Abstract
There is increasing focus on use of resting-state functional connectivity (RSFC) analyses to subtype depression and to predict treatment response. To date, identification of RSFC patterns associated with response to electroconvulsive therapy (ECT) remain limited, and focused on interactions between dorsal prefrontal and regions of the limbic or default-mode networks. Deficits in visual processing are reported in depression, however, RSFC with or within the visual network have not been explored in recent models of depression. Here, we support prior studies showing in a sample of 18 patients with depression that connectivity between dorsal prefrontal and regions of the limbic and default-mode networks serves as a significant predictor. In addition, however, we demonstrate that including visual connectivity measures greatly increases predictive power of the RSFC algorithm (>80% accuracy of remission). These exploratory results encourage further investigation into visual dysfunction in depression, and use of RSFC algorithms incorporating the visual network in prediction of response to both ECT and transcranial magnetic stimulation (TMS), offering a new framework for the development of RSFC-guided TMS interventions in depression.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a severe mental disorder that affects up to 20% of the population worldwide. Approximately 50% of individuals with MDD fail to respond adequately to anti-depressant medications. For individuals with treatment resistant depression (TRD) electroconvulsive therapy (ECT) is the most effective treatment. However, ECT requires general anesthesia and may be accompanied by adverse cognitive effects, reducing its tolerability.
The response and remission rates for patients with depression without psychotic symptoms are 70% and 50%, respectively1. As a result, a significant number of individuals may be exposed to the significant risks of ECT without tangible benefit. At present, there are no measures available that are capable of differentiating responders from non-responders, and thus from preventing unneeded treatments. Also, despite considerable research, neural mechanisms underlying ECT effectiveness remain largely unknown, inhibiting the search for safer or more effective alternatives.
Prominent theories of both TRD and ECT response emphasize disruptions of resting state functional connectivity (RSFC) between large-scale brain networks in TRD that may be reversed with ECT treatment. To date, such studies have focused most prominently on interactions between regions of the fronto-parietal network especially dorsolateral prefrontal cortex (DLPFC; BA46, BA9 and BA8) and limbic regions such as subgenual anterior cingulate cortex (sgACC; BA25) or amygdala, and on fronto-limbic dysconnectivity. Other RSFC networks2, such as default-mode (DMN) and visual (VIS), have been investigated to a more limited extent.
The role of sgACC in TRD was first proposed ~20 years ago3,4,5 based on volumetric analysis, cerebral blood flow (CBF) and glucose metabolism. These findings complemented prior results with DLPFC6,7,8. Subsequent studies suggested an additional role for fronto-limbic dysregulation in both depressive illness and healthy sadness9,10,11. These theories led to development of experimental treatments such as deep brain stimulation (DBS) of the sgACC for treatment of persistent depressive symptoms11,12,13,14. In addition, non-invasive brain stimulation approaches such as transcranial magnetic stimulation (TMS) may be effective only to the degree that they effectively target regions of DLPFC that are anti-correlated with sgACC15.
Successive studies have investigated sgACC connectivity and fronto-limbic balance in relationship to treatment response to antidepressant treatments, including ECT16,17,18,19,20. For example, in one recent study, increased fractional amplitude of low frequency fluctuation (fALFF) involving sgACC significantly predicted ECT response and declined over time during ECT treatment. Functional connectivity between sgACC and additional limbic regions were also significantly reduced over the course of treatment. Nevertheless, the degree of variance explained by sgACC fALFF (~25%) was relatively modest and correlations between reduced fALFF and symptoms were not significant21. Changes in RSFC within dorsal ACC, medial prefrontal (MPFC) and lateral parietal cortex22, and between sgACC and DLPFC23 have also been associated with treatment improvement following ECT, but baseline patterns have not been shown to predict response.
Disturbances in other networks, such as the DMN and VIS, have also been reported in depression and ECT response, but have been less studied24. DMN is activated by internally focused cognitive processes such as mind wandering, self-reference, remembering the past and planning the future25,26,27. Lack of DMN suppression has been reported in depression during performance of cognitive demanding tasks28,29,30,31, and at rest32,33,34,35. DMN contains discrete anterior and posterior nodes in rostral MPFC and posterior cingulate (PCC) cortex, respectively36. Dissociation within the DMN33,37 and anterior-to-posterior hyperconnectivity38 have also been associated with depression.
The VIS cortex plays an important role in facial perception and expression as well as in emotion processing39,40. Processing of facial expressions has a predictive power to discriminate MDD patients from healthy controls41,42,43, and identifying patients who will respond to antidepressants44. Increased BOLD responses to sad stimuli in right sgACC and VIS cortex may be predictive of clinical recovery45, and respond to antidepressant treatment46,47,48. Deficits in VIS activation49,50 and processing of neutral facial51 expressions have also been reported in depression.
VIS perception, including functions such as contrast sensitivity, are altered in patients with depression, and normalized with remission after antidepressive therapy52,53,54. Alterations in VIS association (higher order) regions during perception, attention, working memory and VIS categorization may also occur in MDD55,56,57. Deficits are observed even in tasks involving simple VIS stimuli with no demand on emotion processing49,50, suggesting a basic dysfunction on processing information.
In this exploratory study, we evaluate RSFC pattern prior to and following ECT treatment, and evaluate both predictors and clinical correlates of response. RSFC patterns between DLPFC and both DMN and VIS networks, and between DMN and VIS as well as RSFC within these networks (“network homogeneity”) were computed. Based upon the accumulating data regarding VIS dysfunction in MDD, we hypothesized that including VIS connectivity along with that of more extensively studied fronto-limbic and DMN networks would significantly enhance predictive value of network models.
Results
Mean symptom reduction following ECT was 62% (62.2 ± 5.9). Further, 9 patients out of 18 (50%) were classified as remitters and 9 patients out of 18 (50%) as non-remitters. After treatment, average HDRS-24 in remitter (4.7 ± 0.8) differed from that of non-remitter (15.2 ± 1.3; p < 0.000). No significant differences in sex (60% female vs. 60% female), age (53.2 ± 3.7 vs. 50.2 ± 4.5) or HDRS-24 baseline scores (25.8 ± 1.2 vs. 27.2 ± 1.4) were found between remitters and non-remitters.
Response prediction
Table 1 summarizes findings regarding pretreatment RSFC patterns within and between DLPFC, DMN and VIS networks correlated to treatment response, with p-values greater than 0.05. Reduced connectivity (less anticorrelated) between DLPFC and DMN, or between DMN and VIS, and reduced connectivity (less correlated) between DLPFC and VIS, or within DLPFC, DMN or VIS, were associated with improvement in depression scores. After multiple comparison correction, decreased connectivity within aDMN(10r), between DLPFC(46) and aDMN(s32), or between DLPFC(p9-46v) and VIS(MT+), remained significant. Even if decreased connectivity within VIS(ventral) did not survive multiple comparison correction, it added predictive value to final models. The main model involved pretreatment RSFC within aDMN(10r) and VIS(ventral), with 100% accuracy of remission within this sample. Leave-one-out cross validation (LOOCV) on FDR corrected models, adjusted by motion displacement regressors, showed 0.83–0.89 prediction accuracy.
DLPFC-sgACC/rostral ACC
Consistent with previous work15, connectivity (reduced anticorrelation) between DLPFC(46, p9-46v) and sgACC(25) or rostral ACC(a24) were associated with treatment response but did not survive to multiple comparison correction (Supplementary Results).
DLPFC-DMN
A significant (r = 0.69, p = 0.002; Fig. 1A) correlation was observed between DLPFC(46)-aDMN(s32) connectivity and treatment response, but not for DLPFC(p9-46v). Remitters showed reduced anticorrelation compared to non-remitters (Fig. 1B). A ROC curve of this model showed significant area under the curve (AUC = 0.89, p < 0.001, 95% CI: 0.67–1; Fig. 1C). No significant correlations were observed between DLPFC(46, p9-46v) and pDMN(31pv, v23ab).
DLPFC, sgACC and rostral ACC inter-connectivity at baseline. (A) Correlation plot with baseline RSFC data for DLPFC(46) – aDMN(s32) associated with improvement in depression (% change in depression scores) ([pre-post/pre] × 100); brain images displayed at Cohen’s d values > 0 (Cohen’s d = 2t/sqrt(dfe)), colors represent surface vertices with positive correlation (red-yellow) between RSFC data and improvement in depression (% change in depression scores, [pre-post/pre] × 100). (B) Bar plot with baseline DLPFC(46) – aDMN(s32) comparison between non-remitters and remitters; bars represent connectivity values; **p < 0.01. Error bars represent standard error. (C) ROC curve analysis of DLPFC(46) – aDMN(s32) model. AUC, area under the curve; p-val, from Likelihood Ratio Test.
DLPFC/DMN-VIS
For DLPFC-VIS connectivity, a significant negative correlation was observed for baseline DLPFC(p9-46v)-VIS(MT+) (r = −0.61, p = 0.007; Fig. 2A) and treatment response, but not for DLPFC(46). Remitters showed significantly greater anti-correlation between DLPFC(p9-46v)-VIS(MT+) at baseline than non-remitters (Fig. 2B). In remitters, significant correlations with improvement were observed across multiple parcels. AUC for this model was highly predictive of remission (AUC = 0.89, p < 0.001, 95% CI: 0.70–1; Fig. 2C).
