figure 5. It worth noting that our synthesized TiO2, which possesses a mainly rutile phase and micro-ordered size show even better photocatalytic activity than the nano-ordered P25. The TiO2 prepared under high low φO2 (MW-20, MW-30) with specific core-shell structure show relative lower photocatalytic activity since high concentration of oxygen vacancies causes recombination of photo-excited carriers. In order to confirm the separation and recombination efficiency of photo-excited charge carriers, PL spectra is measured as Figure 9b. In general, the higher the recombination rate is, the stronger the PL peak intensity is14,31. As a result, the as-prepared TiO2 exhibited lower recombination rate than commercial TiO2, since the PL peak intensity decreases. In this case, the well separated electron and hole carriers transfer into the localized level of Ti3+ and VB respectively, leading to sufficient generation of O2· and OH· radicals from the reduction of O2 and oxidation reaction of H2O32,33,34,35. Such active radicals finally contribute to photo-degradation of RhB under visible light. It can be expected that as-synthesized TiO2 perform high photocatalytic efficiency than commercial TiO2 with existence of sufficiently separated photo-excited carriers.
Conclusions
Structurally well-controlled TiO2 are successfully synthesized via one-step SMMW assisted process under Ar and O2 mixed atmosphere. Ti3+ self-doped TiO2 and TiO2+y/TiO2−x-TiO2+z core/shell TiO2 are obtained by altering the volume fraction of O2. The synthesized TiO2 show sufficient light absorption in visible region and narrow band gap. In addition, superior photocatalytic activity for the photo-degradation of RhB under visible light irradiation is observed for the structure-controlled TiO2. Despite a large particle size (micrometer order) and rutile crystal phase, our synthesized TiO2 shows even better performance than commercial P-25 nanoparticle. The SMMW assisted synthesis process can provide new strategy for the preparation of functional metal oxides with well-controlled chemical structure and specific properties.
Methods
Materials and synthesis of TiOx
Titanium powder (3 N, powder under 45 μm mesh, Kojundo Chemical Laboratory, Japan) was used as raw material, and pelletized by uniaxial press. The pressure of 10 MPa was applied to a pellet of 10 mm in diameter. The TiO2 were synthesized by magnetic MW heating using 2.45 GHz single-mode MW applicator for TE103 mode. The MW output was fixed at 100 W and Ti pellets were heated up under mixed atmosphere of argon and oxygen, whose volume fraction were controlled as Ar/O2 = 100-x/x (x = 5, 10, 20 and 30, which named as MW-O5, MW-O10, MW-O20 and MW-O30, respectively).
Characterization
The crystal structure of the raw material and as-prepared samples were analyzed by X-ray diffraction (XRD) measurement with Cu-Kα (Ultima IV, Rigaku, Japan). The crystal structure in particle surface of obtained sample was furtherly analyzed by Raman spectra (NRS-3100, Jasco, Japan) were measured. The surface chemical state was investigated by X-ray photoelectrical spectroscopy (XPS; M-prove, SSI, USA) with Al Kαsource (hν = 1486.6 eV). The shift of the binding energy due to relative surface charge-up was corrected using the Au4f level at 83.98 eV and C1s level at 284.8 eV. UV-vis absorbance spectra and photoluminescence (PL) spectra were measured by commercial UV-Vis spectrophotometer (V-7100, Jasco, Japan) and spectrofluorometer (FP-8500, Jasco, Japan) at an excitation wavelength of 350 nm, respectively.
Photocatalytic degradation of RhB
In photocatalytic experiments, as-prepared TiO2 and commercial available TiO2 pellets catalyst (P-25, Degussa) were loaded into 20 ml of RhB solution (5 ppm). A 200 W Hg-Xe lamp (LA-310UV, HAYASHI, Japan) and Xe lamp (LA-251Xe, HAYASHI, Japan) were used as UV and visible light source, whose powder density was settled as 1 mW cm−2. Prior to irradiation, solutions with samples were left to stand in the dark for at least 180 min to ensure that the surface of photocatalysts were saturated with RhB. The RhB degradation was monitored by measuring the changes of UV-vis absorption spectra at 555 nm.
References
Hoffmann, M. R., Martin, S. T., Choi, W. & Bahnemann, D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69 (1995).
Fujishima, A., Tryk, D. A. & Rao, T. N. Titanium dioxide photocatalysis, Photochem. Photobiol. C, Photochem. Rev. 1, 1 (2000).
Klosek, S. & Raftery, D. Visible Light Driven V-Doped TiO2 Photocatalyst and Its Photooxidation of Ethanol. J. Phys. Chem. B 105, 2815 (2001).
Iwasaki, M., Hara, M., Kawada, H., Tada, H. & Ito, S. Cobalt ion-doped TiO2 photocatalyst response to visible light. J. Colloid. Interf. Sci. 224, 202 (2000).
Ohno, T., Mitsui, T. & Matsumura, M. Photocatalytic Activity of S-doped TiO2 Photocatalyst under Visible Light. Chem. Lett. 32, 364 (2003).
Chen, X. & Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 130, 5018 (2008).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 293, 269 (2001).
Irie, H., Watanabe, Y. & Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2−xNx Powders. J. Phys. Chem. B 107, 5483 (2003).
Xing, M., Zhang, J., Chen, F. & Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 47, 4947 (2011).
Lu, G., Linsebigler, A. & Yates, J. T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. J. Phys. Chem. 98, 11733 (1994).
