Abstract
A rapid, cost-effective and easy method that allows on-site determination of the concentration of live and dead bacterial cells using a fibre-based spectroscopic device (the optrode system) is proposed and demonstrated. Identification of live and dead bacteria was achieved by using the commercially available dyes SYTO 9 and propidium iodide, and fluorescence spectra were measured by the optrode. Three spectral processing methods were evaluated for their effectiveness in predicting the original bacterial concentration in the samples: principal components regression (PCR), partial least squares regression (PLSR) and support vector regression (SVR). Without any sample pre-concentration, PCR achieved the most reliable results. It was able to quantify live bacteria from 108 down to 106.2 bacteria/mL and showed the potential to detect as low as 105.7 bacteria/mL. Meanwhile, enumeration of dead bacteria using PCR was achieved between 108 and 107 bacteria/mL. The general procedures described in this article can be applied or modified for the enumeration of bacteria within populations stained with fluorescent dyes. The optrode is a promising device for the enumeration of live and dead bacterial populations particularly where rapid, on-site measurement and analysis is required.
Similar content being viewed by others
Introduction
Quantifying microorganisms, and especially bacteria, is a vital in task in many fields of microbiology. Traditionally, bacterial viability is determined by the number of colonies (called colony forming units, or CFU) grown from a known volume on solid growth medium after a period of incubation. Inevitably, this method is labour intensive and involves a significant delay of 1 to 5 days. Furthermore, this method can only account for the cells that are culturable under the conditions of the experiment. Hence it cannot give an indication of the number of dead bacteria or the viable but non-culturable (VBNC) cells that retain their metabolic and cellular activity under stress1,2. In addition to detecting live bacteria, the enumeration and differentiation of dead bacteria is valuable or necessary in many applications. For example, in the evaluation of antimicrobial drugs3,4, disinfection procedures5, the viability of starter cultures6, and monitoring of cell proliferation4. In all these applications, accurate and rapid information about the bacterial viability in the sample is desired.
Efficient, culture-independent detection of live and dead bacteria can be achieved using fluorescent dyes SYTO 9 and propidium iodide (PI) that differentially stain live and dead bacteria. Fluorescence detection is most commonly achieved by using microscopy, which allows direct investigation of individual cells. However, only a limited number of cells can be detected simultaneously, thus making the analysis of large sample volumes time consuming7,8,9. Fluorescence-based microplate readers offer more operational ease as the measurement of multiple samples can be automated and obtained in parallel9,10. The fluorescence intensity at discrete wavelengths are measured at the population level using optical filters or monochromats and extra calibration steps are required to obtain the sample concentration11,12. However, the accuracy of the calibration depends on the sensitivity of the plate reader and the quality of its optics, which both increase with cost13. Flow cytometry (FCM) allows study of cells at both the individual and population levels14. However, the application of FCM is restricted by its requirement of expensive and bulky equipment as well as trained technicians. Table 1 summarises the key metrics of alternative standard optical or molecular based microorganism detection methods compared to those of the optrode.
The purpose of this study is to demonstrate the ability of a fibre-based spectroscopic device called the optrode15 (Fig. 1) to obtain differential counts of live and dead bacteria in a mixture of both. This work aims to lay the foundation for future studies that extend the differential counting method to measure live and dead cell counts in mixtures of different bacterial strains. The study outlines a general method of using the optrode to measure fluorescence from mixtures of live and dead Escherichia coli cells that are stained with SYTO 9 and PI. Compared to FCM and microplate methods, the optrode is cost-effective and easy to use while also having a more compact design. Selective sensitivity to enumerate specific bacterial populations can be achieved by using functionalised fibres or surfaces, as applied in other detection systems16,17,18,19. However, the standard optrode system does not require such sophisticated fabrication nor antibody activation. By dipping the fibre probe of the optrode directly into fluorescently tagged bacterial suspensions, the optrode accurately measures the emission signals at the cell population level.
The optrode allows versatile control of exposure times ranging from 8 ms to 10 s, suitable for the sensitive characterisation of various fluorophores. The optrode measures fluorescence spectra across the entire visible range, which is processed in this study to obtain information about the amount and state of bacteria in the samples. To demonstrate, the optrode was used to measure spectra from E. coli samples with concentration of 107 or 108 bacteria/mL, where the proportion of live:dead ranged from 0 to 100% live. Initially, the integrated fluorescence intensity of the SYTO 9 and PI emissions were directly used to model live and dead bacterial concentration, respectively. However, our results showed that the absolute intensities of dyes do not vary linearly across the range of bacterial concentrations investigated, and a more sophisticated processing method was required. Thus, the performance of three multivariate spectral processing methods were evaluated and compared: principal components regression (PCR), partial least squares regression (PLSR) and support vector regression (SVR).
Results and Discussion
In this study, a portable and cost-effective fibre-based spectroscopic device (optrode) was used for the enumeration of fluorescently stained live and dead bacteria. Fluorescence spectra were collected from SYTO 9 and PI-stained E. coli samples where the proportion of live:dead ranged from 0 to 100% live. To model the concentration of live and dead bacteria in samples, the fluorescence spectra were used as predictors in three regression models: PCR, PLSR and SVR. We characterised the errors and investigated limits of detection for the described general optrode protocol in E. coli enumeration. This general protocol can be modified (e.g. by changing the volume or type of fluorescent dyes used) for the enumeration of a lower concentration range of E. coli or different types of bacteria.
