Abstract
Several studies have demonstrated the relevance of endophytic bacteria on the growth and fitness of agriculturally-relevant plants. To our knowledge, however, little information is available on the composition, diversity, and interaction of endophytic bacterial communities in plants struggling for existence in the extreme environments of Chile, such as the Atacama Desert (AD) and Patagonia (PAT). The main objective of the present study was to analyze and compare the composition of endophytic bacterial communities associated with roots and leaves of representative plants growing in Chilean extreme environments. The plants sampled were: Distichlis spicate and Pluchea absinthioides from the AD, and Gaultheria mucronata and Hieracium pilosella from PAT. The abundance and composition of their endophytic bacterial communities was determined by quantitative PCR and high–throughput sequencing of 16S rRNA, respectively. Results indicated that there was a greater abundance of 16S rRNA genes in plants from PAT (1013 to 1014 copies g−1 DNA), compared with those from AD (1010 to 1012 copies g−1 DNA). In the AD, a greater bacterial diversity, as estimated by Shannon index, was found in P. absinthioides, compared with D. spicata. In both ecosystems, the greater relative abundances of endophytes were mainly attributed to members of the phyla Proteobacteria (14% to 68%), Firmicutes (26% to 41%), Actinobacteria (6 to 23%) and Bacteroidetes (1% to 21%). Our observations revealed that most of operational taxonomic units (OTUs) were not shared between tissue samples of different plant species in both locations, suggesting the effect of the plant genotype (species) on the bacterial endophyte communities in Chilean extreme environments, where Bacillaceae and Enterobacteriacea could serve as keystone taxa as revealed our linear discriminant analysis.
Similar content being viewed by others
Introduction
Numerous studies have revealed that bacteria living within plant tissues, collectively called endophytic bacteria, play a crucial role in the growth and fitness of a wide variety of monocot and dicot plant species, among others1,2. Beneficial functions attributed to endophytic bacteria include plant growth promotion by supplying nutrients (e.g., nitrogen fixation), protection against biotic- (e.g., pathogens) and abiotic-stresses (e.g., salinity and drought), detoxification of harmful compounds (e.g., NH3 or CN), and the production of bioactive compounds (e.g., secondary metabolites and hormones)3,4. Various endophytic microorganisms have been categorized as plant growth–promoting bacteria (PGPB) and they are currently used in the formulation of diverse bioproducts (e.g., biofertilizers and biofungicides) or to modify and/or introduce beneficial bacteria into the plant phytomicrobiome for agricultural purposes5,6. To date, however, many microbiome studies have been done using model plant (e.g., Arabidopsis thaliana), commercially relevant plants for agriculture (e.g., wheat, soybean, rice, maize, etc.) and wild plant species (e.g., weeds and trees) grown under laboratory, greenhouse and fields conditions1,2,7,8. Consequently, we only have limited knowledge on the composition and interactions of microbiota and plants, especially endophytic bacterial communities, on native plant vegetation growing in extreme environments, such as hot and/or cold deserts. Thus, our understanding on microbial interactions in plant holobiont will be key in the develop of efficient strategies for native plant conservation and/or exploit the full yield potential of crop plants under climate change scenario9.
The country of Chile is long (4,270 km) and narrow (mean width 177 km) and harbors a great variety of pristine ecosystems. The Atacama Desert (AD) is located in the northern region of Chile (from 18°24′S to 29°55′S) and is considered among the driest places on earth. In contrast, the Chilean Patagonia (from 41°08S to 56°30′S) is located in the far south of the country and is a sub Antarctic region. Both regions have extreme environments and their plant-associated bacterial communities have been barely studied thus far. In this context, we have reported that members of the orders Enterobacteriales, Actinomycetales, and Rhizobiales comprise dominant groups of the bacterial communities in the rhizosphere (the soil influenced by plant roots) of shrubs grown in AD and Patagonia (PAT), namely Atriplex sp. and Chuquiraga sp., respectively10. Results of this study also suggested that some isolates, belonging to the genera Enterobacteria, Pseudomonas, and Bacillus, were putative PGPB. The ability of the native isolates from AD to act as PGPB was confirmed by formulation and inoculation of a bacterial consortium onto plants. These studies revealed that wheat plants inoculated with the consortium produced greater biomass under water shortage and field conditions, compared with uninoculated seedlings11. A recent study also showed a greater protection against salt stress in wheat plants inoculated with rhizosphere bacteria isolated from Andean Altiplano native plant (Parastrephia quadrangularis) in AD12. However, these studies did not take into account the composition and interaction of native endophytic bacteria in Chilean extreme environments, as well as their potential use as PGPB.
