Abstract
Lymphocyte proliferation assays are widely used to assess the cell-mediated immunity. Current in vitro testing methods that are being used have extensive applications but still more problematic, due to the technical complexity and the needs for specialized equipment and reagents. Electrochemical methods such as cyclic voltammetry represent a very promising tool for the development of label-free in vitro assays of cell proliferation and viability. Here, a novel procedure based on voltammetric behaviours of proliferating cells was fabricated. Results indicated that proliferation in cell cultures and whole blood can be monitored electrochemically using cyclic voltammetry. In the comparison with colorimetric (MTT) assay, cyclic voltammetry gave the best correlation with cell count data over a range of 1200–300,000 cells/well of a microplate. Besides the advantages of short assay duration (4 hours) and the rapidness, the possibility use of fresh blood without further processing, would give more accurate results because cells are monitoring in an intact environment. Cyclic voltammetry assay is an efficient analytical method, which can provide a simple platform for the electrochemical study of lymphocyte proliferation.
Similar content being viewed by others
Introduction
Humoral and cell-mediated immunity (CMI) are two major components of the innate and adoptive immune responses. Humoral immune response involves the synthesis and release of macromolecules, such as antibodies, cytokines, antimicrobial peptides, complement and acute phase proteins into the blood and other body fluids. In contrast, CMI is mediated by activated or sensitized reticuloendothelial (Phagocytic) and lymphoid (Lymphocytes) cells1. Most immunological studies necessitate evaluation of both humoral and CMI responses. Humoral components can be easily monitored by use of precipitation, agglutination, neutralization and enzyme-linked immunosorbent assay (ELISA) methods. Assessment of CMI can be undertaken by in vivo, in situ and in vitro methods. Delayed-type hypersensitivity skin testing is an example of in vivo assessment of cellular immunity. Cells of immune system can also be evaluated and enumerated in situ by immunofluorescence microscopy, immunohistochemistry, or flow cytometry. Methods for in vitro evaluating the CMI are broadly grouped into the stimulation (e.g. leucocyte migration technique), proliferation, cytotoxicity and effector activity (cytokine expression) assays.
The proliferation and/or cytotoxic assays is widely used for potency determination of vaccines, diagnosis of infectious disease2,3,4,5, immune deficiencies and drug discoveries4,6,7. In practical terms, cells are stimulating by mitogen, antigen, toxin or drug, which may cause the cell activation or death. Then measurement of proliferating and/or surviving cells is conventionally determined by radiometric or colorimetric (Dye based) assays8. These procedures have widespread potential applications but still more problematic, due to the technical complexity and the needs for specialized equipment and reagents.
Electrochemical methods such as cyclic voltammetry (CV) represent a very promising tool for the development of label-free in vitro assays for evaluation of cell proliferation and viability9,10. Electrochemical sensors operate by reacting with the analyte of interest to produce an electrical signal proportional to the analyte concentrations. A typical electrochemical sensor consists of a sensing electrode (working electrode) and a reference electrode separated by an electrolyte. For most applications, a three-electrode system is used with the reference connected to a high-input-impedance given a constant voltage and a counter electrode is to measure the current flow. The cyclic voltammetry, as a type of potentiodynamic electrochemical measurement, is used for analytes that are redox active within the potential window to be scanned. It is a proficient tool that screen the intrinsic redox reaction of the electrode material as the potential of the electrode is swept in a cyclic manner. Particularly, redox reactions catalyzed by proteins or enzymes to maintain energy and major natural mechanisms. The living cell has its own redox properties that undergo alterations of metabolisms, products and membrane events during the activation and replication or apoptosis (cell death). Therefore, it can be speculated that proliferation of lymphocytes leads to change in their redox properties which might be monitored by electrochemical or conductometer cell sensor. It has been demonstrated that redox properties in living cells can be determined by CV and the peak current increased by increase of cell number11. Using CV for proliferation assays will also provide information on the thermodynamics of redox processes and electron-transfer reactions. Cyclic voltammetry waves virtually fingerprint the individual electrochemical properties of redox systems12,13.
At the present study, we describe cyclic voltammetry measurement of human lymphocyte proliferation, as an alternative means of colorimetric lymphocyte proliferation assay.