DLPFC and VIS inter-connectivity at baseline. (A) Correlation plot with baseline RSFC data for DLPFC(p9-46v) – VIS(MT+) associated with improvement in depression (% change in depression scores) ([pre-post/pre] × 100); brain images displayed at Cohen’s d values < 0 (Cohen’s d = 2t/sqrt(dfe)) for DLPFC(p9-46v) – VIS(MT+), colors represent surface vertices with negative (blue-violet) correlation between RSFC data and improvement in depression (% change in depression scores) ([pre-post/pre] × 100). (B) Bar plot with baseline DLPFC(p9-46v) – VIS(MT+) comparison between non-remitters and remitters; bars represent connectivity values, **p < 0.01. Error bars represent standard error. Brain images display RSFC between DLPFC(p9-46v) and VIS(MT+) for non-remitters and remitters; colors represent surface vertices with negative (blue-violet) correlation (−0.3≤ r ≤0.3) with VIS(MT+). (C) ROC curve analysis of DLPFC(p9-46v) – VIS(MT+) model. AUC, area under the curve; p-val, from Likelihood Ratio Test.
By contrast, for DMN-VIS connectivity, a significant positive correlation with treatment response was found for aDMN(10r)-VIS(MT+), but did not survive to multiple comparison correction (Supplementary Results).
Within-network connectivity
In addition to pairwise connectivity between networks, we also evaluated connectivity within networks. Reduced connectivity within aDMN(10r) (r = −0.70, p = 0.001; Fig. 3A) correlated with clinical improvement. Remitters showed significantly reduced intrinsic connectivity than non-remitters (Fig. 3B). AUC for intra-aDMN(10r) (AUC = 0.89, p < 0.001, 95% CI: 0.67–1; Fig. 3C) significantly predicted remission.
DMN intra-connectivity at baseline. (A) Correlation plot with baseline RSFC data for within aDMN(10r), associated with improvement in depression depression (% change in depression scores) ([pre-post/pre] × 100); brain images displayed at Cohen’s d values < 0 (Cohen’s d = 2t/sqrt(dfe)), colors represent surface vertices with negative (blue-violet) correlation between RSFC data and improvement in depression depression (% change in depression scores) ([pre-post/pre] × 100). (B) Bar plot with baseline RSFC within aDMN(10r) comparison between non-remitters and remitters; bars represent connectivity values; ***p < 0.001. Error bars represent standard error. (C) ROC curve analysis of RSFC within aDMN(10r) model. AUC, area under the curve; p-val, from Likelihood Ratio Test.
For VIS, reduced intrinsic connectivity within the ventral stream (intra-regional connectivity) predicted treatment response (r = −0.52, p = 0.03; Fig. 4A). Remitters showed less intrinsic connectivity than non-remitters (Fig. 4B). The AUC for prediction of remission by intra-regional connectivity within the ventral stream was also significant (AUC = 0.88, p = 0.001, 95% CI: 0.68–1; Fig. 4C).
VIS intra-connectivity at baseline. (A) (from up-down) Correlation plots with baseline RSFC data for within VIS(ventral) associated with improvement in depression (% change in depression scores) ([pre-post/pre] × 100); brain images displayed at Cohen’s d values < 0 (Cohen’s d = 2t/sqrt(dfe)), colors represent surface vertices with negative (blue-violet) correlation between RSFC data and improvement in depression (% change in depression scores) ([pre-post/pre] × 100). (B) (from up-down) Bar plot with baseline RSFC within VIS(ventral) comparison between non-remitters and remitters; bars represent connectivity values; **p < 0.01. Error bars represent standard error. Brain images display RSFC within VIS(ventral) for non-remitters and remitters, colors represent surface vertices with negative (blue-violet) or positive (red-yellow) correlation (−0.5≤ r ≤0.5) within VIS(ventral). (C) ROC curve analyses of RSFC within VIS(ventral) model. AUC, area under the curve; p-val, from Likelihood Ratio Test.
Correlation between connectivity measures at baseline
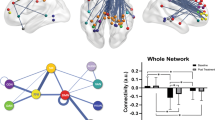
We generated a correlation matrix of pairwise correlation between all pretreatment connections (Fig. 5A) within and between DLPFC, DMN and VIS networks. These analyses most importantly revealed positive correlations between intra-aDMN and DLPFC-VIS connectivity, or negative correlations between intra-aDMN and DLPFC-aDMN connectivity. Conversely, intra-VIS connectivity was not correlated to intra-aDMN, DLPFC-VIS or DLPFC-aDMN connectivity.
Models for prediction of ECT response. (A) Correlation matrix of pairwise correlation between pretreatment connections within and between DLPFC, DMN and VIS networks; colors (red, blue) represent strength of correlations (+1, −1); *<0.05, **<0.01. (B) (from left-right) ROC curve analyses of Model 1: Baseline RSFC within-VIS(ventral) and within-aDMN(10r). Model 2: Baseline RSFC within-VIS(ventral) and between DLPFC(46) – DMN(s32). Model 3: Baseline RSFC between DLPFC(p9-46v) – VIS(MT+) and within-VIS(ventral). AUC, area under the curve; p-val, from Likelihood Ratio Test.
Two Factor Models for prediction of magnitude of improvement
No significant additivity was observed for models involving only DLPFC and DMN regions. Specifically, the combination of sgACC(25) and aDMN(24) did not show significantly increased predictive ability vs. DLPFC(46) connectivity to either region alone (Supplementary Table 1).
By contrast, when either intrinsic ventral VIS connectivity or pairwise connectivity between VIS regions and DLPFC or DMN were included, significant additive predictive values were obtained. Thus, the combination of intrinsic ventral VIS connectivity with either intrinsic aDMN(10r) connectivity (Table 2, Model 1), pairwise connectivity between DLPFC(46)-aDMN(s32) (Table 2, Model 2) or DLPFC(p9-46v)-VIS(MT+) (Table 2, Model 3), all resulted in significant improvements vs. the corresponding one-predictor models (R2 change ≥ 0.3, p < 0.05). Each of these models accounted for ~60% of the variance in treatment response.
Two factor models for prediction of remission
Logistic regression analyses and LOOCV were used in order to assess the degree to which the models shown above predicted remission. Intrinsic ventral VIS connectivity alone predicted 77.8% of remitters and non-remitters. The addition of intrinsic aDMN(10r) connectivity in Model 1 (Table 3), increased predictive value to 100% accuracy for both remitters and non-remitters. In Model 2 (Table 3), the addition of DLPFC(46)-DMN(s32) connectivity, also improved predictive value, with 94% accuracy of remission.
DLPFC(p9-46v)-VIS(MT+) connectivity alone predicted 83.3% (88.9% of remitters and 77.8% of non-remitters). The addition of ventral VIS connectivity in Model 3 (Table 3), also increased predictive value to 94% accuracy of remission. In ROC analyses, Models 1–3 (Fig. 5B) accounted for AUC values of 100%. In LOOCV analyses, Models 1–3 showed 0.83–0.89 accuracy (Table 4).
Parcelwise analysis
Given the overall significant involvement of reduced connectivity with the MT+ complex and within the ventral VIS region in prediction of ECT response, follow-up analyses explored connectivity related to individual parcels of these regions.
At baseline, within the MT+ VIS region, strongest (negative) correlations were observed between the Fundal area of the Superior Temporal (FST) sulcus (r = −0.66, p = 0.003) or the Lateral Occipital area 1 (LO1) (r = −0.61, p = 0.008) and the DLPFC(p9-46v) (Table 1). DLPFC(p9-46v)-FST connectivity was highly predictive of remission (AUC = 0.91, p < 0.001, 95% CI: 0.75–1). By contrast, DLPFC(p9-46v)-LO1 connectivity had only a modest predictive value (AUC = 0.77, p < 0.03; 95% CI: 0.51–0.99).
Within the ventral VIS region, strongest (negative) correlations were observed between the Fusiform Face complex (FFC) (r = −0.52, p = 0.03), area V8 (r = −0.53, p = 0.02) or the PIT complex (r = −0.56, p = 0.015) and the ventral region as a whole (Table 1). The AUC for prediction of remission was highly significant for PIT (AUC = 0.90, p < 0.001, 95%CI: 0.72–1). The predictive value for other parcels within the ventral region - e.g., FFC (AUC = 0.79, p = 0.008, 95%CI: 0.56–0.96) or V8 (AUC = 0.82, p = 0.005, 95%CI: 0.58–1) - were also significant but below for that of PIT.
Two factor models involving specific parcels within MT+ or ventral VIS regions were also assessed with multiple linear and logistic regression. These models were stronger than models involving the overall regions from which the parcels were taken (Supplementary Results).
Connectivity measures, motion displacement regressors and multiple comparison correction at baseline
No significant correlation between the mean relative motion displacement from frame-to-frame and percentage change in depression scores (r = −0.15, p = 0.55), nor between the mean absolute motion displacement from the first fMRI frame and percentage change in depression scores (r = 0.04, p = 0.87), were observed.