During the last several years, advances in high–throughput DNA sequencing (HTS) technologies (e.g., Illumina®, PacBio® and Oxford Nanopore®) have opened new windows into the microbial ecology of a variety of environments, allowing the detailed study of complex bacterial communities in nature as never seen before. Thus, HTS platforms have widely been used to decipher the structure and function of microbiota in different compartments of plants, including as the rhizosphere, endosphere (inner tissues of plants), and phyllosphere (the aerial part of plant leaves)1,13. Results of 454‒pyrosequencing studies showed that the Proteobacteria (mainly Gammaproteobacteria) were the dominant taxa in the rhizospheres of Atriplex sp. and Stipa sp. (shrubs) grown in the AD14. These authors also postulated that native plants from Chilean extreme environments may attract, select, and conserve specific bacterial groups in order to sustain plant growth and tolerance to local harsh conditions. Based on this supposition, the main goal of the present study was to describe and compare the relative abundances and composition of bacterial communities associated with roots and leaves of plants grown in the AD and PAT regions of Chile by using HTS of 16S rRNA genes.
Material and Methods
Sampling
Plant specimens were collected in the AD (23°1′59″S, 68°11′59″W; and PAT (53°28′0″S, 71°0′59″W) regions of Chile (Fig. 1). The plants sampled were: Distichlis spicate (Poacea; Fig. 1A) and Pluchea absinthioides (Asteraceae; Fig. 1B) from AD, and Gaultheria mucronata (Ericaceae; Fig. 1C), and Hieracium pilosella (Asteraceae; Fig. 1D) from PAT. Three specimens of each plant species were randomly taken in a 10 m transect by using a clean spade to remove intact roots from soil. Specimens were placed in plastic bags and immediately transported, on ice, to the Applied Microbial Ecology Laboratory at La Frontera University for microbiological analyses.
DNA extraction
Roots and leaves samples were separated and surface sterilized by repeated immersion in 70% (v/v) ethanol for 3 min, followed by 2.5% (v/v) sodium hypochlorite (NaOCl) for 5 min as described by Barra et al.15. Roots were exhaustively rinsed with sterile distilled water. Triplicate portions of roots and leaves were aseptically cut, frozen in liquid nitrogen, macerated and homogenized with a mortar and pestle, and stored at −80 °C until DNA extraction. Samples of the homogenized tissues (0.25 g) were used for DNA extraction with Quick‒DNATM Plant/seed Miniprep kits according to manufacturer instructions (Zymo Research, CA, USA). The quantity and purity of DNA extracts were determined by measuring absorbance at 260 nm and 280 nm by using a microplate spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Inc., MA, USA).
Quantitative PCR
The abundance of endophytic bacteria in each tissue sample was determined by quantitative PCR (qPCR) by using a universal primer set for the bacterial 16S rRNA gene (Bac1369F 5′-CGG TGA ATA CGT TCY CGG-3′) and Prok1492R (5′-GGW TAC CTT GTT ACG ACT-3′) as previously described16,17. Briefly, PCR conditions were run with an enzyme activation step at 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C, and 1 min of annealing plus extension at 60 °C. PCR reactions were performed in triplicate per plant species (including technical triplicates) with 20 µg L−1 of total DNA in a StepOnePlusTM Real–Time PCR System (Applied Biosystems, Inc., CA, USA) using PowerUpTM SYBRTM Green Master Mix (Applied Biosystems, Inc.), by following the manufacturer instructions. The numbers obtained were normalized and analyzed by using one‒way ANOVA, and comparisons were done by using Tukey’s post‒hoc test. Differences were considered to be significant when the P value was ≤0.05.
High‒Throughput DNA Sequencing
The distribution and relative abundances of endophytic bacteria in root and leaf tissues was assessed by HTS using triplicate samples of each plant species as follow. The V4 hypervariable region of the 16S rRNA genes were amplified, for bacteria and archaea, by using primer set 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). Sequencing was done by the University of Minnesota Genomics Center (UMGC, Minneapolis, MN, USA)18 using barcode primers and the dual indexing method. Amplicons were gel purified, pooled, and paired‒end sequenced at a read length of 300 nt on the Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) from UMGC.