Results
Cyclic voltammetry was used to characterize the cell proliferation and to determine the cell concentration. To evaluate the optimum test parameters and CV index cutoff, we used ROC curves approach for comparing different potential sets (i.e. 250, 500, 750 and 1000 mV) (Fig. 1), and to find a criterion for determining mitogen-induced responses in the cell proliferation assay (Table 1). The area under the ROC curve (AUC) represents the test accuracy and is equal to the probability of correctly discriminating between affected and non-affected cells. The best AUC (0.9887) obtained for the electrochemical responses between 0 and 500 mV, at 5 mV/sample sweep rate and 100 mV/sec slope.
Determining a cut-off value for assigning lymphocyte proliferation responses. Graph represents ROC curves analysis of the results obtained from cyclic voltammetry assay. Four different potential sets including 250, 500, 750 and 1000 mV, at 5 mV/sample, 100 mV/sec slope were tested. In each assay at least 40 test and 40 control well were tested. AUC, area under the ROC curve.
When the electrode potential was set to 0 and 500 mV (AUC = 0.99%), the best pairs of values for highest sensitivity and specificity was found at several current values (Table 1; Fig. 2). Our results indicated that the current values obtained at the range of 348–454 mV (23 spot) appear to have the best discriminating power to differentiate mitogen-induced cells from control subjects. To enable comparison between treated and control cells in proliferation assays the formula 1 was used to calculate CV index:
Where µ is a general mean; cis the current of the CV ith spot in the sample (test or control); crpmi the current of the CV ith spot in the RPMI media; N the number of spots. Finally, the stimulation index for CV method (CVSI) is expressed with average CVI value in the mitogen induced group divided by average CVI value in negative controls.
Differential voltammograms and MTT OD values for different concentrations of PBMC ranging from 3 × 106 to 1.2 × 104 cells/mL (3 × 105 to 1.2 × 103 cells/well). Complete RPMI (RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin) used to dilute the cells. Complete RPMI was used as the blank and an equal amount of PHA (5 µg/mL) were added to the RPMI in wells corresponding to PHA + RPMI.
The cyclic voltammogram measurement of PBMC displayed a quasi-reversible pattern. The relationship between the cell numbers and voltammograms pulse obtained in CV is shown in Fig. 2. The peak current revealed a linear relationship with cell number ranging 3 × 105 to 1.2 × 103 per well. The colorimetric assay (MTT OD values) also revealed a linear relationship with the same concentration of cells (number ranging 3 × 105 to 1.2 × 103 per well). No significant difference was observed between different concentrations of PHA. The cyclic voltammogram measurement of fresh blood also displayed a quasi-reversible pattern. The voltammograms obtained for several concentrations of mitogen-induced blood as well as non-induced blood is shown in Fig. 3.
Differential voltammograms for mitogen-induced versus non-induced blood sample. Several dilutions of fresh blood in complete RPMI (RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin) used to dilute the blood samples. Wells corresponding to mitogen-induced blood received an equal amount of PHA (5 µg/mL).
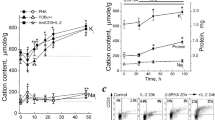
The effect of 4, 24, 48 and 72 hours incubation of cells was tested by either the CV (0–500 mV, 5 mV/sample) or the optical density of the formazan produced in MTT assay. The calculated CV index (CVI), MTT OD values, and stimulation indexes for CV (CVSI) and MTT method (MTTSI) are presented in Table 2. Relationship between the voltammogram pulse in the limited current values between 348 and 454 mV (23 spot) and assay duration is shown in Fig. 4.
Discussion
There is growing demand for developing low cost, rapid and simple analysis tools for practical applications in cellular immunology. Electrochemical techniques are an attractive candidate which provided a valuable platform for a wide range of medical applications, in particular the study of the effects of biomaterial and drugs on cell viability and toxicity9,11. We present here a novel application of electrochemical cell sensor for the study of lymphocyte proliferation. In this approach, cyclic voltammetry was compared with colorimetric MTT assay applicable to cell proliferation or cytotoxicity assays as an in vitro test.