Fluorescence profile and interdependence of SYTO 9 and PI
The spectral training data (N = 56 samples; n = 159 measurements, on average three per sample) were obtained from seven experiments where standard bacterial samples containing varying ratios of live:dead bacteria were prepared. The FCM measurements of the concentration of live and dead cell suspensions before they were combined, and the volumes combined to make the final bacterial samples are outlined in Supplementary Tables 1 and 2, respectively. The optrode measured spectral profiles of stained bacteria exhibit peak maxima at c. 520 nm and 620 nm, corresponding to SYTO 9 and PI fluorescence, respectively. Figure 2a shows exemplar spectra to demonstrate the changes in peak intensity, position and shape when samples containing different proportions of live and dead bacteria are analysed for a total concentration of 108 bacteria/mL. Initially, the integrated fluorescence intensity of SYTO 9 and PI were directly used to model the concentration of live and dead bacteria, respectively. However as shown in Fig. 3, the dye intensities were variable and did not vary linearly with bacterial concentration. These results demonstrate that when used in combination, the intensity of SYTO 9 and PI varies with changes in the presence of live and dead bacteria in the sample, but these intensity variations are not directly proportional to the change in bacterial concentration. This complexity is expected due to the interactions of the nucleic acid dyes as they compete for the same target area and the possibility for Förster resonance energy transfer (FRET) to occur, which also has been documented in other studies20,21. Thus for this experiment, quantitative information about the presence of a bacterial subset cannot be obtained from direct correlation with the intensity of a fluorescence peak.
An alternative way to predict bacterial concentration from fluorescence spectra is to use information from the full spectrum instead of only the integrated intensity from discrete sections of the spectrum. Analysis of the full spectrum will take into account features such as the relative intensity of both dyes, the shifts in wavelength and the changes in spectral shape, which provides information about the bacterial content of the samples. Numerous techniques including both traditional (e.g. PCR and PLSR) and machine learning approaches (e.g. SVR) allow multivariate inputs for the regression problem. To illustrate, the loadings plot in Fig. 2b shows the principal component analysis (PCA) signal corresponding to each wavelength that contributed to the PCR analysis. PC1 and PC2 explained 65.6% and 34.2% of the variance in the spectral dataset, respectively. PC1 and PC2 both encompass the signals from SYTO 9 and PI, which is further evidence for the interdependence of these two dyes. The weights for the SYTO 9 and PI peaks are shaped differently between PC1 and PC2, which accounts for the spectral changes that occur as the proportion of live and dead bacteria varies. Compared to peak intensity alone, methods such as PCR, PLSR and SVR that use information from the entire spectral window allow better characterisation of the spectral changes relative to changes in bacterial content of the sample.
Comparison of the models: PCR, PLSR and SVR
The group K-fold cross validation (GKCV) analysis of PCR showed that the lowest root mean square error (RMSE) values for live and dead prediction models were 2 and 3 principal components (PCs), respectively. However, PCA decomposition of the spectral training dataset showed that the first two PCs accounted for more than 99.8% of the variance with PC3 only explaining 0.01% of the variance. As a result, the first two PCs were used in multiple linear regression to build the PCR models for predicting the concentrations of live and dead bacteria. On the other hand, two and four latent variables were included in the PLSR models of live and dead E. coli, respectively. The choice of latent variables for PLSR was based on the lowest values of RMSE from GKCV, which were 7.2 and 6.8 for the live and dead prediction models, respectively. An initial examination of the models’ linearity was done by correlating the predicted concentration of the training dataset with the expected concentration measured by FCM. For all three regression models, the predicted concentrations correlated linearly with the FCM-measured concentrations down to c. 106.2 and c. 106 bacteria/mL for live and dead bacteria, respectively. The R2 and standard error of each regression model is summarised in Table 2, these values were calculated excluding data below the linear range.
PLSR and SVR performed slightly better than PCR in modelling the live and dead bacteria in training samples, as they had higher R2 and lower standard error values than PCR. However, the performance of PCR was better than both PLSR and SVR in predicting the concentration of test set samples, as shown in Table 3. PLSR and SVR are heavily dependent on identifying the precise patterns and relationships between the variations in spectral dataset and the expected bacterial concentration. However, the changes in spectral dataset become too subtle if the fluorescence signals are low, such as in the training samples with low concentrations or low percentages of the population of interest. As shown in the validation results, when there are spectral variations in the test set samples that are inconsistent with those in the training samples, PLSR and SVR predict bacterial concentration poorly and return invalid negative concentrations. In comparison, PCR is more robust against inconsistent spectral variations as shown by its performance in evaluating test set samples. Rather than focusing on the pattern between spectral predictors and target values, the predictive features used in PCR were chosen based on the amount of variance they explain in the spectral dataset. As PCR has less dependency on established patterns between predictors and targets, it demonstrated a superior ability to extrapolate the results for test set data.
Validation of the models using test set samples
The regression models were validated and compared using external test set samples (N = 27 samples, n = 80 measurements, on average three per sample) obtained from two blind experiments. The test set consisted of 24 samples within the concentration range of the training set (i.e. OD-estimated concentration of 107 to 108 bacteria/mL), and 3 extra samples with OD-estimated total concentration of 106.5 bacteria/mL. All three regression models performed significantly better in predicting live than the dead bacterial concentration. PCR performed the best overall, in predicting the concentration of both live and dead bacteria in test set samples. In some instances, the models returned negative concentration values which were considered as invalid predictions. The results for the prediction of the test set samples are summarised in Table 3.