Bioinformatics and Statistical Analysis
Sequences were analyzed by using mothur program ver. 1.34.0 (https://www.mothur.org)19. The first 150 nt were trimmed from sequences to remove low–quality regions at the ends of reads. Fastq–join software was used to join paried–end sequencing reads20, the joined sequencing reads were trimmed to maintain an average quality score >35, a homopolyer length >8 nt. Sequences with >2 mismatches in primer sequences and ambiguous bases were removed. High quality sequencing reads were aligned on the basis of the SILVA database ver. 12321, and subjected to a 2% pre–clustering step to remove possible sequence errors22. The UCHIME software was used to identify and remove probable chimeric sequences23. To avoid the influence of non-microbiota (e.g., Chloroplast and mitochondria), the sequences were futher filtered by Qiime to remove non-microbiota taxa before a subsequent analysis. Sequence data was rarefied to 700 and 4,500 sequence reads per data set prior to statistical analysis for AD and PAT, respectively. Raw sequencing data were deposited in the Sequence Read Archive (SRA) of NCBI under Accession Number SRP156290.
For statistical analysis, Alpha diversity indices, as well as Good’s coverage, were calculated using the Shannon index and the abundance–based coverage estimate (ACE) through the mothur program. Visualization of the taxonomic distribution of microbial communities was performed using the “ggplot2” package in R24. Differences in beta diversity was evaluated by using analysis of similarity (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA). Principal coordinate analysis (PCoA) was performed based on unweighted unifrac distance for the ordination25. The VennDiagram package in R was used to identify shared OTUs of endophytic bacterial communities between root and leave tissues26. Variations in taxa associating with root and leaf tissues were evaluated using linear discriminant analysis (LDA) of effect sizes27, which employs Kruskal–Wallis and Wilconxon rank–abundance tests and then utilizes linear discriminant analysis (LDA) to estimate effect sizes of the features.
Results
Abundances of Bacteria
In general terms, plant tissues from Patagonia (G. mucronata and H. pilosella) had greater abundances of bacteria (from 1013 to 1014 16S rRNA gene copies g−1 template DNA), compared with those from AD (D. spicata and P. absinthioides) (from 1010 to 1012 16S rRNA genes copies g−1 template DNA), except roots from G. mucronata (Table 1). In AD plants, a significantly (P ≤ 0.05) greater abundance of bacteria in both tissues was found in P. absinthioides (2.6 × 1011 and 2.7 × 1012 16S rRNA gene copies g−1 template DNA in roots and leaves, respectively) compared with those from D. spicata (4.4 × 1010 and 5.4 × 1011 16S rRNA gene copies g−1 template DNA in roots and leaves, respectively). In PAT plants, a significantly (P ≤ 0.05) greater abundance of bacteria in both tissues was found in H. pilosella (8.1 × 1013 and 5.4 × 1014 16S rRNA gene copies g−1 template DNA in roots and leaves, respectively) compared with those from G. mucronata (1.3 × 1013 and 3.0 × 1010 16S rRNA gene copies g−1 template DNA in roots and leaves, respectively).
Composition of Endophytic Bacterial Community in Extreme Environments
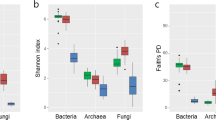
Sequence analyses showed a lower estimated coverage in AD (from 91 to 95%) compared with PAT (from 98 to 99%) (Table 2). The values of observed OTUs (Sobs) were lower in plant tissues from AD compared with those from PAT, ranging in values from 68 to 113 and 208 to 220 in roots, and 103 to 152 and 151 to 160 in leaves, respectively. Similarly, lower ACE values were also observed in AD compared to PAT plants, ranging in values of 148 to 188 and 263 to 314 in roots, and 139 to 211 and 190 to 245 in leaves, respectively. However, and as revealed by the Shannon index, there was significant differences (Tukey’s post‒hoc test, P < 0.05) in bacterial diversity in both tissues of P. absinthioides compared with tissues from D. spicata in AD (Table 2). In contrast, no significant differences in bacterial diversity were found in plant tissues from PAT plants.