Further inspection of the association between baseline functional connections and change in depression scores revealed no significant effects of motion displacement (mean absolute or mean relative) on correlation values (Table 1). After FDR controlled multiple comparison correction, connectivity within aDMN(10r), or between DLPFC(46) and aDMN(s32), or DLPFC(p9-46v) and VIS(MT+), remained significant (Table 1). Of note, DLPFC(p9-46v)-VIS(MT+) survived multiple comparison correction after adjusting by motion displacement regressors, in line with the notion of distance-dependence bias58. Within the MT+ region, connectivity between FST and DLPFC(p9-46v), adjusted by motion displacement regressors, also remained significant after FDR correction (Table 1).
Finally, LOOCV analyses on FDR corrected models showed 0.83–0.89 accuracy (Table 4).
Changes in connectivity associated with clinical improvement
In order to identify correlates of improvement, changes in depression scores were correlated with change in RSFC measures (Supplementary Table 4). Consistent with predictor models, significant negative correlations were observed between change in DLPFC(46)-sgACC(25) connectivity and change in symptoms (r = −0.63, p = 0.005), suggesting that increases in anticorrelation between the parcels correlate with improvements in symptoms.
Similarly, change in connectivity between DLPFC(46) and aDMN(a24) (r = −0.63, p = 0.005), aDMN(s32) (r = −0.61, p = 0.008; Fig. 6B) or aDMN(10r) (r = −0.60, p = 0.009) all correlated with response, with increases in anticorrelation between parcels correlating with greater improvement. Similar magnitude correlations were also observed between treatment response and increase in DLPFC(p9-46v) anticorrelation to these same regions (aDMN(a24): r = −0.58, p = 0.01; aDMN(s32): r = −0.52, p = 0.03; aDMN(10r): r = −0.55, p = 0.02).
Baseline vs. change within and between DLPFC, DMN and VIS connectivity. (A,B) (from up-down, left-right) Schematic representation of RSFC structure at baseline (A) or change after ECT (B) associated with improvement in depression (% change in depression scores) ([pre-post/pre] × 100); colored straight lines show significant connections, with positive (+) or negative (−) RSFC correlation with ECT response. Correlation matrix of pairwise correlation between most significant connections at baseline (A), or change after ECT (B), within and between DLPFC, DMN and VIS networks and improvement in depression scores (% change in depression scores) ([pre-post/pre] × 100); colors (red, blue) represent strength of correlations (+1, −1); *p < 0.05, **p < 0.01. Correlation plots with baseline (A), or change after ECT (B), RSFC data for within and between DLPFC, DMN and VIS associated with improvement in depression (% change in depression scores) ([pre-post/pre] × 100); colored dot lines show significant connections with positive (+) or negative (−) correlation from final models.
Consistent with predictor models (Fig. 6A), positive correlations were also observed between treatment response and both increased correlation between DLPFC(p9-46v)-VIS(MT+) (r = 0.56, p = 0.015; Fig. 6B), and increased anticorrelation between VIS(dorsal)-pDMN(31pv) (r = −0.62, p = 0.007; Fig. 6B).
For within-network correlations, significant positive correlations with treatment response were observed for intra-DLPFC(46) connectivity (r = 0.65, p = 0.004; Fig. 6B), or connectivity between DLPFC(46) and DLPFC(p9-46v) (r = 0.69, p = 0.001); for intra-aDMN(10r) connectivity (r = 0.63, p = 0.005; Fig. 6B) or intra-pDMN(31pv/v23ab) connectivity (r = 0.61, p = 0.007); and for intra-VIS ventral connectivity (i.e., between PIT and the ventral region as a whole) (r = 0.48, p = 0.04; Fig. 6B).
Parcelwise analysis after ECT
We also examined correlation within individual parcels of MT+ and ventral or dorsal VIS regions (Supplementary Results). Within the MT+ region, strongest (positive) correlations were observed between LO1 or FST and DLPFC(p9-46v) (Supplementary Table 4). Within the dorsal stream, strongest (negative) correlations were observed between V3A, V3B, V6A and pDMN(31pv) (Supplementary Table 4).
Change in connectivity measures, motion displacement regressors and multiple comparison correction
Finally, no significant effects of motion displacement (mean relative or mean absolute) on the association between change in connectivity and depression scores were observed (Supplementary Table 4). After FDR controlled multiple comparison correction, connectivity between DLPFC(46, p9-46v) and aDMN(a24); or between DLPFC(46) and sgACC(25), aDMN(10r), or aDMN(s32); or between VIS(dorsal) and pDMN(31pv); or within aDMN(10r), pDMN(31pv/v23ab) or DLPFC(46, p9-46v/46), all remained significant (Supplementary Table 4).
Cortical Thickness and RSFC
No significant effect of treatment response on cortical thickness was found. Cortical thickness was not associated with remission nor with treatment response.
Discussion
This study evaluated RSFC patterns prior to and following ECT, with the goals of both identifying individuals who would benefit maximally from ECT treatment and evaluating RSFC changes associated with successful ECT. As in prior studies of this type23,59,60,61, pretreatment reduced connectivity (decreased negative correlation) within the fronto-limbic network, i.e., between DLPFC(46) and area 25, correlated with the magnitude of subsequent response and significantly predicted which subjects would enter remission. Similar relationships were observed for connectivity between DLPFC and DMN, also consistent with prior observations59,62,63,64,65,66; our findings regarding the fronto-limbic and DMN networks are further discussed in Supplementary Discussion.
A novel finding of the present study is that baseline reductions in RSFC between DLPFC and VIS regions, as well as reduced local connectivity within VIS, also significantly predicted subsequent treatment response, and changed along with improvement in depression. Moreover, inclusion of pretreatment connectivity reductions within the ventral VIS region along with other measures in the two-factor prediction algorithm significantly improved the accuracy of prediction and permitted ~100% discrimination between remitters and non-remitters within this sample, and >80% accuracy in a confirmatory LOOCV analysis.
VIS disturbances in TRD have previously been demonstrated using task-based activation and functional connectivity45,56,67,68, or metabolite concentration approaches69,70, but RSFC with or within VIS regions and treatment response in TRD has not been previously investigated. These findings thus provide strong evidence both for VIS cortical involvement in the pathophysiology of TRD and for the utility of RSFC as a predictor of ECT response in depression.
In the present study, we used a recently published multimodal parcellation map that permits sub-fractionation of regional networks into constituent components (“parcels”)2. This allowed us to identify discrete sub-regions of cortex (e.g., p9-46v, 46, FST, FFC, V8 or PIT; Fig. 7A,B) that may serve as appropriate targets for lower energy, non-invasive15,71,72 brain stimulation alternative to ECT; e.g., TMS using figure-8 field coils73 is focal (~1.3 cm) and superficial (0.9–3.4 cm). fMRI-guided TMS may allow identification and targeting of circuit-based targets using RSFC between specific DLPFC, DMN or VIS parcels, such as connectivity between DLPFC(p9-46v) and MT+ (FST), or between DLPFC(46) and DMN(s32).
DLPFCpos versus DLPFCneg and potential targets for TMS. (A) (from left-right) sgACC RSFC map from an independent cohort of 1200 healthy subjects from HCP was generated to identify more correlated (DLPFCpos) and anticorrelated (DLPFCneg) left DLPFC coordinates in the same sample of healthy subjects; colors represent surface vertices with negative (blue-violet) or positive (red-yellow) correlation with the sgACC. Geodesic map showing the geodesic distance (dg) in mm from average 5-cm MNI coordinates to the rest of the brain. (B) Potential VIS targets for FMRI-guided TMS.
To our knowledge, this is the first study to evaluate RSFC involving VIS cortex as a predictor of treatment response. Although sensory functions were once considered to be intact in neuropsychiatric disorders, over recent years there has been increased documentation of VIS cortical dysfunction in conditions such as schizophrenia74, autism spectrum disorder75 and aging76. Higher order VIS2 areas are divided into 3 regions, i.e., dorsal, ventral and MT+ complex. Consistent with our a priori hypothesis, pretreatment reduced connectivity (reduced positive correlation) between DLPFCneg and VIS was highly predictive of ECT treatment response including remission (Fig. 2C). Within DLPFCneg, parcel DLPFC(p9-46v) showed the highest correlation, while within MT+ complex, parcels FST and LO1 were most involved. Accuracy of models based on VIS connectivity were similar to models involving DLPFC and DMN.
Furthermore, when RSFC involving ventral VIS regions was included in two-predictor models, significant added predictive value was obtained over and above contributions of DLPFC and DMN (Fig. 5B). Thus, reduced positive RSFC within ventral VIS (Fig. 4C) and aDMN(10r) (Fig. 3C), or between DLPFC(p9-46v) and VIS(MT+) (Fig. 2C), or negative RSFC between DLPFC(46) and aDMN(s32) (Fig. 1C), at baseline significantly predicted ECT response.
Pretreatment DMN-VIS reduced connectivity (reduced negative correlation) also significantly predicted remission, although to a lesser extent than DLPFC-VIS connectivity; i.e., reduced connectivity between aDMN(10r) and MT+ VIS (e.g., parcels FST, LO1). After treatment, increased positive RSFC between VIS and DLPFC, or increase negative between VIS and DMN, were highly correlated to symptom improvement (Fig. 6B).