Like other methods of proliferation assays, there are some limitations concerning the general applicability of CV method. Cyclic voltammetry is likely influenced by biologically active ingredients that may exist in different type of cells or samples (PBMC or fresh blood). Drugs or medications as well as reducing compounds are important factors to interfere with the CV assay. It has been also revealed that lymphocyte proliferation differs between individuals and in the same individual changed by the age14. The assay chemistry interference can be greatly reduced by using the appropriate controls and calculating the stimulation index (Treated/control ratio).
To evaluate the optimum test parameters and CVI cutoff, ROC curves were generated. ROC analysis revealed that the electrochemical responses between 0 and 500 mV, at 5 mV/sample, 100 mV/sec slope more accurately classified mitogen induced cells versus non-induced cells (Fig. 1). In order to select cut-off points, the operating characteristics of the test was adjusted for the highest sensitivity (91.6–97.6%) and specificity (97.6–100%). Accordingly, the cut-off value that maximized both sensitivity and specificity was found at the range of 348–454 mV (Fig. 4). The calculation of data derived for selected cut-off points (21 spot) by CMI formula allowed us to make an intra/inter-assay validation, as well as comparative evaluation of CV and colorimetric assays at different time points (Table 2).
To assess the electrochemical response to the cell numbers, different concentrations of cells were examined by CV assay. Each concentration showed a distinct voltammogram with minimum detectable cell number (LOD) of 1.2 × 103 (Fig. 2). We also demonstrated that the same linear relationship with cell concentrations could be obtained by testing fresh blood samples. Based on the electrochemical interaction findings, one interpretation for the correlation between the cell number and current increase is the extracellular/membrane electron transfer that might be enhanced by cell intensity. Mechanisms of extracellular electron transfer and electron exchange have been well defined for bacteria and yeast15,16. Electroactive microorganisms can exchange electrons with other organisms or electrodes. So far, cytoplasm conductivity and membrane capacitance, as a biophysical marker, have been investigated for either white blood cells or tumor cells in suspension17,18,19,20. Mitogen-stimulated lymphocytes undergo alterations of their redox active products and cell surface molecules. Application of Alamar Blue (i.e. an oxidation–reduction indicator) for a lymphocyte proliferation assay, further show evidences of effectively reduction capacity in the cells undergoing proliferation8,21. However, the specific indirect redox mediators or direct redox cell surface-associated molecules have not been clearly identified.
In the comparison between blood induced by mitogen and non-induced blood, regarding to peak currents, two different patterns were identified (Fig. 3). A linear relationship with blood concentrations was only observed for mitogen-induced samples, diluted up to 1/81. By using CV assay a considerable significant difference was found between isolated lymphocyte and fresh blood. Our results further indicated that, although LOD differed between two experimental conditions, but voltammogram behavior drastically changed when fresh blood was induced by mitogen. According to data reported by Petty et al. (1995), MTT assay cannot detect less than 2.5 × 104 cells per microplate well while the ATP-based assay is able to detect 1.6 × 103 cells/well (i.e.) closer to CV assay22. The number of lymphocyte in human blood is about 2 × 103 lymphocytes in 1 µL (i.e. 200 × 103 per well). Intrestingly, lymphocyte number in blood at the minimum limit of detection (1/27) includes around 7 × 103 cells/well that indicates the sensitivity of CV assay is still much better than the MTT method.
The effect of cell incubation time was tested by comparing values at four time points with the same concentration of PHA (5 µg/mL) and CV parameters. In the CV assay the experimental error (SEM) slightly increased at 24 hours but remained less than MTT assay (Fig. 5). Incubation for longer time decreased the error of stimulation index. In comparison with colorimetric assay, the observed CVSI values were higher than that of MTT assay and significantly (P < 0.05) increased with time up to 48 hours after stimulation. The CV assay was able to detect the proliferative response to mitogen as early as 4 hours after induction and found to be very sensitive and rapid under the conditions examined. This will allow to analyze plates before possible chemical or physical changes during the long incubation time. Although longer incubation time will increase sensitivity, the number of cells and their metabolism can be affected by culture conditions such as altered pH, depletion of essential nutrients and accumulation of toxic by-products23. The eventual decline in CVSI after prolonged incubation (72 hours) might be attributed to accumulation of toxic metabolites that reduce cell proliferation. In colorimetric assay, optical density versus cell number showed a lower sensitivity to change in cell number and proliferation response. Lower test sensitivity and discrimination power was further indicated by comparing the MTTSI between different time points of incubation (Fig. 5).