In both ecosystems, the assignment of taxonomic affiliation to endophytic bacterial communities at the phylum level indicated that there were high relative abundances of Proteobacteria (14.88% to 68.53%), Firmicutes (26.03% to 41.59%), Actinobacteria (6.45% to 23.69%), and Bacteroidetes (1.09% to 21.21%) in both tissues (Fig. 2A). It is noteworthy that the lowest relative abundance of Bacteroidetes was found in roots from D. spicata. This tissue, however, presented a relative abundance of 31.01% of members belonging to Euryarchaeota phylum (Fig. 2A).
Mean relative abundances of major (A) and minor (B) taxa (at the phylum level) among endophytic bacterial communities from plant root and leaf tissues obtained from the Atacama Desert (Distichlis spicata and Pluchea absinthioides) and Patagonia (Gaultheria mucronata and Hieracium pilosella). Legend: DSR: root tissue from D. spicata, PAR: root tissue of P. absinthioides, DSL: leaf tissues from D. spicata, PAL: leaf tissues from P. absinthioides, GMR: root tissue from G. mucronata, HPR: root tissue from H. pilosella, GML: leaf tissues from G. mucronate, and HPL: leaf tissues from H. pilosella. Values represent means of 3 replicates.
With respect to minor taxa, broad taxonomic diversity among samples was found. The tissues of AD plants (D. spicata and P. absinthioides) showed large relative abundances of Cyanobacteria, Lentisphaerae and Chloroflexi in roots, and Verrucomicrobia and Fusobacteria and Cyanobacteria in leaves (Fig. 2B). The tissues from PAT plants (G. mucronata and H. pilosella) showed a large relative abundance of Elusimicrobia, Fusobacteria, Spirochaetes and Acidobacteria in roots, and Elusimicrobia, Fusobacteria, TM7 and Verrucomicrobia in leaves (Fig. 2B). At family level, a wide taxonomic diversity among samples was also found. In AD plants, higher relative abundances of Halobacteriaceae (31.01%), Bacillaceae (24.67%) and Nocardiopsaceae (17.78%) are highlight in roots of D. spicata whereas a higher relative abundance of Halomonadaceae (25.81%) is highlight in leaves of P. absinthioides (Fig. 3). In PAT plants, a higher relative abundance of members belonging to Pseudomonaceae was found in roots (21%) and leaves (57.93%) from G. mucronata.
Mean relative abundances of taxa (at the family level) among endophytic bacterial communities in root and leaf tissues obtained from plants in the Atacama Desert (Distichlis spicata and Pluchea absinthioides) and Patagonia (Gaultheria mucronata and Hieracium pilosella). Legend: DSR: root tissue from D. spicata, PAR: root tissue of P. absinthioides, DSL: leaf tissues from D. spicata, PAL: leaf tissues from P. absinthioides, GMR: root tissue from G. mucronata, HPR: root tissue from H. pilosella, GML: leaf tissues from G. mucronate, and HPL: leaf tissues from H. pilosella. Values represent means of 3 replicates.
Differences between tissues and plant species were also confirmed by PCoA. In AD plants, a clear grouping between roots and leaves from D. spicata, and roots from P. absinthioides was observed (Fig. 4, AD). Similarly, a clear grouping between roots and leaves from H. pilosella, and roots from G. mucronata was also observed in PAT plants (Fig. 4, PAT).
Principal coordinate analysis (PCoA) of endophytic bacterial communities from plant roots and leaf tissues obtained from the (AD) Atacama Desert (Distichlis spicata and Pluchea absinthioides), and (PAT) Patagonia (Gaultheria mucronata and Hieracium pilosella). Legend: DSR: root tissue from D. spicata, PAR: root tissue of P. absinthioides, DSL: leaf tissues from D. spicata, PAL: leaf tissues from P. absinthioides, GMR: root tissue from G. mucronata, HPR: root tissue from H. pilosella, GML: leaf tissues from G. mucronate, and HPL: leaf tissues from H. pilosella. Analyses were done by using unweighted UniFrac distances.