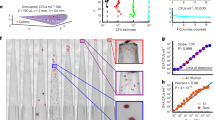
Figure 4a shows the live bacterial concentration of test set samples analysed using PCR, which are mostly within the 95% confidence interval of the 1:1 line, as represented by the region of ±2 standard errors22. Enumeration of live bacteria in the 3 extra test set samples with OD-estimated total concentration of 106.5 bacteria/mL was achieved. Furthermore, although the live bacteria in training samples modelled by PCR begin to flatten from c. 106.2 bacteria/mL, test data with concentration down to 105.7 bacteria/mL were predicted within 2 SE. Close inspection of the training data revealed that the subset which flattened from 106.2 bacteria/mL contained low percentages of live bacteria. The FCM measurements on this subset of training samples showed that the proportion of live bacteria they contained were below 10% and 25% for the c. 108 and 107 bacteria/mL samples, respectively.
The log of the live (a) and dead (b) E. coli concentrations predicted using the PCR model (each model used 2 PCs) against the log of the E. coli concentrations measured by FCM. The spectral training data and test set data are represented by crosses and circles, respectively. Extra test samples containing lower total bacterial concentrations than the concentration range of the training set were also evaluated and represented in triangles. The dashed line marks the ideal 1:1 relationship between predicted concentration and that measured by FCM, and the shaded area represents the region of plus or minus two standard errors of the regression model. The vertical and horizontal error bars represent the standard error in replicate measurements. Samples with invalid predictions were excluded.
The PCR model predicted dead bacterial concentrations of test set samples are shown in Fig. 4b. Although all three regression techniques modelled the dead bacteria in training samples down to c. 106 bacteria/mL, none of the three models were able to predict the concentration in test set samples below c. 107 bacteria/mL regardless of the proportion of live:dead bacteria that was contained.
Limit of detection (LOD) of the optrode method
The LOD of the PCR model for live and dead bacterial enumeration were found experimentally by examining their performance in modelling and predicting the training and test samples, respectively. PCR was able to model the live bacteria in training samples down to c. 106.2 bacteria/mL. The samples below this threshold corresponded to the 107 and 108 bacteria/mL samples that contained 10% and 25% live, respectively. However, we observed that the test samples which contained higher %live bacteria were successfully predicted down to c.105.7 bacteria/mL. Thus, in samples with low proportions of live bacteria, the SYTO 9 signal can be overwhelmed by PI signal from the dead bacteria. This weak signal makes it difficult to obtain reliable measurements of the SYTO 9 signal, which contains the information about the presence of live bacteria. In addition, the amount of dyes added remained the same while the total bacterial concentration was lowered from 108 to 107 bacteria/mL. Thus, at lower bacterial concentrations there will be more dye available per cell which could potentially lead to more FRET. This will further decrease the SYTO 9 emission and increase the PI emission intensity in samples with lower concentrations, making it difficult to measure the live bacteria.
Overall, the regression models performed better at enumerating live bacteria than dead bacteria. One explanation for this is that in solutions of DNA, the increase in PI fluorescence upon binding is up to c. 9 times which is low compared to that of SYTO 9 which is more than 360 times21. The initial linearity examination of the PCR model showed that the predicted concentration of dead bacteria in the training dataset correlated with the expected concentration down to c. 106 bacteria/mL. However, the predictions of dead bacteria in test samples below c. 107 bacteria/mL could not be achieved regardless of the %dead bacteria contained. When used in combination with SYTO 9, we observed that the peak intensity of PI in saline is comparable to that in dead bacterial samples at a concentration of 107 bacteria/mL (Fig. 5). As dead bacteria are primarily stained by PI, the strong background signal from unbound PI may account for the difficulty in enumerating dead bacteria below c. 107 bacteria/mL. This was not a problem for 108 bacteria/mL samples due to the quantum yield enhancement of PI upon binding and the large number of PI-nucleic acid complexes formed. Thus, strong PI signals above the background level were obtained from 108 bacteria/mL samples, with peak PI intensity that changes according to changes in the proportions of dead bacteria present. On the other hand, there is 10-fold less nucleic acid available in 107 bacteria/mL samples for PI to bind to and hence, the overall PI signal from these samples were not enough to exceed the background signal of unbound PI. Thus, to enumerate dead bacteria below c. 107 bacteria/mL, the regression algorithms would have to rely on the information from other sections of the spectrum to make its predictions, such as the changes in SYTO 9 intensity. However, these other spectral changes are not a direct result of the change in abundance of dead bacteria. For example, the decrease in SYTO 9 intensity would depend on the process of FRET, which depends on the abundance and proximity of PI, that then in turn depend on the abundance of dead bacteria and ease of access to its nucleic acids by PI. In addition, compared to SYTO 9 with its excitation maxima of 480 nm, PI has an excitation maxima of 540 nm and is less optimally excited using the 473 nm laser in the optrode21. With more optimal excitation of PI, the signals from the dead cells would appear stronger, more distinct and it may be possible to obtain more accurate predictions for dead bacterial concentration.