Finally, reduced positive RSFC within-VIS regions at baseline, especially within the ventral stream (e.g. parcels FFC, V8, PIT), was highly predictive of remission, with similar classifier performance (AUC) to that for connectivity between DLPFC-aDMN(s32), DLPFC-VIS(MT+) or intra-aDMN(10r). Following ECT, increased positive correlation within ventral VIS also correlated with magnitude of treatment response (Fig. 6B).
Although the physiological basis for the correlations between VIS connectivity and ECT response are unknown, regions such as MT+ complex are directly or indirectly coupled to parietal and frontal regions associated with sensory-motor integration77. Multisynaptic inputs in non-human primates from the frontal cortex to MT+ complex and ventral stream are derived from ventral area 4678, which is possibly involved in carrying functionally diverse top-down signals to each VIS region (i.e., dorsal, ventral and MT+).
The MT+ complex incorporates areas in lateral occipital and posterior temporal cortex, and is extensively activated during both motion and social cognition tasks2. Within MT+, the connectivity in non-human primates between area FST (dorsal) and the dorsal processing stream directed to posterior parietal cortex is important in spatial aspects of vision, while FST (ventral) connectivity to the ventral processing stream is related to object vision79. Thus, the MT+ parcels FST and LO might function as an intermediary of top-down regulation of dorsal and ventral streams of VIS.
The ventral stream incorporates face-sensitive regions such as FFC2 and PIT80 and color sensitive regions such as V881. Patients with MDD show increased FFC activity to sad faces and decreased activation to happy faces82, and slowed face emotion recognition83. Moreover, alterations in color processing are known both scientifically53,84,85,86,87 and colloquially (“seeing the world through grey tinted glasses”)88 in depression. More basic deficits in VIS function, such as alterations in sensory event-related potentials or center-surround inhibition of motion processing89 are also reported, consistent with our VIS findings.
A surprising result of our study is that the strongest model for prediction of ECT response involved connectivity within ventral VIS by itself, rather than connectivity between VIS and other brain regions, in combination with connectivity within aDMN. Although the basis for the intraregional dysconnectivity is unknown, both serotonergic and GABAergic theories can be considered. In humans, VIS cortex receives dense serotonergic innervation. Serotonergic fibers arise in the raphe nuclei and project anteriorly to innervate inferior and medial prefrontal cortex along with looping posteriorly to terminate in VIS regions. Treatment of depression with the serotonin 5-HT2A receptor agonist psilocybin improves emotional processing83, suggesting potential serotonergic mediation of the effects. Additionally, ECT-induced changes in several 5-HT-receptor subtypes18,90,91 in the central nervous system, have also been reported.
Occipital GABA levels are also reduced in MDD92, consistent with center-surround inhibition abnormalities89. Furthermore, such levels increase following ECT93. Both serotonin94 and GABA95,96,97, may modulate RSFC in healthy individuals. The present study suggests that disruption of intrinsic connectivity within both VIS and DMN may indicate both need for and successful response to treatments such as ECT that may function in part through modulation of serotonergic and GABAergic activity. Thus, to the extent that serotonergic and GABAergic dysfunction is involved in TRD, disturbances in RSFC between VIS cortex and DLPFC/DMN are expected as part of a larger RSFC disturbance.
The findings of impaired connectivity within ventral VIS also converge with reported alterations in attention to happy vs. sad faces in depression. Connectivity within VIS cortex, including ventral VIS, may be modulated by top-down connections from DLPFC and limbic regions such as amygdala or sgACC98,99,100,101. Thus, even though connectivity was obtained during the resting state, it is possible that decreased top-down DLPFC or increased top-down limbic modulation of VIS may have contributed to the present results. Future studies evaluating modulation of RSFC as a function of attention to affective stimuli are therefore required.
Alternatively, intrinsic abnormalities of ventral VIS connectivity may lead to intrinsic biases in VIS function that cannot be overcome through usual top-down control mechanisms. VIS connectivity may also be indexed by generation of the posterior alpha rhythm, with reduced connectivity associated to an alpha synchronization deficit102. Alpha rhythms in parieto-occipital cortex are controlled by top-down signals103 and their disruption might lead to a suboptimal state of neural synchronization. Future studies would thus be needed to evaluate the potential utility of resting posterior alpha for prediction of ECT response.
Based upon the present pattern of results, we propose a dysfunction in the hierarchical organization within and between VIS, DMN, and DLPFCneg subnetworks as a new biomarker of ECT response, which also sheds light on underlying mechanisms. Specifically, we observed that TRD patients who show the best response to ECT show a pretreatment pattern characterized by reduced anti-correlation between DLPFCneg-DMN and DMN-VIS, as well as reduced positive correlation between VIS-DLPFCneg or within VIS and DMN (Fig. 6A).
Moreover, effective ECT increases the strength of anti-correlations between DMN and DLPFC or VIS, and of the positive correlation between VIS and DLPFCneg (Fig. 6B), or within VIS and DMN. The resting anticorrelation between DLPFC and DMN is extensively studied and serves as the basis for suppression of DMN during active task conditions. Our results suggest that failures of deactivation may result in part from reduced connectivity both within DMN and between DMN and DLPFC. The VIS-DLPFC correlation is also studied in the context of attentional filtering of distracting information (bottom-up/top-down inhibitory control of the distractor related processing). The VIS-DMN anti-correlation is less studied, but may underlie the ability of VIS stimuli also to suppress DMN activity104.
Aberrant DMN connectivity is well established in MDD29,105,106, including TRD32,107. The present findings suggest that ECT may function primarily to restore the normal108 anti-correlation of DMN with both “top down” and “bottom up” networks, in order to suppress ongoing DMN hyperactivity that may be associated with increased “mind wandering” relative to goal-directed activity. Positive connectivity between DLPFC and VIS regions is critical for normal goal-directed VIS activity109. ECT-induced changes in connectivity may therefore restore both baseline VIS biases and top-down VIS control. Interestingly, our study implicates both face- (e.g. FFC, PIT) and color- (e.g. V8) parcels within ventral VIS, consistent with popular depictions of the disorder.
The only model to obtain 100% accuracy in both logistic regression and ROC analyses involved connectivity within ventral VIS and aDMN(10r) (Model 1, Table 3), or aDMN(s32/10r) (Model 5, Supplementary Table 3). The rostral MPFC (i.e., 10r) is considered a focal point of neural communication, participating in multiple functional networks and enabling high levels of functional diversity (functional hub)110. Disturbances of this hub region and their interconnections are likely to cause severe impairments due to their influential role in global integrative processes.
Our results showed abnormal functioning of this hub region was associated to disturbances in the pattern of dynamic interactions (outgoing projections) with central regions of attentional networks, such as DLPFC and VIS, with decreased connectivity intra-aDMN(10r) associated to decreased connectivity intra-DLPFC or DLPFC-VIS, and DLPFC-DMN or DMN-VIS (connected components) (Fig. 5A), reinforcing the central role for 10r hub node in the overall network structure and dynamical organization. Depression has also been associated with abnormal topological organization of brain networks, including disrupted global integrity and regional connectivity111.
On the other hand, decreased intra-VIS connectivity was only associated to decreased intra-DLPFC connectivity (Fig. 5A), consistent with the observation of primary regions (e.g., VIS) to participate in small number of functional networks compared to the participation of 10r in multiple functional networks110. Consistent with current results, Zhang et al.112 showed decreased regional connectivity (degree efficiency and betweenness) in the DLPFC and occipital regions. As such, the model combining both, RSFC within 10r and ventral VIS, includes two core non-overlapping components of the overall network dysfunction in TRD.
The posterior alpha network comprises the occipital lobe and parts of the temporal and medial posterior-parietal cortex (including posterior cingulate gyrus and precuneus) and temporal lobes113. After effective treatment, connectivity changes within (increased correlation) and between VIS and pDMN (increased anticorrelation) were associated with good response. Connectivity changes propagated through the entire VIS network including MT+ complex (e.g., FST, L03, V3CD), dorsal stream (e.g., V3A, V3B, V6A, V7) and ventral stream (e.g., PIT), extending to pDMN (e.g., 31pv, v23ab), suggesting modulatory effects in the intrinsic activity of VIS system.
In addition to predicting ECT response, the present results argue for a move in scalp location of the standard TMS probe placement ≥ 20–50 mm of geodesic distance (dg) (defined as the length of the shortest directed path from the current canonical location) from the present location which is defined based upon the 5cm-rule (corresponding to vertex 30641) (Fig. 7A). Furthermore, it argues strongly for individual targeting of rTMS to areas 46 or p9-46v, which correspond to the parcels of DLPFC region that are anti-correlated to sgACC (DLPFCneg). These findings are consistent with those of Fox et al.15,71,72, who also suggested that the DLPFCneg (BA46) region may serve as a critical treatment target in depression, and support the role of DLPFC-sgACC, DLPFC-aDMN, and DLPFC-VIS RSFC both as predictors of need for ECT, and correlates of change.
There are several limitations of the present study. First, the sample size is relatively small, necessitating replication in larger cohorts. There were also no cohorts receiving other treatment types (e.g. antidepressants, ketamine) so that it is unknown whether the network effects predict general treatment response or response specifically to ECT.