Here we indicated that proliferation in cell cultures and whole blood can be monitored electrochemically using a cyclic voltammetry assay. CV assay is an efficient analytical method, since it provides a label-free, rapid, simple, high-throughput and conceivable continuous measurements. Also we could use low cost disposable electrodes to maintain the quality of results in each run. We found that this method gave the best correlation with cell count data and the sensitivity is more than colorimetric assays. In vitro lymphocyte proliferation assays generally require the isolation of cells, followed by a prolonged incubation time. We also demonstrated that the same clonal results could be obtained by testing fresh blood samples. In contrast, besides the advantages of short assay duration (4 hours) and rapidness, possibility use of whole blood without further processing in CV assay, would provide more accurate results because cells are monitoring in an intact environment.
Methods
Isolation of cells
Human blood was collected into 10 ml EDTA-anticoagulated tubes and mononuclear cells isolated by the Ficoll density gradient centrifugation method. The peripheral blood mononuclear cells (PBMC) were isolated from whole blood within 8 h of collection by centrifugation at 600 × g for 15 min through Ficoll-Hypaque Plus (Sigma–Aldrich). The white layer of cells at the plasma-Ficoll interface was harvested and washed three times with RPMI-1640 medium (Biosera, Nuaille, France) without fetal bovine serum (FBS). The cell count was measured with an automatic blood cell counter (Nihon Kohden, Tokyo, Japan). Cell viability was determined with a hemacytometer, using trypan blue exclusion method. Samples with the number of viable cells less than 95% and red blood cell contamination more than 2% were excluded from further tests.
This study was approved and monitored by National Research Ethics Committee, University of Tehran. All methods were performed in accordance with the relevant guidelines and regulations of the institution. Informed consent was obtained from subjects.
Lymphocyte proliferation studies
Duplicate wells in each plates were set-up for concurrent determination of proliferation by colorimetric MTT (tetrazolium) and cyclic voltammetry (CV) assays. One hundred microliters of diluted cell suspension (2 × 106 cells/mL) in complete RPMI (RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin) were dispersed and incubated in 96-well microtiter plate. Cells were seeded in 10 wells per sample for each test (mitogen added) and control negative experiment. The mitogen phytohemagglutinin-L (PHA-L; Sigma) at the final working concentrations of 5, 10 and 20 µg/mL was added to test wells. In addition, cell-free media containing complete RPMI were run in parallel to test and control groups. The inoculated plates were incubated at 37 °C in a 5% CO2 incubator and checked for growth after 4, 24, 48 and 72 h using both colorimetric and CV assays. Each assay is further evaluated for sensitivity to change in cell number and different concentrations of mitogen. Six different concentrations of cells in complete RPMI media ranging from 3 × 106 to 1.2 × 104 cells/mL (3 × 105 − 1.2 × 103 cells/well) were tested by both colorimetric MTT and CV assays. All the experiments reported here were repeated four times.
Colorimetric MTT assay
Colorimetric lymphocyte proliferation was evaluated using MTT (3-(4, 5-dimethyl thiazol-2-yl) 2, 5-diphenyl tetrazolium bromide) method. After cell culture incubation (4, 24, 48 and 72 h), 10 μl MTT (dissolved in RPMI-1640, 5 mg/mL) was added to well, and plates were further incubated at 37 °C for 4 h. Finally, the purple formazan crystals were dissolved by adding 100 μl acid-isopropanol (0.04 N HCI in isopropanol) into each well. Absorbance was then measured at 550 nm against reference wavelength of 630 nm and stimulation index (SI) was determined. The SI is expressed with average OD value in the test group divided by average OD value in negative controls.