Exemplar spectra obtained from two fluorescently stained samples containing c. 107 bacteria/mL, and one stained saline sample with no bacteria. There is an increase in SYTO 9 intensity in the samples containing bacteria. On the other hand, the intensity of PI in saline is comparable to that in samples containing different proportions of dead bacteria at concentration of 107 bacteria/mL.
To investigate whether there were useful spectral patterns in samples that have concentration of live bacteria below the threshold of 106.2 bacteria/mL, the spectral training data for these samples were analysed independently of the higher concentration samples. Out of PCR, PLSR and SVR, only PLSR showed the potential to model live bacterial concentration below c. 106.2 bacteria/mL. However, evaluation using test set samples showed that the PLSR model built using low bacterial concentration data was unable to predict in this low concentration range. The fluorescence emission of the bacterial samples is affected by numerous factors including the ratio of dye molecules to nucleic acid (and hence the availability of unbound dye). As the stained bacterial samples were not washed to remove unbound dyes, the intensity change of spectral peaks in accordance to the changes in the low percentages of live or dead bacteria is limited. From the results we describe, the present method would favour situations where a controlled input concentration of bacteria is used to test the effectiveness of an antimicrobial intervention, e.g. a test of antimicrobial efficacy.
Future work
In the future, adjustments will be made to improve the LOD that goes beyond the scope of the current study. The main drawback in the method we describe is the strong background signal from unbound dyes, which has also been a challenge for previous studies using microplate readers to analyse SYTO 9 and PI dual-stained bacteria11,23. To overcome this limitation, the volume of dyes used can be decreased to target the detection of low concentrations of bacteria, as this will help reduce background fluorescence. Washing the sample to remove unbound dye is an option, however doing this at the laboratory bench level adds extra sample processing time and is difficult to achieve without losing bacteria in the process24. Instead, we plan on implementing ways to automate the staining and washing procedures efficiently using microfluidics25. Suitability of dyes with higher enhancement in quantum yield upon binding, and less broadband fluorescence excitation/emission that selectively stain dead bacteria could also be explored26. In addition, it is worth investigating the relationship between changes in spectral shape and the percentage of live or dead bacteria present in a sample to provide information that can be used in combination with the predicted concentration to yield a more accurate and precise measurement of bacterial content.
Ultimately, the ability of the optrode technique to enumerate low concentrations of bacteria will be limited due to the dependence on the bacteria being in the collection volume of the fibre which is c. 0.028 mm2 27. In this case, optrode faces the same difficulty as the microscope because to overcome the statistical counting error, a large number of measurements must be taken. To achieve this, the optrode could be programmed to sequentially record a series of measurements whereupon each series of spectral dataset can be analysed using a modified algorithm to account for the statistical fluctuations.
In addition to lowering the LOD, we also plan to further validate the optrode method using different species of bacteria, and specifically to study the fluorescence response of different groupings of microbes, including slow-growing bacteria, e.g. species of mycobacteria that cause skin infections28; microbes showing some natural fluorescence at the same wavelengths, e.g. plant-probiotic pseudomonads29; aggregating microorganisms, e.g. some pathogenic species of E. coli30. We do not expect major difficulties adapting the optrode method to study slow-growing bacteria, as the measurements are taken as a snapshot in time. Stained microbes which have intrinsic fluorescence will have more complex emission spectra and this effect would need to be accounted for in calibration development. We expect that it will be challenging to enumerate aggregating organisms using the optrode, as it is already a challenge to do using established methods such as plate counts and FCM31,32,33. Aggregation of cells may affect the penetration of laser excitation or fluorescence emission light, and hinder the penetration of dyes thus preventing homogeneous staining of the aggregated cells31. Thus, sample preparation may be difficult and need additional steps that could include surfactant34 or salt30 treatments.
The sensitivity of most optical detection methods, including fluorescence-based microplate readers, FCM and the optrode, is limited by the optical transparency of the suspension medium. If the suspension solution is fluorescent, opaque or contains particulates, this will interfere with the measured fluorescence emission and increase the noise11,31,35. Hence, in this study the FCM and optrode measurements were performed on bacterial samples suspended in saline. In order to analyse bacteria in different sample matrices, we are currently investigating automating the filtering and washing process by using microfluidic devices25,36. With automation of the numerous sample processing steps, it will be possible to obtain a substantially larger training dataset in which case the performance of SVR may improve37, and it also opens the possibilities to try selected deep learning algorithms38. We believe that future improvements on the LOD and sample processing procedures will contribute to the development of a device with sensitivity and robustness relevant for the medical and food industries.
To conclude, a rapid, cost-effective and easy method that allows on-site determination of the abundance of live and dead bacteria is important in numerous fields including pharmacodynamic studies3,4 and monitoring of cell proliferation4. In this study, we demonstrated that the fibre-based spectroscopic device (optrode) can be used to analyse a sample containing various ratios of live and dead E. coli, and obtain the concentration of each population. Of the three regression models investigated, PCR performed the best in predicting the live and dead bacterial concentrations in test set samples. The current optrode protocol with PCR is able to reliably enumerate live bacteria ranging from 108 down to 106.2 bacteria/mL, and there is potential to detect as low as 105.7 bacteria/mL if there is a large proportion of live bacteria in the sample. On the other hand, enumeration of dead bacterial concentration can be achieved within the range of 108 to 107 bacteria/mL.