Second, in order to obtain 100% correct classification, we required use of two independent predictors. Because the model was not pre-specified, the present results must be considered exploratory and must be confirmed in a future sample. Nevertheless, analyses were constrained using a pre-planned parcellation scheme, which limited the number of comparisons. Specifically, DLPFCneg, sgACC, aDMN, ventral VIS and MT+ complex were all identified a priori. DLPFCneg-sgACC and DLPFCneg were selected for analyses based upon prior literature. Although 3 VIS regions were used in analyses, the findings regarding DLPFC-MT+ connectivity were sufficiently strong to survive FDR correction for multiple comparisons. The findings with intra-ventral connectivity were weaker, but were additive to several other connectivities (DLPFC-sgACC, DLPFC-aDMN, DLPFC-VIS, intra-aDMN), providing convergent support. Predictive models showed 0.83–0.89 accuracy in LOOCV analyses even following control by motion regressors and FDR correction, and thus support further studies with this algorithm.
As opposed to the present study, a recent study114 evaluated changes in patients with schizophrenia treated with combined antipsychotics and ECT, and showed a five-predictor algorithm (i.e., DMN, temporal lobe network, language network, cortico-striatal network and fronto-parietal network) that correctly classified 83.8% of 13 patients, suggesting that multiple predictors do not, of necessity, produce high predictive values. Here, by uniquely incorporating VIS RSFC measures in our algorithm, we obtain 100% correct classification in a sample of comparable size and with only one- or two-predictors.
Finally, since there is not a multi-modal subcortical parcellation available at this time, we considered only cortical structures in the present study. By contrast, a recent study demonstrated that DLPFC-striatal connectivity significantly predicted TMS response115. Thus, future studies incorporating both cortical and subcortical structures are needed.
Methods
Participants
We studied 18 TRD patients receiving ECT for clinical indications at New York State Psychiatry Institute (NYSPI) or Columbia University Medical Center (CUMC) (ages: 18–75; mean = 52, SD = 12), who met DSM-IV criteria for a Major Depressive Episode (MDE) according to the diagnostic assessment by the Structured Clinical Interview Patient Edition (SCID-P), with scores of 18 or greater (mean = 26.5, SD = 3.9) on the 24-Hamilton Depression Scale (HDRS-24). Patients with comorbid other Axis I or Axis II psychiatric disorders were excluded. All subjects were right-handed and without severe medical conditions. The NYSPI/CUMC Institutional Review Board approved this study. All participants provided written informed consent. This study was performed in accordance with all relevant guidelines and regulations.
Electroconvulsive therapy
Participants received a full course of right unilateral, frontal ECT. ECT and anesthesia procedures complied with APA Guidelines116. Blood pressure, pulse, ECG, and pulse oximetry were monitored prior to anesthetic induction and continuously during the procedure. Seizure manifestations were recorded with two frontal-mastoid EEG channels, as well as motor manifestations with the cuff technique. Using conservative criteria (≥15 seconds), generalized seizures of adequate duration were elicited at each treatment. Ultra-brief right unilateral (RUL) ECT was given with a Thymatron System IV brief pulse device. Seizure threshold (ST) was determined at the first treatment using the dose titration method117. Dose at subsequent treatments was at 6xST or maxim output, whichever was lower. Patients remained on pre-existing antidepressant medication without alteration over the course of the study.
The Hamilton118 rating scale (HDRS, 24 items) was obtained pre/post ECT and used for outcome analyses.
fMRI Data Acquisition and Processing
High resolution anatomical images and resting-state functional MRI were collected pre/post ECT. Post study imaging was performed approximately 48–72 hours after the last treatment to allow sufficient time for recovery from treatment. Anatomical and functional imaging data from each subject were collected and processed using acquisition guidelines and processing pipelines provided by the Human Connectome Project (HCP)119. Collected data was also compared to the healthy individuals from the HCP database (https://www.humanconnectome.org/study/hcp-young-adult/data-releases). High-resolution functional imaging scans were completed at the MRI facility at NYSPI using a GE Discovery MR750 3.0 Tesla full body MR system equipped with a 32-channel phased array head coil (Nova Medical, Wilmington MA).
Subjects were placed in the scanner with head cushioning to restrict head movement. Following localizer scans (5 minutes), distortion correction scans (B0 fieldmap), a pair of T1-weighted images, and a T2-weighted image are acquired over 25 minutes, followed by one 10-minute resting state fMRI scan. Total scan time was 45 min. Sessions were conducted prior to and following completion of the course of ECT. T1-weighted images acquired for anatomical co-registration were transverse T1-weighted BRAVO sequence with the following acquisition parameters: 3D sagittal, 0.8 mm isotropic, matrix size = 300 × 300, slices = 220, TR = 7.856 ms, TE = 3.108 ms, flip angle = 12°, TI = 450 ms and CUBE T2-weighted image was acquired with these parameters; 3D sagittal, 0.8 mm isotropic, matrix = 320 × 320, # of slices = 220, TR = 2500 ms, TE = 95.708 ms, flip angle = 90°.
Functional images were acquired with a GE-EPI sequence (2.5 mm isotopic, slice plane = transverse, TR = 2500 ms, SENSE factor = 2, TE = 22 ms, matrix = 96 × 96, slices = 54, phase encode = A −> P); an instruction was given to all patients before the functional MRI sequence to have their eyes opened. Images were transferred to a workstation with the HCP processing pipeline version 3.4 installed. The pipeline implements standard automated structural and fMRI processing (movement correction, atlas realignment, creation of cortical surface model etc.) with FSL 5.0.6, FreeSurfer v5.3.0-HCP, and Connectome Workbench v1.0, and two additional procedures that improve upon standard preprocessing: required use of field-mapping sequences to “undistort” GE-EPI images and creation of a “gray-ordinate” CIFTI-format files that only contained data from cortical and subcortical gray matter. The HCP pipelines were modified to a) process the B0 fieldmaps produced by the GE MR scanner and b) to implement slice-time correction (fMRIVolume processing pipeline set to ODD for interleaved).
Resulting structural and functional data were aligned in volume space to the MNI152 atlas and in surface space to the HCP-generated Conte69 surface atlas based on cortical folding patterns. Resting-state data files have undergone removal by regression of CSF, white matter, whole brain, and movement parameters (6 translation/rotation parameters + quadratic combinations of the 6 parameters + derivatives of these 12 parameters); to avoid slice-time correction affecting the movement parameters, movement parameters were derived from the data before HCP processing120. Frames with FD >0.2 mm and/or DVARS with >(75percentile) + (1.5) × (interquartile range) (fsl defaults) were then censored and interpolated121,122, and then a bandpass filter with a low cutoff of 0.0005 Hz and high cutoff of 0.2 Hz was applied. Subsequent analyses only used the non-censored frames (they did not include the interpolated frames).
Even though the most common approach for removing global structured noise is to remove the mean (across space) fMRI timecourse from the data using global signal regression123, there are some limitations associated to this approach given that global signal may differ between patients with psychiatric disorders124,125, and because the removal of neural signal may distort the resulting connectivity measures in network-specific ways126.
Definition of ROIs
Regions of interest (ROI) for these analyses were defined using the multi-modal parcellation2 of human cortex (Supplementary Table 5); all ROIs used for analyses were bilateral. DLPFC, DMN and VIS networks were created manually following grouped regions from the multi-modal parcellation (Supplementary Fig. 1A–C).
The sgACC ROI (area 25) was first used as a seed on an independent cohort of 1200 healthy subjects from the HCP database. The sgACC RSFC map generated across 1200 healthy subjects from the HCP dataset was used to find parcels within the DLPFC region2 with highest negative (DLPFCneg) correlation with the sgACC (Supplementary Fig. 2), based on previous findings15. These negative areas (46, p9-46v, a9-46v, 9-46d) within the DLPFC were used to create our DLPFCneg masks.
Regions within the anterior (aDMN: a24, s32, 10r) or posterior (pDMN: 31pv, v23ab) medial locus of DMN were selected based on their strongest association in the resting-state with task negative, as shown in Glasser et al.2. Main parcels within rostral ACC (a24, s32) were also selected based on early studies on predictors of treatment response64,127. MNI coordinates from the core set of hubs, rostral MPFC (−6 52 −2) or PCC (−8 −56 26) based on previous work36, were approximately128 mapped to Glasser parcels rostral MPFC (10r; surface vertex 28169) or PCC (31pv, v23ab; surface vertex 26238), respectively. These cortical areas were used to create our aDMN or pDMN masks.
The VIS network has been divided into primary VIS (V1), early VIS (V2-V3), dorsal stream, ventral stream and MT+ complex regions. We exclusively focused on higher order VIS regions, grouped into three separate subsystems: dorsal and ventral streams and MT+ complex. These separate subsystems were used to create our VIS masks.
Seed-based RSFC analyses
Baseline RSFC maps of areas within the DLPFCneg (46, p9-46v, a9-46v, 9-46d) were first created for each TRD patient to explore patterns of connectivity with sgACC (25) and rostral ACC (a24) as predictors of treatment response. Cortical areas within the DLPFCneg with predictive power (46, p9-46v) were then selected to further explore patterns of connectivity with DMN and VIS networks.
RSFC maps of areas within the aDMN (s32, 10r) and pDMN (31pv, v23ab) were created for each TRD patient to further explore patterns of connectivity with VIS network.