Cyclic voltammetry (CV) assays
Cyclic voltammogram measurement was performed using a home-made potentiostat which was designed according to the needs. One of which was to be able to measure currents up to 10 micro amps, and the other was to be able to change slope, voltage boundaries applied to the sample and also the sampling rate. A mini-electrochemical system containing three-electrode system was constructed according to the previously described method24,25. A polished pencil graphite was used as the working electrode, a platinum wire as the auxiliary electrode and a saturated Ag/AgCl electrode as the reference electrode. The voltage applied to the samples was set in a way to prevent cells from dying due to oxidation. So the cells kept alive along sampling time and after. To gain more information on the voltammetric behaviors of cells, current-voltage (I-V) spectra were obtained at four different potential sets including 0–250 V, 0–500 mV, 0–750 mV and 0–1000 mV with the same sweep rate of 5 mV/sample at the slope of 100 mV/sec. Voltammograms were obtained at room temperature (22 ± 2 °C) and data analyzed to find the best voltage ranges and other specifications.
Testing fresh blood by CV assay
Two hundred microliter of collected blood sample containing 1.8 × 106 lymphocyte/mL (1.8 × 105/well) was placed in 96-well sterile culture plates and serially diluted three-fold with complete RPMI. The PHA at the final working concentrations of 5 µg/mL was added to test wells. As a negative control, the same serially diluted blood in RPMI media without PHA were run in parallel to test group. The plate was incubated at 37 °C in a 5% CO2 incubator and test by CV after 4 h.
Data analysis
Since the using CV for the lymphocyte proliferation assay represents a novel format, for all the possible and appropriate cut-off values of the test, receiver-operator characteristic (ROC) curves are constructed. Accordingly, for each point of CV data the sensitivity was plotted against one minus specificity for all the possible cut-off values. The values that provides an operating position nearest that of 100% sensitivity and 100% specificity were selected based on Youden’s index (J). The Youden’s J statistic, which shows the performance of a diagnostic test, was calculated as sensitivity + specificity −1; maximum value of the index was used as a criterion for selecting the optimum cut-off point.
Between-group comparisons were performed by one-way analysis of variance (ANOVA) using Fisher’s protected least significant difference set to the 95% confidence level. Correlations were assessed by linear regression analysis. Repeated measure analysis was conducted to test the PHA treatment effect on the cell proliferation data during the different time point of experiment. Results are expressed as mean ± S.E.M. of absolute values. All statistical analyses were carried out with the software SPSS ver. 23.0.0.1 (Chicago, IL, USA).
Data Availability
The datasets generated and/or analysed during the current study are not publicly available due [because it is not general data] but are available from the corresponding author on reasonable request.
References
Abbas, A. K., Lichtman, A. H., Pillai, S., Baker, D. L., & Baker, A. Cellular and molecular immunology (2017).
Braun, K. et al. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. In Vitro 52, 214–221 (2018).
Long, X. et al. Memory CD4(+) T cells are suppressed by CD8(+) regulatory T cells in vitro and in vivo. Am. J Transl. Res 9, 63–78 (2017).
Sitz, K. V. & Birx, D. L. Lymphocyte proliferation assay. Methods Mol. Med 17, 343–353 (1999).
Spohr, C. et al. A new lymphocyte proliferation assay for potency determination of bovine tuberculin PPDs. ALTEX. 32, 201–210 (2015).
Greene, N., Aleo, M. D., Louise-May, S., Price, D. A. & Will, Y. Using an in vitro cytotoxicity assay to aid in compound selection for in vivo safety studies. Bioorg. Med Chem. Lett. 20, 5308–5312 (2010).
El-Said, W. A., Yoon, J. & Choi, J. W. Nanostructured surfaces for analysis of anticancer drug and cell diagnosis based on electrochemical and SERS tools. Nano. Converg. 5, 11 (2018).
Zhi-Jun, Y., Sriranganathan, N., Vaught, T., Arastu, S. K. & Ahmed, S. A. A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J Immunol. Methods 210, 25–39 (1997).
Yu, C. et al. A new disposable electrode for electrochemical study of leukemia K562 cells and anticancer drug sensitivity test. Biosens. Bioelectron. 53, 142–147 (2014).