The optrode is portable and requires little operator expertise which compares favourably with other forms of bacterial enumeration such as plate counting, fluorescence microscopy and FCM. The optrode procedure takes about 20 min, with 15 min allotted for staining and the spectral measurements required less than 15 s. The method is potentially applicable to the enumeration of live and dead bacteria in a wide range of disciplines, particularly the medical and food industries. The current study outlined the protocols and precautions of using the optrode for bacterial enumeration and serves to lay the foundation for future improvements and analysis of different bacterial mixtures.
Material and Methods
Bacterial growth conditions
E. coli strain ATCC 25922 (American Type Culture Collection, Virginia, USA) was incubated overnight in Difco tryptic soy broth (TSB; Fort Richard Laboratories, Auckland, New Zealand) then diluted 20 times and subcultured in fresh TSB for c. 1 h to yield a culture with an optical density (OD) at 600 nm, 1 cm path length of 0.6, equating to 4 × 108 bacteria/mL of exponentially growing cells. All broth cultures were grown at 37 °C and aerated with orbital shaking at 200 rpm.
Preparation of live:dead bacterial mixtures
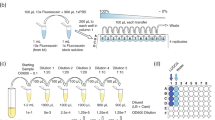
Bacterial suspensions were made using a modified protocol based on the instructions from the BacLight LIVE/DEAD Bacterial Viability and Counting Kit manual39. 10 mL of subcultured E. coli cells were harvested by centrifugation (4302 × g, 10 min, 21 °C) followed by removal of supernatant and resuspension in 3 mL of saline (0.85% w/v). Subsequently, 1 mL of the harvested subculture was diluted in 9 mL of saline (live bacterial suspension) and another 1 mL diluted in 9 mL of 70% isopropyl alcohol (dead bacterial suspension). Each suspension was incubated for 1 h at 28 °C and shaken at 200 rpm. Live and dead cells were harvested via three cycles of the washing process: centrifugation (4302 × g, 10 min, 21 °C) followed by removal of supernatant and resuspension in 20 mL of saline. After the final wash, the cells were resuspended in saline to achieve a concentration of c. 108 bacteria/mL; equivalent to diluting the sample to an OD of c. 0.2 at 600 nm. To make the standard bacterial samples, live and dead bacterial suspensions were combined in various volume ratios, giving c. 0, 2.5, 5, 10, 25, 50, 75, 100% live bacteria. The final bacterial suspensions contained varying live:dead ratios of either 108 or 107 bacteria/mL, with the total concentration estimated from OD measurements.
In addition to the set of standard bacterial samples for training the regression models, test set samples with OD-estimated total concentration of either 108 or 107 bacteria/mL were used as external validation for assessing the validity of the models. The live:dead ratios of the test samples did not coincide with that of the training set and to avoid bias, the preparation of the test set samples were blinded to the individual who measured and analysed them.
Fluorescent dye staining and microsphere protocol
BacLight LIVE/DEAD Bacterial Viability and Counting Kit (Invitrogen, Molecular Probes, Carlsbad, CA, USA; L34856) was used in our experiments. The kit contains a reference bead suspension and two nucleic dyes, SYTO 9 and PI. SYTO 9 is membrane permeant while PI is membrane impermeant39. Saline was used for diluting the stock dyes to make working solutions of SYTO 9 and PI with concentration of 0.0334 mM and 0.4 mM, respectively. For each sample, 50 µL of the working solution of SYTO 9, 50 µL of the working solution of PI and 10 µL of homogenised reference beads were aliquoted into an empty microcentrifuge tube. Then, 900 µL of each bacterial sample was added to the dyes and beads, followed by gentle vortexing at 500 rpm in the dark for 15 min in room temperature. The final concentration of SYTO 9 and PI in each sample was 1.65 µM and 19.8 µM, respectively.
Enumeration using the reference flow cytometry method
All samples were evaluated using a LSR II Flow Cytometer (BD Biosciences, San Jose, CA, USA), with previously established protocols that evaluated its sensitivity against plate counting24. Briefly, excitation was achieved by a 488 nm laser with 20 mW power. SYTO 9 fluorescence was collected using a 505 nm longpass filter and a 530/30 nm bandpass filter. PI fluorescence was collected using a 685 nm longpass filter and bandpass filter with 695/40 transmission. Threshold was set to side scatter at 200. The flow rate was set to c. 6 μL/min and the duration of each measurement was 150 s. The number of microsphere beads added to each sample was used to calculate the absolute concentration of live and dead bacteria measured, via the bead-based FCM method24,39,40.
This FCM method was able to achieve reliable enumeration of live E. coli when its proportions ranged from 100% to 2.5% live; and reliable enumeration of dead E. coli in the concentration range of 100% to c. 20% dead24. Thus to construct regression models, only the samples within the reliable enumeration range were used, i.e. the 2.5 to 100% and 0 to 75% live samples to model live and dead bacteria, respectively.