RSFC maps of each separate region of VIS network (dorsal, ventral and MT+) were created for each TRD patient to explore patterns of intra-VIS connectivity (intra-regional connectivity). First, the spatial map of each VIS region was used as the predefined mask. Then, RSFC of each VIS region to all voxels in each predefined mask was computed and averaged as the intra-regional connectivity (intra-ventral, intra-dorsal and intra-MT+). Following detection of significant within regional correlations, secondary analyses evaluated the relative contribution of individual parcels within each region by computing parcel-to-region connectivity within region (e.g., FFC to ventral).
Network analyses
Initial RSFC analyses explored patterns of connectivity between 4 cortical areas within the DLPFCneg (46, p9-46v, a9-46v, 9-46d) and 2 cortical areas within the sgACC (25) or the rostral ACC (a24); 8 correlation analyses were conducted. Subsequent RSFC analyses were based on a network configuration of 9 ROIs: 2 cortical areas within the DLPFCneg (46, p9-46v), 2 cortical regions within aDMN (s32, 10r), 2 cortical regions within pDMN (31pv, v23ab), and 3 VIS subsystems focused on higher order VIS regions (dorsal, ventral and MT+). Using the RSFC measures obtained from this network, we analyzed (1) within-network RSFC, averaged across hemispheres, and (2) between-network RSFC, averaged across hemispheres; 38 correlation analyses were conducted. Analyses focused first on prediction of the degree of treatment response and remission across individuals; and subsequently on correlates of change. Follow-up VIS analyses focused on significant patterns of connectivity between DLPFC(p9-46v) or DMN(31pv) and VIS(MT+, dorsal), or within VIS(ventral), to evaluate the relative contribution of individual parcels within each VIS region (7 parcels for ventral, 6 parcels for dorsal, 9 parcels for MT+).
Fisher’s z transform was applied to individual RSFC maps before group level analyses; uncorrected p-values were reported as only 8 (for initial analyses) and 38 (for network-analyses) comparisons were made; corrected p-values using FDR129 controlled multiple comparison correction were computed to see whether any of the uncorrected p-values survived. Leave-one-out cross validation (LOOCV) analysis was conducted on FDR corrected predictive models to estimate potential predictive value when applied to larger samples.
Influence of motion regressors on depression scores and connectivity
We evaluated if depression scores or connectivity measures were linked to motion estimators. The HCP pipeline provides an estimate of average displacement from initial frame (mean absolute) and frame-to-frame (mean relative) displacement for each run and fMRI session. We evaluated linear trends between percentage change in depression scores and subject’s motion displacement values (Corr), or between percentage change in depression scores and baseline/change in connectivity measures controlling by subject’s motion displacement values (PCorr). Corrected p-values using FDR129 were also computed to see whether any of the uncorrected p-values, adjusted by subject’s motion displacement values, survived.
Statistics
All analyses were conducted using R-package (version 3.5.1). To investigate association between continuous variables, Pearson’s correlation and partial correlation analyses were used. Partial correlation analyses were adjusted by motion displacement values (mean absolute and mean relative).
Initial analyses were performed using simple correlations between each pretreatment RSFC measure and percent change in depressive symptoms, defined as percentage change in HDRS-24 ([pre-post]/pre] × 100%). Multiple linear regression analyses were then conducted to evaluate the potential additive value of significant functional connections to identify best two-factor predictive models of percentage change in HDRS-24. The ability of pretreatment RSFC patterns to predict symptom remission, defined as final HDRS-24 score ≤ 7, was assessed using multiple logistic regression and the area under the receiver operating characteristic (ROC) curve. ROC analyses were conducted using R-package pROC (version 1.8)130,131; confidence interval (95% CI) used bootstrapping (2000 stratified bootstrap replicates). Leave-one-out cross validation (LOOCV) analysis was conducted on FDR corrected predictive models; confidence interval (95% CI) of accuracy measure used bootstrapping (1000 iteration). Subsequent analyses used simple correlation analyses to asses ECT-induced changes on baseline connectivity measures by computing correlation between (post-pre) connectivity measures and percent change in depressive symptoms. For two group comparisons, two-sample t-test were used. Sample descriptive statistics for continuous variables are reported as mean ± s.e. All statistics are two-tailed, with preset alpha level for significance of p < 0.05, and n value of 18.
Cortical Thickness and RSFC
To confirm that changes in cortical thickness did not affect RSFC analyses, cortical thickness was evaluated (Supplementary Methods) as a function of both time (pre/post ECT) and group (remitter/non-remitter), using repeated ANOVA. In addition, we computed correlation analyses between changes in cortical thickness and improvement in depression. Across analyses, no significant relationships were observed between cortical thickness and ECT response.
References
van Diermen, L. et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry 212, 71–80, https://doi.org/10.1192/bjp.2017.28 (2018).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178, https://doi.org/10.1038/nature18933 (2016).
Drevets, W. C., Ongur, D. & Price, J. L. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Molecular psychiatry 3, 220–226, 190–221 (1998).
Drevets, W. C., Ongur, D. & Price, J. L. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Molecular psychiatry 3, 190–191 (1998).
Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827, https://doi.org/10.1038/386824a0 (1997).
Buchsbaum, M. S. et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord 10, 137–152 (1986).
Baxter, L. R. Jr. et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry 42, 441–447 (1985).
Baxter, L. R. et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 46, 243–250 (1989).
Mayberg, H. S. et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156, 675–682, https://doi.org/10.1176/ajp.156.5.675 (1999).
Mayberg, H. S. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9, 471–481, https://doi.org/10.1176/jnp.9.3.471 (1997).
Mayberg, H. S. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British medical bulletin 65, 193–207 (2003).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69, 150–158, https://doi.org/10.1001/archgenpsychiatry.2011.1456 (2012).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660, https://doi.org/10.1016/j.neuron.2005.02.014 (2005).
Riva-Posse, P. et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biological psychiatry 76, 963–969, https://doi.org/10.1016/j.biopsych.2014.03.029 (2014).
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D. & Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological psychiatry 72, 595–603, https://doi.org/10.1016/j.biopsych.2012.04.028 (2012).
DeRubeis, R. J., Siegle, G. J. & Hollon, S. D. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature reviews. Neuroscience 9, 788–796, https://doi.org/10.1038/nrn2345 (2008).
Goldapple, K. et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61, 34–41, https://doi.org/10.1001/archpsyc.61.1.34 (2004).
Lanzenberger, R. et al. Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Molecular psychiatry 18, 93–100, https://doi.org/10.1038/mp.2012.93 (2013).
Mottaghy, F. M. et al. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res 115, 1–14 (2002).
Nobler, M. S. et al. Decreased regional brain metabolism after ect. Am J Psychiatry 158, 305–308, https://doi.org/10.1176/appi.ajp.158.2.305 (2001).
Argyelan, M. et al. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl Psychiatry 6, e789, https://doi.org/10.1038/tp.2016.54 (2016).
Leaver, A. M. et al. Modulation of intrinsic brain activity by electroconvulsive therapy in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 1, 77–86, https://doi.org/10.1016/j.bpsc.2015.09.001 (2016).
Cano, M. et al. Modulation of Limbic and Prefrontal Connectivity by Electroconvulsive Therapy in Treatment-resistant Depression: A Preliminary Study. Brain Stimul 9, 65–71, https://doi.org/10.1016/j.brs.2015.08.016 (2016).
Wang, L., Hermens, D. F., Hickie, I. B. & Lagopoulos, J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord 142, 6–12, https://doi.org/10.1016/j.jad.2012.04.013 (2012).
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R. & Schooler, J. W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America 106, 8719–8724, https://doi.org/10.1073/pnas.0900234106 (2009).
D’Argembeau, A. et al. Modulation of medial prefrontal and inferior parietal cortices when thinking about past, present, and future selves. Social neuroscience 5, 187–200, https://doi.org/10.1080/17470910903233562 (2010).
Spreng, R. N., Mar, R. A. & Kim, A. S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of cognitive neuroscience 21, 489–510, https://doi.org/10.1162/jocn.2008.21029 (2009).
Bartova, L. et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res 64, 9–18, https://doi.org/10.1016/j.jpsychires.2015.02.025 (2015).
Sheline, Y. I. et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America 106, 1942–1947, https://doi.org/10.1073/pnas.0812686106 (2009).
Disner, S. G., Beevers, C. G., Haigh, E. A. & Beck, A. T. Neural mechanisms of the cognitive model of depression. Nature reviews. Neuroscience 12, 467–477, https://doi.org/10.1038/nrn3027 (2011).
Anticevic, A. et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci 16, 584–592, https://doi.org/10.1016/j.tics.2012.10.008 (2012).
Li, B. et al. A treatment-resistant default mode subnetwork in major depression. Biological psychiatry 74, 48–54, https://doi.org/10.1016/j.biopsych.2012.11.007 (2013).
Sambataro, F., Wolf, N. D., Pennuto, M., Vasic, N. & Wolf, R. C. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychological medicine 44, 2041–2051, https://doi.org/10.1017/S0033291713002596 (2014).
Dutta, A., McKie, S. & Deakin, J. F. Resting state networks in major depressive disorder. Psychiatry Res 224, 139–151, https://doi.org/10.1016/j.pscychresns.2014.10.003 (2014).