Shared and Unique Operational Taxonomic Units and Keytone Taxa in Extreme Ecosystems
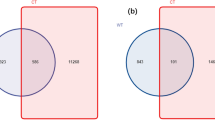
In relation to the distribution of shared and unique OTUs among endophytic bacterial communities in ecosystems, 53 out of 1075 OTUs were shared in AD, while 115 out of 1713 OTUs shared in PAT ecosystem (Fig. 6 and Table 3). In AD plants, among these 53 shared OTUs, most belonged to the Firmicutes (20), followed by Proteobacteria (15) and Actinobacteria (13). In PAT plants, among these 115 shared OTUs, most belonged to the Firmicutes (37), followed by the Actinobacteria (29), Bacteroidetes (25) and Proteobacteria (24). In the PAT, there were a greater number of unique OTUs, relative to those seen at AD, where 305 and 341 and 306 and 195 plant specific OTUs were found in root and leaves from G. mucronata and H. pilosella, respectively (Table 3 and Fig. 5). In the AD, 234 and 224, and 218 and 93 plant specific OTUs were found in root and leaves from D. spicata and P. absinthioides, respectively (Table 3 and Fig. 5). In AD plants, most of unique OTUs belonged to the Firmicutes (353), followed by the Proteobacteria (213), Actinobacteria (90) and Bacteroidetes (87). Similarly, in PAT plants, most of unique OTUs belonged to the Firmicutes (512), followed by Proteobacteria (397), Actinobacteria (159) and Bacteroidetes (119) (Table 3).
Shared OTUs among endophytic bacterial communities in roots and leaves of plants from (AD) Atacama Desert (Distichlis spicata and Pluchea absinthioides), and (PAT) Patagonia (Gaultheria mucronata and Hieracium pilosella) determined in each plant species (n = 3). Legend: DSR: root tissue from D. spicata, PAR: root tissue of P. absinthioides, DSL: leaf tissues from D. spicata, PAL: leaf tissues from P. absinthioides, GMR: root tissue from G. mucronata, HPR: root tissue from H. pilosella, GML: leaf tissues from G. mucronate, and HPL: leaf tissues from H. pilosella.
Linear discriminant analysis (LDA) of effect size was performed to determine which taxa varied among plant components at two ecosystems. Several bacterial taxa, which belong to the Bacillacea, Nocardiopsaceae, Ectothiorhodospiraceae, and Moraxellaceae (at family level), elucidative served as the keystone taxa in roots of D. spicata, while only the Propionivacteriaceae and Corynebacteriaceae could be used to indicate leaves of D. spicata and P. absinthioides, respectively (Fig. 6). Moreover, the Bacillaceae had the greatest effect size among the plant components in AD. Contrastingly, taxa belonging to the family level of the Enterobacteriaceae, Pseudomonadaeceae, Dermabacteraceae, Coriobacteriaceae, and Bacteroidaceae were the keystones presenting endophytic microbiota among plant compartments in PAT ecosystem (Fig. 6). Additionally, the Enterobacteriaceae had the highest effect size in PAT.
Key phylotypes of endophytic microbiota residing within roots and leaves of plants growing in the (AD) Atacama Desert and (PAT) Patagonia. Differences between plant compartments were assessed by using linear discriminant analysis (LDA) of effect size and Kruskal–Wallis and Wilconxon rank–abundance tests. The histogram shows the linear discriminant analysis scores, computed for features (at the operational taxonomic unit level), of differentially abundant taxa (at the family level) on root and leaf tissues of plants. Histogram bars representing the same bacterial families were deduced from different taxa. Legend: DSR: root tissue from D. spicata, PAR: root tissue of P. absinthioides, DSL: leaf tissues from D. spicata, PAL: leaf tissues from P. absinthioides, GMR: root tissue from G. mucronata, HPR: root tissue from H. pilosella, GML: leaf tissues from G. mucronate, and HPL: leaf tissues from H. pilosella.
Discussion
Microbial endophytes play a central role in the ecology, evolution, and growth promotion of plants2,4,28. However, despite their importance, there is scant knowledge concerning endophytic microbial populations in plants living in extreme environments, including Chile. Our study showed that the abundances of bacteria from the endosphere of root and leaf tissues ranged from 1010 to 1012 and from 1010 to 1014 16S rRNA gene copies g−1 template DNA in AD and PAT, respectively; corresponding from 103 to 107 copies of 16S rRNA genes copies g−1 fresh tissue.