Optrode system
Bacterial fluorescence emission were recorded using a fibre-based spectroscopic system called the optrode15. Fluorescence excitation was achieved by a 473 nm solid state laser with c. 10 mW power. A data acquisition (DAQ) card synchronises the laser shutter with the spectrometer to minimise experimental variation from photobleaching. Using a 2 × 2 fibre coupler, laser light irradiates both the sample and a photodiode which monitors power fluctuations. A single probe made from multimode low OH silica fibre (diameter 200 μm, NA 0.22; Thorlabs Inc., Newton, NJ, USA) was used for excitation and fluorescence collection. The excitation line was removed by a 495 nm long-pass filter before reaching an Ocean Optics QE65000 CCD spectrometer which recorded the fluorescence spectra.
Spectra acquisition and preprocessing
The standard bacterial samples (N = 56 samples and n = 159 spectra, on average three per sample) and test set samples (N = 27 samples and n = 80, on average three per sample) were measured by the optrode with an integration time of 20 ms. The instrument dark noise was removed from each measurement, and the spectra were normalised to 10 mW laser power and 8 ms integration time. Then, the background spectrum acquired from saline was subtracted from each bacterial sample spectrum. Due to hasty cleaning of the probe in a few instances, one spectrum showed noticeably higher or lower intensity compared to the others that were recorded from the same sample, the abnormal spectrum was noted and excluded from the analysis. The remaining fluorescence spectra were mean-centered with respect to the average training spectra. These preprocessed fluorescence spectra were subsequently used in algorithms to correlate to bacterial concentration measured by FCM.
Data analysis
Initially, the integrated intensity of SYTO 9 and PI were directly compared to the live and dead bacterial concentration, respectively. The regions of intensity integration corresponded to the fluorescence peak of the dyes bound to E. coli, which was between 509–529 nm for SYTO 9 and 609–629 nm for PI.
Subsequently, the full fluorescence spectrum was used as input to evaluate performance of three regression techniques for predicting the concentration of live and dead bacteria: PCR, PLSR and SVR. The three models were evaluated by external validation using test set samples. The R2, standard error and the RMSE of the regression models were found. These algorithms were computed in Python codes using packages from NumPy41, Matplotlib42 and Scikit-learn43. Log-log plots (Fig. 4) were used to present the predicted versus measured E. coli concentrations clearly.
PCA was applied to reduce the multidimensional fluorescence spectral data to PCs, with the majority of the variance explained by the initial PCs. Multiple linear regression was performed using the initial PCs to build PCR models which correlates the spectral profiles to concentration of live and dead bacteria in the sample.
Similar to PCR, PLSR decomposes multidimensional input data into a smaller set of uncorrelated components called latent variables44. In contrast to PCA which decomposes the predictor data to obtain principal components that best explains variance in the predictor data itself, the first step of PLSR decomposes the predictor data to find latent variables that maximise covariance between predictor data and the response dataset (i.e. the expected values)44. This is followed by a regression step where a subset of latent variables are used to predict the response44.
To determine the number of PCs and latent variables to use, the amount of variance explained and GKCV were used to evaluate the performance of PCA and PLSR models built with varying numbers of PCs and latent variables, respectively. The spectral training dataset was split into groups that corresponded to the seven experiments performed to collect the data. Seven iterations were performed for the GKCV and for each iteration, one group was held out as the internal test set while the remaining six were used as the training set. The number of PCs or latent variables that returned the lowest RMSE and appreciably increased the amount of variance explained were chosen.
The ε-SVR from Python’s Scikit-learn library43 was applied using the linear kernel. In SVR, the input spectral data are mapped onto a high-dimensional feature space through nonlinear mapping, and subsequently a linear regression model is constructed in this feature space45. Hyper parameters ε and C which defines the margin of tolerance and penalty factor, respectively, were determined using the grid search function with GKCV43. Grid search performed exhaustive search over various values of both parameters to find the best estimators that minimised the mean squared error of the predictions.
Data Availability
The datasets generated and/or evaluated during the current study are available from the corresponding author on reasonable request.
References
Zhang, S., Ye, C., Lin, H., Lv, L. & Yu, X. UV Disinfection Induces a VBNC State in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 49, 1721–1728 (2015).
Colwell, R. R. et al. Viable but Non-Culturable Vibrio cholerae and Related Pathogens in the Environment: Implications for Release of Genetically Engineered Microorganisms. Nat. Biotechnol. 3, 817–820 (1985).
Gant, V. A., Warnes, G., Phillips, I. & Savidge, G. F. The application of flow cytometry to the study of bacterial responses to antibiotics. J. Med. Microbiol. 39, 147–154 (1993).
Ugolini, M., Gerhard, J. & Burkert, S. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat. Immunol. 19, 386–396 (2018).
Lee, Y., Imminger, S., Czekalski, N., von Gunten, U. & Hammes, F. Inactivation efficiency of Escherichia coli and autochthonous bacteria during ozonation of municipal wastewater effluents quantified with flow cytometry and adenosine tri-phosphate analyses. Water Res. 101, 617–627 (2016).
Bensch, G. et al. Flow cytometric viability assessment of lactic acid bacteria starter cultures produced by fluidized bed drying. Appl. Microbiol. Biotechnol. 98, 4897–4909 (2014).
Hammes, F. et al. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42, 269–277 (2008).
Jin, C., Mesquita, M. M. F., Deglint, J. L., Emelko, M. B. & Wong, A. Quantification of cyanobacterial cells via a novel imaging-driven technique with an integrated fluorescence signature. Sci. Rep. 8, 9055 (2018).