Ho, T. C. et al. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biological psychiatry 78, 635–646, https://doi.org/10.1016/j.biopsych.2014.09.002 (2015).
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562, https://doi.org/10.1016/j.neuron.2010.02.005 (2010).
Zhu, X. et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological psychiatry 71, 611–617, https://doi.org/10.1016/j.biopsych.2011.10.035 (2012).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA. Psychiatry 72, 603–611, https://doi.org/10.1001/jamapsychiatry.2015.0071 (2015).
Haxby, J. V., Hoffman, E. A. & Gobbini, M. I. The distributed human neural system for face perception. Trends Cogn Sci 4, 223–233 (2000).
Wang, L., McCarthy, G., Song, A. W. & Labar, K. S. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion 5, 12–22, https://doi.org/10.1037/1528-3542.5.1.12 (2005).
Hahn, T. et al. Integrating neurobiological markers of depression. Arch Gen Psychiatry 68, 361–368, https://doi.org/10.1001/archgenpsychiatry.2010.178 (2011).
Fu, C. H. et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biological psychiatry 63, 656–662, https://doi.org/10.1016/j.biopsych.2007.08.020 (2008).
Rosa, M. J. et al. Sparse network-based models for patient classification using fMRI. NeuroImage 105, 493–506, https://doi.org/10.1016/j.neuroimage.2014.11.021 (2015).
Furey, M. L. et al. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry 70, 280–290, https://doi.org/10.1001/2013.jamapsychiatry.60 (2013).
Keedwell, P. A. et al. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord 120, 120–125, https://doi.org/10.1016/j.jad.2009.04.031 (2010).
Fu, C. H. et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 61, 877–889, https://doi.org/10.1001/archpsyc.61.9.877 (2004).
Keedwell, P. et al. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol 23, 775–788, https://doi.org/10.1177/0269881108093589 (2009).
Davidson, R. J., Irwin, W., Anderle, M. J. & Kalin, N. H. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160, 64–75, https://doi.org/10.1176/appi.ajp.160.1.64 (2003).
Moratti, S., Rubio, G., Campo, P., Keil, A. & Ortiz, T. Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch Gen Psychiatry 65, 532–541, https://doi.org/10.1001/archpsyc.65.5.532 (2008).
Hasler, G. et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry 65, 521–531, https://doi.org/10.1001/archpsyc.65.5.521 (2008).
Leppanen, J. M., Milders, M., Bell, J. S., Terriere, E. & Hietanen, J. K. Depression biases the recognition of emotionally neutral faces. Psychiatry Res 128, 123–133, https://doi.org/10.1016/j.psychres.2004.05.020 (2004).
Bubl, E., Ebert, D., Kern, E., Van Elst, L. T. & Bach, M. Effect of antidepressive therapy on retinal contrast processing in depressive disorder. Br J Psychiatry 201, 151–158, https://doi.org/10.1192/bjp.bp.111.100560 (2012).
Bubl, E., Kern, E., Ebert, D., Bach, M. & Tebartz van Elst, L. Seeing gray when feeling blue? Depression can be measured in the eye of the diseased. Biological psychiatry 68, 205–208, https://doi.org/10.1016/j.biopsych.2010.02.009 (2010).
Bubl, E., Tebartz Van Elst, L., Gondan, M., Ebert, D. & Greenlee, M. W. Vision in depressive disorder. World J Biol Psychiatry 10, 377–384, https://doi.org/10.1080/15622970701513756 (2009).
Kastner, S. & Ungerleider, L. G. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23, 315–341, https://doi.org/10.1146/annurev.neuro.23.1.315 (2000).
Le, T. M., Borghi, J. A., Kujawa, A. J., Klein, D. N. & Leung, H. C. Alterations in visual cortical activation and connectivity with prefrontal cortex during working memory updating in major depressive disorder. Neuroimage Clin 14, 43–53, https://doi.org/10.1016/j.nicl.2017.01.004 (2017).
Fu, C. H. et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological psychiatry 64, 505–512, https://doi.org/10.1016/j.biopsych.2008.04.033 (2008).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154, https://doi.org/10.1016/j.neuroimage.2011.10.018 (2012).
van Waarde, J. A. et al. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Molecular psychiatry 20, 609–614, https://doi.org/10.1038/mp.2014.78 (2015).
Perrin, J. S. et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proceedings of the National Academy of Sciences of the United States of America 109, 5464–5468, https://doi.org/10.1073/pnas.1117206109 (2012).
Abbott, C. C. et al. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front Psychiatry 4, 10, https://doi.org/10.3389/fpsyt.2013.00010 (2013).
Dougherty, D. D. et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg 99, 1010–1017, https://doi.org/10.3171/jns.2003.99.6.1010 (2003).
Ketter, T. A. et al. Baseline cerebral hypermetabolism associated with carbamazepine response, and hypometabolism with nimodipine response in mood disorders. Biological psychiatry 46, 1364–1374 (1999).
Mayberg, H. S. et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8, 1057–1061 (1997).
Saxena, S. et al. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry 160, 522–532, https://doi.org/10.1176/appi.ajp.160.3.522 (2003).
Wu, J. et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 156, 1149–1158, https://doi.org/10.1176/ajp.156.8.1149 (1999).
Desseilles, M. et al. Abnormal neural filtering of irrelevant visual information in depression. The Journal of neuroscience: the official journal of the Society for Neuroscience 29, 1395–1403, https://doi.org/10.1523/JNEUROSCI.3341-08.2009 (2009).
Desseilles, M. et al. Depression alters “top-down” visual attention: a dynamic causal modeling comparison between depressed and healthy subjects. NeuroImage 54, 1662–1668, https://doi.org/10.1016/j.neuroimage.2010.08.061 (2011).
Sanacora, G. et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56, 1043–1047 (1999).
Bhagwagar, Z. et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol 11, 255–260, https://doi.org/10.1017/S1461145707007924 (2008).
Fox, M. D., Halko, M. A., Eldaief, M. C. & Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). NeuroImage 62, 2232–2243, https://doi.org/10.1016/j.neuroimage.2012.03.035 (2012).
Fox, M. D., Liu, H. & Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage 66, 151–160, https://doi.org/10.1016/j.neuroimage.2012.10.082 (2013).
Deng, Z. D., Lisanby, S. H. & Peterchev, A. V. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 6, 1–13, https://doi.org/10.1016/j.brs.2012.02.005 (2013).
Javitt, D. C., Zukin, S. R., Heresco-Levy, U. & Umbricht, D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38, 958–966, https://doi.org/10.1093/schbul/sbs069 (2012).
Robertson, C. E. et al. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain 137, 2588–2599, https://doi.org/10.1093/brain/awu189 (2014).
Kuchinsky, S. E. et al. Word intelligibility and age predict visual cortex activity during word listening. Cerebral cortex 22, 1360–1371, https://doi.org/10.1093/cercor/bhr211 (2012).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125–1165, https://doi.org/10.1152/jn.00338.2011 (2011).
Ninomiya, T., Sawamura, H., Inoue, K. & Takada, M. Segregated pathways carrying frontally derived top-down signals to visual areas MT and V4 in macaques. The Journal of neuroscience: the official journal of the Society for Neuroscience 32, 6851–6858, https://doi.org/10.1523/JNEUROSCI.6295-11.2012 (2012).
Kaas, J. H. & Morel, A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. The Journal of neuroscience: the official journal of the Society for Neuroscience 13, 534–546 (1993).
Ponce, C. R., Hartmann, T. S. & Livingstone, M. S. End-Stopping Predicts Curvature Tuning along the Ventral Stream. The Journal of neuroscience: the official journal of the Society for Neuroscience 37, 648–659, https://doi.org/10.1523/JNEUROSCI.2507-16.2016 (2017).
Nunn, J. A. et al. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words. Nature neuroscience 5, 371–375, https://doi.org/10.1038/nn818 (2002).
Stuhrmann, A., Suslow, T. & Dannlowski, U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 1, 10, https://doi.org/10.1186/2045-5380-1-10 (2011).
Stroud, J. B. et al. Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology (Berl) 235, 459–466, https://doi.org/10.1007/s00213-017-4754-y (2018).
Bedwell, J. S. et al. The P1 visual-evoked potential, red light, and transdiagnostic psychiatric symptoms. Brain research 1687, 144–154, https://doi.org/10.1016/j.brainres.2018.03.002 (2018).
Lavoie, M. P. et al. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biological psychiatry 66, 253–258, https://doi.org/10.1016/j.biopsych.2008.11.020 (2009).
Fountoulakis, K. N., Fotiou, F., Iacovides, A. & Kaprinis, G. Is there a dysfunction in the visual system of depressed patients? Ann Gen Psychiatry 4, 7, https://doi.org/10.1186/1744-859X-4-7 (2005).
Normann, C., Schmitz, D., Furmaier, A., Doing, C. & Bach, M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biological psychiatry 62, 373–380, https://doi.org/10.1016/j.biopsych.2006.10.006 (2007).
Fitzgerald, P. J. Gray colored glasses: is major depression partially a sensory perceptual disorder? J Affect Disord 151, 418–422, https://doi.org/10.1016/j.jad.2013.06.045 (2013).