Oz, S., Breiling, A. & Maercker, C. Measurement of Cellular Behavior by Electrochemical Impedance Sensing. Methods Mol. Biol. 1601, 267–273 (2017).
Kafi, M. A., Kim, T. H., An, J. H. & Choi, J. W. Electrochemical cell-based chip for the detection of toxic effects of bisphenol-A on neuroblastoma cells. Biosens. Bioelectron. 26, 3371–3375 (2011).
Hu, X. B. et al. Biomimetic Graphene-Based 3D Scaffold for Long-Term Cell Culture and Real-Time Electrochemical Monitoring. Anal. Chem. 90, 1136–1141 (2018).
Guo, M. et al. Monitoring of cell growth and assessment of cytotoxicity using electrochemical impedance spectroscopy. Biochim. Biophys. Acta 1760, 432–439 (2006).
Dupont, B. & Good, R. A. Lymphocyte transformation in vitro in patients with immunodeficiency diseases: use in diagnosis, histocompatibility testing and monitoring treatment. Birth Defects Orig. Artic. Ser. 1975, 477–485 (2011).
Hubenova, Y. & Mitov, M. Extracellular electron transfer in yeast-based biofuel cells: A review. Bioelectrochemistry. 106, 177–185 (2015).
Simonte, F., Sturm, G., Gescher, J., & Sturm-Richter, K. Extracellular Electron Transfer and Biosensors. Adv. Biochem. Eng Biotechnol.(2017).
Bicher, H. I. The “membrane capacitance” aggregometer–a method for measuring platelet aggregation in whole blood. Angiology 22, 285–294 (1971).
Wang, J. T. et al. Detection of the cell viability and proliferation using two-signal electrochemical method. Analyst 137, 3230–3233 (2012).
Wang, K. et al. Membrane capacitance of thousands of single white blood cells. J R. Soc. Interface 14 (2017).
Zhao, Y. et al. Tumor cell characterization and classification based on cellular specific membrane capacitance and cytoplasm conductivity. Biosens. Bioelectron. 57, 245–253 (2014).
Petrenko, Y. A., Gorokhova, N. A., Tkachova, E. N. & Petrenko, A. Y. The reduction of Alamar Blue by peripheral blood lymphocytes and isolated mitochondria. Ukr. Biokhim. Zh. (1999.) 77, 100–105 (2005).
Petty, R. D., Sutherland, L. A., Hunter, E. M. & Cree, I. A. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin. Chemilumin. 10, 29–34 (1995).
Wright, M. M., Ortega, F., Breitling, R., Bendtsen, C., & Westerhoff, H. V. Rational cell culture optimization enhances experimental reproducibility in cancer cells. Sci. Rep. 2018. Feb. 143029–21050 (2008).
Guo, X. et al. A mini-electrochemical system integrated micropipet tip and pencil graphite electrode for detection of anticancer drug sensitivity in vitro. Biosens. Bioelectron. 64, 594–596 (2015).
Wu, D. M. et al. A Mini-Electrochemical System with Integrated Micropipet Tip and Pencil Graphite Electrode for Measuring Cytotoxicity. Methods Mol. Biol. 1572, 153–167 (2017).
Acknowledgements
University of Tehran.
Author information
Authors and Affiliations
Contributions
All authors conceived of the methods. G.N. supervised the project and provided guidance and assistance in cell culture and the manuscript writing. M.N. and B.P. performed the experiments and wrote the main manuscript with the input from all other authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nikbakht, M., Pakbin, B. & Nikbakht Brujeni, G. Evaluation of a new lymphocyte proliferation assay based on cyclic voltammetry; an alternative method. Sci Rep 9, 4503 (2019). https://doi.org/10.1038/s41598-019-41171-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41171-8
This article is cited by
-
Em14-3-3 delivered by PLGA and chitosan nanoparticles conferred improved protection in chicken against Eimeria maxima
Parasitology Research (2022)
-
Immunogenicity analysis of conserved fragments in Plasmodium ovale species merozoite surface protein 4
Malaria Journal (2020)
-
Probiotic Lactobacillus fermentum strain JDFM216 improves cognitive behavior and modulates immune response with gut microbiota
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.