Alakomi, H.-L., Mättö, J., Virkajärvi, I. & Saarela, M. Application of a microplate scale fluorochrome staining assay for the assessment of viability of probiotic preparations. J. Microbiol. Methods 62, 25–35 (2005).
Bajorath, J. Integration of virtual and high-throughput screening. Nat. Rev. Drug Discov. 1, 882–894 (2002).
Feng, J., Wang, T., Zhang, S., Shi, W. & Zhang, Y. An Optimized SYBR Green I/PI Assay for Rapid Viability Assessment and Antibiotic Susceptibility Testing for Borrelia burgdorferi. PLoS One 9, 111809 (2014).
Duedu, K. O. & French, C. E. Two-colour fluorescence fluorimetric analysis for direct quantification of bacteria and its application in monitoring bacterial growth in cellulose degradation systems. J. Microbiol. Methods 135, 85–92 (2017).
Beal, J. et al. Quantification of bacterial fluorescence using independent calibrants. PLoS One 13, e0199432 (2018).
Blasi, T. et al. Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 7 (2016).
Guo, R., McGoverin, C., Swift, S. & Vanholsbeeck, F. A rapid and low-cost estimation of bacteria counts in solution using fluorescence spectroscopy. Anal. Bioanal. Chem. 409, 3959–3967 (2017).
Schiff, D., Aviv, H., Rosenbaum, E. & Tischler, Y. R. Spectroscopic Method for Fast and Accurate Group A Streptococcus Bacteria Detection. Anal. Chem. 88, 2164–2169 (2016).
Guo, Q. et al. Silicon-on-Glass Graphene-Functionalized Leaky Cavity Mode Nanophotonic Biosensor. ACS Photonics 1, 221–227 (2014).
Shi, X., Kadiyala, U., VanEpps, J. S. & Yau, S.-T. Culture-free bacterial detection and identification from blood with rapid, phenotypic, antibiotic susceptibility testing. Sci. Rep. 8, 3416 (2018).
Templier, V. et al. Biochips for Direct Detection and Identification of Bacteria in Blood Culture-Like Conditions. Sci. Rep. 7, 9457 (2017).
Barbesti, S. et al. Two and three-color fluorescence flow cytometric analysis of immunoidentified viable bacteria. Cytometry 40, 214–218 (2000).
Stocks, S. M. Mechanism and use of the commercially available viability stain. BacLight. Cytom. Part A 61, 189–195 (2004).
Altman, D. G. & Bland, J. M. Standard deviations and standard errors. BMJ 331, 903 (2005).
Stiefel, P., Schmidt-Emrich, S., Maniura-Weber, K. & Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 15, 1 (2015).
Ou, F., McGoverin, C., Swift, S. & Vanholsbeeck, F. Absolute bacterial cell enumeration using flow cytometry. J. Appl. Microbiol. 123, 464–477 (2017).
Julich, S. et al. Evaluation of a microfluidic chip system for preparation of bacterial DNA from swabs, air, and surface water samples. Biologicals 44, 574–580 (2016).
ThermoFisher Scientific. SYTOX ® Dead Cell Stain Sampler Kit for Flow Cytometry (S34862) Product Manual (2011).
Tai, D. C. S., Hooks, D. A., Harvey, J. D., Smaill, B. H. & Soeller, C. Illumination and fluorescence collection volumes for fiber optic probes in tissue. J. Biomed. Opt. 12, 034033 (2007).
Franco-Paredes, C. et al. Cutaneous Mycobacterial Infections. Clin. Microbiol. Rev. 32 (2018).
Picard, C. & Bosco, M. Genotypic and phenotypic diversity in populations of plant-probiotic Pseudomonas spp. colonizing roots. Naturwissenschaften 95, 1–16 (2007).
Rowe, M. C., Withers, H. L. & Swift, S. Uropathogenic Escherichia coli forms biofilm aggregates under iron restriction that disperse upon the supply of iron. FEMS Microbiol. Lett. 307, 102–109 (2010).
Jansson, J. K. & Prosser, J. I. Quantification of the presence and activity of specific microorganisms in nature. Mol. Biotechnol. 7, 103–120 (1997).
Jennison, M. W. The Relations Between Plate Counts and Direct Microscopic Counts of Escherichia coli During the Logarithmic Growth Period. J. Bacteriol. 33, 461–77 (1937).
Van Nevel, S. et al. Flow cytometric bacterial cell counts challenge conventional heterotrophic plate counts for routine microbiological drinking water monitoring. Water Res. 113, 191–206 (2017).
Caceres, N. et al. Evolution and role of corded cell aggregation in Mycobacterium tuberculosis cultures. Tuberculosis 93, 690–698 (2013).
He, S. et al. Rapid quantification of live/dead lactic acid bacteria in probiotic products using high-sensitivity flow cytometry. Methods Appl. Fluoresc. 5, 24002 (2017).
Marty, A., Roques, C., Causserand, C. & Bacchin, P. Formation of bacterial streamers during filtration in microfluidic systems. Biofouling 28, 551–562 (2012).
Smola, A. J. & Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 14, 199–222 (2004).
Goodacre, R. Explanatory analysis of spectroscopic data using machine learning of simple, interpretable rules. Vib. Spectrosc. 32, 33–45 (2003).
ThermoFisher Scientific. LIVE/DEAD ® BacLight TM Bacterial Viability and Counting Kit (L34856) Product Information. (2004).