Norton, D. J., McBain, R. K., Pizzagalli, D. A., Cronin-Golomb, A. & Chen, Y. Dysregulation of visual motion inhibition in major depression. Psychiatry Res 240, 214–221, https://doi.org/10.1016/j.psychres.2016.04.028 (2016).
Lanzenberger, R. et al. Impact of electroconvulsive therapy on 5-HT1A receptor binding in major depression. Molecular psychiatry 18, 1, https://doi.org/10.1038/mp.2012.177 (2013).
Ishihara, K. & Sasa, M. Mechanism underlying the therapeutic effects of electroconvulsive therapy (ECT) on depression. Jpn J Pharmacol 80, 185–189 (1999).
Schur, R. R. et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Human brain mapping 37, 3337–3352, https://doi.org/10.1002/hbm.23244 (2016).
Sanacora, G. et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160, 577–579, https://doi.org/10.1176/appi.ajp.160.3.577 (2003).
Hahn, A. et al. Differential modulation of the default mode network via serotonin-1A receptors. Proceedings of the National Academy of Sciences of the United States of America 109, 2619–2624, https://doi.org/10.1073/pnas.1117104109 (2012).
Muthukumaraswamy, S. D., Edden, R. A., Jones, D. K., Swettenham, J. B. & Singh, K. D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proceedings of the National Academy of Sciences of the United States of America 106, 8356–8361, https://doi.org/10.1073/pnas.0900728106 (2009).
Donahue, M. J., Near, J., Blicher, J. U. & Jezzard, P. Baseline GABA concentration and fMRI response. NeuroImage 53, 392–398, https://doi.org/10.1016/j.neuroimage.2010.07.017 (2010).
Qin, P. et al. GABA(A) receptors in visual and auditory cortex and neural activity changes during basic visual stimulation. Frontiers in human neuroscience 6, 337, https://doi.org/10.3389/fnhum.2012.00337 (2012).
Mathewson, K. E. et al. Dynamics of alpha control: preparatory suppression of posterior alpha oscillations by frontal modulators revealed with combined EEG and event-related optical signal. Journal of cognitive neuroscience 26, 2400–2415, doi:10.1162/jocn_a_00637 (2014).
Pessoa, L., Kastner, S. & Ungerleider, L. G. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res 15, 31–45 (2002).
Rotshtein, P., Malach, R., Hadar, U., Graif, M. & Hendler, T. Feeling or features: different sensitivity to emotion in high-order visual cortex and amygdala. Neuron 32, 747–757 (2001).
Kibleur, A. et al. Stimulation of subgenual cingulate area decreases limbic top-down effect on ventral visual stream: A DBS-EEG pilot study. NeuroImage 146, 544–553, https://doi.org/10.1016/j.neuroimage.2016.10.018 (2017).
Jiang, H. et al. Predictability of depression severity based on posterior alpha oscillations. Clin Neurophysiol 127, 2108–2114, https://doi.org/10.1016/j.clinph.2015.12.018 (2016).
Capotosto, P., Babiloni, C., Romani, G. L. & Corbetta, M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. The Journal of neuroscience: the official journal of the Society for Neuroscience 29, 5863–5872, https://doi.org/10.1523/JNEUROSCI.0539-09.2009 (2009).
Huang, S. et al. Multisensory Competition Is Modulated by Sensory Pathway Interactions with Fronto-Sensorimotor and Default-Mode Network Regions. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 9064–9077, https://doi.org/10.1523/JNEUROSCI.3760-14.2015 (2015).
Hamilton, J. P. et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological psychiatry 70, 327–333, https://doi.org/10.1016/j.biopsych.2011.02.003 (2011).
Sheline, Y. I., Price, J. L., Yan, Z. & Mintun, M. A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America 107, 11020–11025, https://doi.org/10.1073/pnas.1000446107 (2010).
Liston, C. et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological psychiatry 76, 517–526, https://doi.org/10.1016/j.biopsych.2014.01.023 (2014).
Kwon, S., Watanabe, M., Fischer, E. & Bartels, A. Attention reorganizes connectivity across networks in a frequency specific manner. NeuroImage 144, 217–226, https://doi.org/10.1016/j.neuroimage.2016.10.014 (2017).
Chadick, J. Z. & Gazzaley, A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature neuroscience 14, 830–832, https://doi.org/10.1038/nn.2823 (2011).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn Sci 17, 683–696, https://doi.org/10.1016/j.tics.2013.09.012 (2013).
Gong, Q. & He, Y. Depression, neuroimaging and connectomics: a selective overview. Biological psychiatry 77, 223–235, https://doi.org/10.1016/j.biopsych.2014.08.009 (2015).
Zhang, J. et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological psychiatry 70, 334–342, https://doi.org/10.1016/j.biopsych.2011.05.018 (2011).
Hindriks, R. et al. Role of white-matter pathways in coordinating alpha oscillations in resting visual cortex. NeuroImage 106, 328–339, https://doi.org/10.1016/j.neuroimage.2014.10.057 (2015).
Li, P. et al. Erratum: Electroconvulsive therapy-induced brain functional connectivity predicts therapeutic efficacy in patients with schizophrenia: a multivariate pattern recognition study. NPJ Schizophr 3, 33, https://doi.org/10.1038/s41537-017-0024-6 (2017).
Avissar, M. et al. Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul 10, 919–925, https://doi.org/10.1016/j.brs.2017.07.002 (2017).
The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. 2nd edn, (2001).
Sackeim, H., Decina, P., Prohovnik, I. & Malitz, S. Seizure threshold in electroconvulsive therapy. Effects of sex, age, electrode placement, and number of treatments. Arch Gen Psychiatry 44, 355–360 (1987).
Williams, J. B. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 45, 742–747 (1988).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124, https://doi.org/10.1016/j.neuroimage.2013.04.127 (2013).
Power, J. D. A simple but useful way to assess fMRI scan qualities. NeuroImage, https://doi.org/10.1016/j.neuroimage.2016.08.009 (2016).
Power, J. D. et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84, 320–341, https://doi.org/10.1016/j.neuroimage.2013.08.048 (2014).
Power, J. D., Schlaggar, B. L. & Petersen, S. E. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage 105, 536–551, https://doi.org/10.1016/j.neuroimage.2014.10.044 (2015).
Power, J. D., Plitt, M., Laumann, T. O. & Martin, A. Sources and implications of whole-brain fMRI signals in humans. NeuroImage 146, 609–625, https://doi.org/10.1016/j.neuroimage.2016.09.038 (2017).
Yang, G. J. et al. Altered Global Signal Topography in Schizophrenia. Cerebral cortex 27, 5156–5169, https://doi.org/10.1093/cercor/bhw297 (2017).
Yang, G. J. et al. Altered global brain signal in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 111, 7438–7443, https://doi.org/10.1073/pnas.1405289111 (2014).
Glasser, M. F. et al. Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. NeuroImage 181, 692–717, https://doi.org/10.1016/j.neuroimage.2018.04.076 (2018).
Mulert, C. et al. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci 38, 78–81, https://doi.org/10.1177/155005940703800209 (2007).
Coalson, T. S., Van Essen, D. C. & Glasser, M. F. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proceedings of the National Academy of Sciences of the United States of America 115, E6356–E6365, https://doi.org/10.1073/pnas.1801582115 (2018).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 57, 289–300 (1995).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12, 77, https://doi.org/10.1186/1471-2105-12-77 (2011).
Carpenter, J. & Bithell, J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 19, 1141–1164 (2000).
Author information
Authors and Affiliations
Contributions
S.R., J.P. and D.C.J. designed the experiment and supervised the project; S.R. and J.P. recruited patients and performed ECT treatments; G.H.P. adapted HCP pipeline for GE image format, designed imaging protocol, and together with J.G. and M.F.G. assisted in technical details of imaging postprocessing analyses; A.K. supervised image acquisition quality and data analysis; M.M.O. collected data, performed data analyses and conceived the original idea; M.M.O. and S.L. designed statistical analyses; T.P. and T.P. assisted with clinical implications of ECT and depression; M.M.O. and D.C.J. wrote the paper. All co-authors contributed intellectually to the final version of the paper
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_41175_MOESM1_ESM.docx
Supplementary Documentation for: Resting state functional connectivity predictors of treatment response to electroconvulsive therapy in depression.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreno-Ortega, M., Prudic, J., Rowny, S. et al. Resting state functional connectivity predictors of treatment response to electroconvulsive therapy in depression. Sci Rep 9, 5071 (2019). https://doi.org/10.1038/s41598-019-41175-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41175-4
This article is cited by
-
Functional connectivity changes between amygdala and prefrontal cortex after ECT are associated with improvement in distinct depressive symptoms
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Rostral anterior cingulate network effective connectivity in depressed adolescents and associations with treatment response in a randomized controlled trial
Neuropsychopharmacology (2022)
-
Noninvasive neuromodulation of the prefrontal cortex in mental health disorders
Neuropsychopharmacology (2022)
-
Magnetic resonance imaging for individual prediction of treatment response in major depressive disorder: a systematic review and meta-analysis
Translational Psychiatry (2021)
-
Prefrontal resting-state connectivity and antidepressant response: no associations in the ELECT-TDCS trial
European Archives of Psychiatry and Clinical Neuroscience (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.