A wide range of endophytic prokaryote densities have been reported in tissues of different plants thus far. Similar to our finding, 1014 16S rRNA gene copies g−1 template DNA of total endophytic prokaryotes were reported in endospheres of olive tree (Olea europaea L.) leaves collected from diverse Mediterranean ecosystems29. Higher abundances than those observed in this current study were reported to be present in the endosphere of rice (107 to 108 16S rRNA gene copies g−1 root) and crops (1010 to 1013 copies of 16S rRNA genes g−1 root) by Ruppel et al.30 and Breidenbach et al.31, respectively. Under stress conditions, Blain et al.32 recently reported abundances of endophytic bacteria from 103 to 105 16S rRNA gene copies g−1 from fresh roots of natural vegetation growing in a hydrocarbon‒contaminated site. In addition, and to our knowledge, there are no studies reporting the abundances of endophytic bacterial populations in plants from Chilean extreme environments. It should be noted, however that a previous study of the rhizosphere of plants from AD and PAT revealed values of 109 and 1011 16S rRNA genes copies g−1 of soil, respectively33.
With respect to analyses of alpha diversity of endophytic bacterial communities in root and leave tissues, the number of OTUs observed (97% similarity) range from 68 to 152 and from 151 to 220 in the endospheres of plants from AD and PAT, respectively. These values are in accordance with other studies reporting OTUs values from plant endospheres ranging from 100 to 3002,28,34,35. However, a significantly greater number of OTUs (from 450 to 3700) have also been reported in plant endospheres by other authors29,36,37.
The Shannon index values ranged from 2.28 to 4.41. and from 2.76 to 4.41 in the endospheres of plants from AD and PAT, respectively. The diversity of bacteria in root and leaf tissues of tree peony (Paeonia Sect. Moutan) had greater Shannon index values (7 to 9) compared to our study37. In contrast, a recent study reported Shannon index values that were similar to those we found (3 to 4) in root and leaf endospheres of groundsel (Senecio vulgaris L., Asteraceae), also by using the Illumina platform34. Similarly, but by using 454‒pyrosequencing, Correa-Galeote et al.35 reported Shannon index values of 3 to 4 in the root endosphere of maize cultivated at 3,537 meters above sea level in Perú. Interestingly, the Shannon index values found in endosphere tissues from AD plants were generally lower than those reported in other AD habitats, such as soil, lakes and sediments of flat mats, with Shannon index values ranging from 3 to 938,39,40,41. This suggests that endospheres of AD plants may harbor less bacterial diversity compared to other niches in the AD, which is considered oldest and driest place on the Earth42.
Interestingly, compared with G. mucronata, H. pilosella had high bacterial abundances and diversity at the PAT ecosystem, as determined by qPCR and Shannon index, respectively. H. pilosella is an exotic weed at the Chilean Patagonia, which recognized by its explosive expansion in Patagonian grasslands, often replacing forage plants with the concomitant economic loss for livestock and soil degradation by overgrazing43. Therefore, the higher abundance and diversity of endophytic bacteria in H. pilosella might give this plant species a competitive advantage against other Patagonian plants. Thus, alteration of the composition and activity of endophytic bacteria may be a useful strategy for biological control of invasive plants. However, our study is limited to few sampled plants and major efforts are required to validate this statement and evaluate the potential biocontrol of H. pilosella expansion in Chilean Patagonia grasslands.
Our Illumina-based analyses revealed the dominance of members of the phyla Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes in the endosphere. It was previously reported that Proteobacteria and Firmicutes are common inhabitants of plant endospheres with relative abundances ranging from 39% to 97% and 14% to 44%, respectively4,28,36. Studies done to analyze root and leave endosphere tissues have also shown a great dominance (over 86%) by the Proteobacteria, Firmicute and other phyla (such as Bacteroidetes, Acidobacteria and Actinobacteria) in olive trees, peony and groundsel29,34,37. Interestingly, a high relative abundance (31%) of Euryarchaeota was found in roots of D. spicata. Member of Euryarchaeota have also been found colonizing the endospheres of Mediterranean olive trees29 and compartments (rhizosphere, endosphere and phyllosphere) of a halophyte plant (Salsola stocksii) from Pakistan44.