Khan, M. M. T., Pyle, B. H. & Camper, A. K. Specific and Rapid Enumeration of Viable but Nonculturable and Viable-Culturable Gram-Negative Bacteria by Using Flow Cytometry. Appl. Environ. Microbiol. 76, 5088–5096 (2010).
Oliphant, T. E. A guide to NumPy. 1 (Trelgol Publishing USA, 2006).
Hunter, J. D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 9, 90–95 (2007).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip. Rev. Comput. Stat. 2, 97–106 (2010).
Vapnik, V. N. The Nature of Statistical Learning Theory (Springer New York, 1995).
Jarvis, B., Corry, J. E. L. & Hedges, A. J. Estimates of measurement uncertainty from proficiency testing schemes, internal laboratory quality monitoring and during routine enforcement examination of foods. J. Appl. Microbiol. 103, 462–267 (2007).
Delaire, C. et al. How Much Will It Cost To Monitor Microbial Drinking Water Quality in Sub-Saharan Africa? Environ. Sci. Technol. 51, 5869–5878 (2017).
Wang, Y., Hammes, F., De Roy, K., Verstraete, W. & Boon, N. Past, present and future applications of flow cytometry in aquatic microbiology. Trends Biotechnol. 28, 416–424 (2010).
Fu, C. J., Carter, J. N., Li, Y., Porter, J. H. & Kerley, M. S. Comparison of agar plate and real-time PCR on enumeration of Lactobacillus, Clostridium perfringens and total anaerobic bacteria in dog faeces. Lett. Appl. Microbiol. 42, 490–494 (2006).
Vero, S., Fremaux, B., Kralik, P. & Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 8, 108 (2017).
Luedtke, B. E. & Bosilevac, J. M. Comparison of methods for the enumeration of enterohemorrhagic Escherichia coli from veal hides and carcasses. Front. Microbiol. 6, 1062 (2015).
Ricchi, M. et al. Comparison among the Quantification of Bacterial Pathogens by qPCR, dPCR, and Cultural Methods. Front. Microbiol. 8, 1174 (2017).
Hasan, M. M., Alam, M. W., Wahid, K. A., Miah, S. & Lukong, K. E. A Low-Cost Digital Microscope with Real-Time Fluorescent Imaging Capability. PLoS One 11, e0167863 (2016).
Pettipher, G. L., Mansell, R., McKinnon, C. H. & Cousins, C. M. Rapid membrane filtration-epifluorescent microscopy technique for direct enumeration of bacteria in raw milk. Appl. Environ. Microbiol. 39, 423–9 (1980).
Gutierrez, R. et al. Monoclonal Antibodies and an Indirect ELISA for Detection of Psychrotrophic Bacteria in Refrigerated Milk. J. Food Prot. 60, 23–27 (1997).
Gracias, K. S. & Mckillip, J. L. A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can. J. Microbiol. 50, 883–890 (2004).
Pascaud, A., Amellal, S., Soulas, M.-L. & Soulas, G. A fluorescence-based assay for measuring the viable cell concentration of mixed microbial communities in soil. J. Microbiol. Methods 76, 81–87 (2008).
Bartholomew, C. Prices of CD4 assays and viral load tests must be reduced for developing countries. BMJ 323, 809–10 (2001).
Imade, G. E. et al. Comparison of a new, affordable flow cytometric method and the manual magnetic bead technique for CD4 T-lymphocyte counting in a northern Nigerian setting. Clin. Diagn. Lab. Immunol. 12, 224–7 (2005).
Acknowledgements
We are grateful to the New Zealand Ministry of Business, Innovation and Employment for funding the Food Safe; real time bacterial count (UOAX1411) research programme. This work is in partial fulfilment of Fang Ou’s PhD thesis, who is grateful for the University of Auckland Doctoral Scholarship, the Todd Foundation Award for Excellence, the RHT Bates Scholarship and the Claude McCarthy Fellowship. The authors thank Stephen Edgar, Dr. Julia Robertson, Zak Whiting and Janesha Perera for their laboratory support.
Author information
Authors and Affiliations
Contributions
F.O. conducted the experiments and analysed the data. F.V., S.S. and C.M. supervised the research and conceived the aim of the experiments. F.V. and S.S. obtained funding for the work. F.O. wrote the manuscript. All authors interpreted the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ou, F., McGoverin, C., Swift, S. et al. Near real-time enumeration of live and dead bacteria using a fibre-based spectroscopic device. Sci Rep 9, 4807 (2019). https://doi.org/10.1038/s41598-019-41221-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41221-1
This article is cited by
-
What happens to Bifidobacterium adolescentis and Bifidobacterium longum ssp. longum in an experimental environment with eukaryotic cells?
BMC Microbiology (2024)
-
Abundance of Live and Dead Bacteriopsammon Inhabiting Sandy Ecosystems of Recreational Marine Beaches of the Southern Baltic Sea
Microbial Ecology (2023)
-
Biosensors, modern technology for the detection of cancer-associated bacteria
Biotechnology Letters (2022)
-
Early detection of drug-resistant Streptococcus pneumoniae and Haemophilus influenzae by quantitative flow cytometry
Scientific Reports (2021)
-
Development of a nanoparticle-based gradient method for simple and fast quantification of bacteria-nanoparticle conjugates
Journal of Nanoparticle Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.