Sasikala, R. et al. Highly dispersed phase of SnO2 on TiO2 nanoparticles synthesized by polyol-mediated route: Photocatalytic activity for hydrogen generation. Int. J. Hydrogen Energy 34, 3621 (2009).
Zuo, F. et al. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 132, 11856 (2010).
Nakamura, I. et al. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 161, 205 (2000).
Ozer, L. Y. et al. Long-Lasting Non-hydrogenated Dark Titanium Dioxide: Medium Vacuum Anneal for Enhanced Visible Activity of Modified Multiphase Photocatalysts. ChemCatChem 10, 2949 (2018).
Kumar, S. G. & Koteswara Rao, K. S. R. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 391, 124 (2017).
Kato, K., Yunzi, X. & Shirai, T. A novel single-mode microwave assisted synthesis of metal oxide as visible-light photocatalyst. materials letter 235, 125 (2019).
Santara, B., Giri, P. L., Imakita, K. & Fujii, M. Microscopic origin of lattice contraction and expansion in undoped rutile TiO2 nanostructures. J. Phys. D; Appl. Phys. 47, 215302 (2014).
Narayanan, P. S., Raman spectra of titanium dioxide, Proc. Indian Acad. Sci. Sect. A 32, 279 (1950).
Lan, T., Tang, X. & Fultz, B. Phonon anharmonicity of rutile TiO2 studied by Raman spectrometry and molecular dynamics simulations. Phys. Rev. B. 85, 094305 (2012).
Parker, J. C. & Siegel, R. W. Raman microprobe study of nanophase TiO2 and oxidation-induced spectral changes. J. Mater. Res. 5, 1246 (1990).
Parker, J. C. & Siegel, R. W. Calibration of the Raman spectrum to the oxygen stoichiometry of nanophase TiO2. Appl. Phys. Lett. 57, 943 (1990).
Bringgs, D. & Seah, M. P. Aurger and X-ray photoelectron spectroscopy, Practical Surface Analysis, (Wiley, New York, 1990).
Petigny, S. & Bourgeois, S. Superficial defects induced by argon and oxygen bombardments on (110) TiO2. surfaces, Surf. Sci. 410, 250 (1998).
Kumar, P. M., Badrinarayanan, S. & Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: correlation to presence of surface states. Thin Solid Films. 358, 122–130 (2000).
Erdem, B. et al. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 17, 2664 (2001).
Schaub, R. et al. Oxygen Vacancies as Active Sites for Water Dissociation on Rutile TiO2(110). Phys. Rev. Lett. 87, 2661041 (2001).
Etacheri, V., Seery, M. K., Hinder, S. J. & Pillai, S. C. Oxygen rich titania: A dopant free, high temperature stable, and visible‐light active anatase photocatalyst. Adv. Funct. Mater. 21, 1 (2011).
Liang, C. H., Shimizu, Y., Sasaki, T. & Koshizaki, N. Preparation of ultrafine TiO2 nanocrystals via pulsed-laser ablation of titanium metal in surfactant solution. Appl. Phys. A 80, 819 (2005).
Niu, K. Y. et al. Morphology control of nanostructures via surface reaction of metal nanodroplets. J. Am. Chem. Soc. 132, 9814 (2010).
Khosroshahi, H. R., Edalati, K., Arita, M., Horita, Z. & Fuji, M. Plastic strain and grain size effect on high-pressure phase transformations in nanostructured TiO2 ceramics. Scripta Materialia. 124, 59 (2016).
Li, F. B. & Li, X. Z. Photocatalytic properties of gold/gold ion-modified titanium dioxide for wastewater treatment. Appl. Catal. A: General 228, 15 (2002).
Turchi, C. S. & Ollis, D. F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. Journal of Catalysis 122, 178 (1990).
Carter, E., Carley, A. F. & Murphy, D. M. Evidence for O2 − radical stabilization at surface oxygen vacancies on polycrystalline TiO2. J. Phys. Chem. C 111, 10630 (2007).
Hirakawa, T., Yawata, K. & Nosaka, Y. Photocatalytic reactivity for O2 − and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A 325, 105 (2007).
Cao, Y. Q. et al. TiOxNy modified TiO2 powders prepared by plasma enhanced atomic layer deposition for highly visible light photocatalysis. Scientific Reports 8, 12131 (2018).
Author information
Authors and Affiliations
Contributions
K. Kato planned the work, performed the experiments, characterized the results and wrote the manuscript. Y. Xin helps in the interpretation of characterization and writing manuscript. T. Shirai supervised the project, contributed to planning the work and provided the interpretation of characterization.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kato, K., Xin, Y. & Shirai, T. Structural-Controlled Synthesis of Highly Efficient Visible Light TiO2 Photocatalyst via One-Step Single-Mode Microwave Assisted Reaction. Sci Rep 9, 4900 (2019). https://doi.org/10.1038/s41598-019-41465-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41465-x
This article is cited by
-
Aluminium foil-assisted NaBH4 reduced TiO2 with surface defects for photocatalytic degradation of toxic fuchsin basic dye
Applied Nanoscience (2023)
-
Facile synthesis and defect optimization of 2D-layered MoS2 on TiO2 heterostructure for industrial effluent, wastewater treatments
Scientific Reports (2020)
-
Effect of Pd and Cu co-catalyst on the charge carrier trapping, recombination and transfer during photocatalytic hydrogen evolution over WO3–TiO2 heterojunction
Journal of Materials Science (2020)
-
Visible light sensitization of TiO2/Ag/N nanostructures synthesized by microwave irradiation for oxidative degradation of organic dyes
SN Applied Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.