Relative to what was seen in tissues from the AD and PAT as minor taxa, both ecosystems had a great diversity, represented by members of the Lentisphaerae, Chloroflexi, Verrucomicrobia, Elusimicrobia, Fusobacteria, Spirochaetes and Acidobacteria. Low numbers of sequences of these phyla were observed by Hardoim et al.4, when endophytic data sets from all peer–reviewed publications were revised in the ISI Web of Science and PubMed databases. Similarly, our analyses interestingly revealed the presence of members of the phylum Cyanobacteria in roots and leaves of AD plants. High abundances (24% to 47%) of members of this phyla have also been reported in the endospheres of grasses (Spartina alterniflora) and mangrove (Kandelia obovata)45. Lower abundances (1.7%) of Cyanobacteria have also been reported in the endosphere and other compartments (rhizosphere and rhizoplane) of the medicinal perennial plant Stellera chamaejasme L.46. It is noteworthy that Cyanobacteria are a diverse group of photosynthetic bacteria (some of them nitrogen‒fixing) that live in a great variety of extreme environments, including soils and rocks from the AD and hot springs and lakes from Argentine Patagonia42,47,48. However, and to our knowledge, there have been no previous reports of Cyanobacteria associated with the endosphere of plants in AD and PAT.
At the family level, bioinformatic analyses indicated that there was high diversity in the endosphere of plants from both ecosystems, highlighting some dominant groups as the Halobacteriaceae, Bacillaceae, Nocardiosaceae and Halomonadaceae in AD plants, and Pseudomonaceaea in G. mucronata in PAT. Halobacteriaceae and Halomonadaceae are phylogenetically diverse groups of Archaea and Eubacteria able to survive and proliferate in hypersaline habitats, such as soils from AD49,50, and including some endophytes, such as Euryarchaeota, Kushneria endophytica, Salinicola tamaricis44,51,52,53. Similarly, Bacillaceae and Pseudomonaceae families are commonly found as inhabitant of plant endospheres34,47,54. It is noteworthy that the contrast in the family diversity among AD and PAT plants reveals significant differences in the composition of endophytic bacterial communities between locations.
A recent study postulated that plant species are able to recruit specific endophytic bacterial communities2,55. Similarly, Gadhave et al.56 reported that soil inoculation with Bacillus spp. modified the diversity, evenness, and community composition of endophytic bacterial in roots of broccoli. However, the composition of endophytic bacterial communities might not only to be ruled by biotic factors (such as plant genotype or competition with other microbes), but by abiotic factors including climate (temperature and drought)57 and soil cultivation history35. In this context, significant differences between plant species and locations were also observed in rhizosphere bacterial communities in plants from the AD, PAT and Antarctic14. In addition, differences in endophytic bacterial community composition may be due to a great portion of unique OTUs (93 to 234 and 195 to 341 in AD and PAT endospheres, respectively) compared with the shared OTUs (53 and 115 in AD and PAT endospheres, respectively) between the plant species as revealed by diagram Venn. Bacteria belonging to the Firmicutes, Proteobacteria, Actinobacteria and Bacteroidetes, were mainly observed among the unique OTUs observed in plants. The low number of shared OTUs, compared with unique OTUs, is in contrasts with observations in tree peony grass and mangrove, where Venn analysis showed overlap patterns of OTUs between endophytic bacteria in root and leaves samples37,45. Therefore, our results suggest that endosphere of the plant species we studied in each Chilean extreme environment harbored specific bacterial communities unique to their location and plant genotype (species), where Bacillaceae and Enterobacteriacea could serve as keystone taxa as revealed our LDA analysis.
References
Turner, T. R., James, E. K. & Poole, P. S. The plant microbiome. Genome Biol. 14, 209 (2013).
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. & Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206 (2015).
Brader, G., Compant, S., Mitter, B., Trognitz, F. & Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotechnol. 27, 30–37 (2014).
Hardoim, P. R. et al. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79, 293–320 (2015).
de Souza, R., Ambrosini, A. & Passaglia, L. M. P. Plant growth‒promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38, 401–419 (2015).
Mitter, B. et al. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 8, 11 (2017).
Beckers, B., Op De Beeck, M., Weyens, N., Boerjan, W. & Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 5, 25 (2017).
Hassani, M. A., Durán, P. & Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 27, 6:58 (2018).
Schlaeppi, K. & Bulgarelli, D. The plant microbiome at work. Mol. Plant. Microbe Interact. 28, 212–217 (2015).
Jorquera, M. A. et al. Bacterial community structure and detection of putative plant growth-promoting rhizobacteria associated with plants grown in Chilean agro-ecosystems and undisturbed ecosystems. Biol. Fertil. Soils 50, 1141–1153 (2014).
Inostroza, N. G., Barra, P. J., Wick, L. Y., Mora, M. L. & Jorquera, M. A. Effect of rhizobacterial consortia from undisturbed arid- and agro-ecosystems on wheat growth under different conditions. Lett. Appl. Microbiol. 64, 158–163 (2016).
Acuña, J. J., Jaisi, D., Campos, M., Mora, M. L. & Jorquera, M. A. ACCD-producing rhizobacteria from an Andean Altiplano native plant (Parastrephia quadrangularis) and their potential to alleviate salt stress in wheat seedling. Appl. Soil Ecol. 136, 184–190 (2019).
Lagos, M. L. et al. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: a mini-review. J. Soil Sci. Plant. Nutr. 15, 504–523 (2015).
Jorquera, M. A. et al. Rhizobacterial community structures associated with native plants grown in Chilean extreme environments. Microb. Ecol. 72, 633–646 (2016).
Barra, P. J. et al. Formulation of bacterial consortia from avocado (Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. Appl. Soil Ecol. 102, 80–91 (2016).
Jorquera, M. A. et al. Effect of nitrogen and phosphorus fertilization on the composition of rhizobacterial communities of two Chilean Andisol pastures. World J. Microbiol. Biotechnol. 30, 99–107 (2014).
Suzuki, M. T., Taylor, L. T. & DeLong, E. F. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 66, 4605–4614 (2000).
Gohl, D. M. et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 34, 942–949 (2016).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Aronesty, E. Comparison of sequencing utility programs. Open Bioinforma. J. 7, 1 (2013).
Pruesse, E. et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196 (2007).
Huse, S. M., Welch, D. M., Morrison, H. G. & Sogin, M. L. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ. Microbiol. 12, 1889–1898 (2010).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Wickham, H. ggplot2: elegant graphics for data analysis, Springer (2009).
Bray, J. R. & Curtis, J. T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monographs 27, 325–349 (1957).
Chen, H. & Boutros, P. C. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12, 35 (2011).
Segata, N. & Huttenhower, C. Toward an efficient method of identifying core genes for evolutionary and functional microbial phylogenies. PLoS One 6, e24704 (2011).
Santoyo, G., Moreno-Hagelsieb, G., Orozco-Mosqueda, M. C. & Glick, B. R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99 (2016).
Müller, H. et al. Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front. Microbiol. 6, 138 (2015).
Breidenbach, B., Brenzinger, K., Brandt, F. B., Blaser, M. B. & Conrad, R. The effect of crop rotation between wetland rice and upland maize on the microbial communities associated with roots. Plant Soil 419, 435–445 (2017).
Ruppel, S., Rühlmann, J. & Merbach, W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil 286, 21–35 (2006).
Blain, N. P., Helgason, B. L. & Germida, J. J. Endophytic root bacteria associated with the natural vegetation growing at the hydrocarbon-contaminated Bitumount Provincial Historic site. Can. J. Microbiol. 63, 502–515 (2017).
Acuña, J. J. et al. Bacterial alkaline phosphomonoesterase in the rhizospheres of plants grown in Chilean extreme environments. Biol. Fertil. Soils 52, 763–773 (2016).
Cheng, D., Tian, Z., Feng, L., Xu, L. & Wang, H. Diversity analysis and function prediction of rhizo- and endophytic bacterial communities of Senecio vulgaris L. (Asteraceae) in an invasive range. Peer J. Preprints 6, e26701v1 (2018).
Correa-Galeote, D., Bedmar, E. J. & Arone, G. J. Maize endophytic bacterial diversity as affected by soil cultivation history. Front. Microbiol. 9, 484 (2018).
Proença, D. N. et al. The microbiome of endophytic, wood colonizing bacteria from pine trees as affected by pine wilt disease. Sci. Rep. 7, 4205 (2017).
Yang, R., Liu, P. & Ye, W. Illumina-based analysis of endophytic bacterial diversity of tree peony (Paeonia Sect. Moutan) roots and leaves. Braz. J. Microbiol. 48, 695–705 (2017).
Crits-Christoph, A. et al. Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome 1, 28 (2013).
Mandakovic, D. et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci. Rep. 8, 5875 (2018).
Mandakovic, D. et al. Microbiome analysis and bacterial isolation from Lejía Lake soil in Atacama Desert. Extremophiles 22, 665–673 (2018).
Rasuk, M. C. et al. Bacterial Diversity in Microbial Mats and Sediments from the Atacama Desert. Microb. Ecol. 71, 44–56 (2016).
Azua-Bustos, A., Urrejola, C. & Vicuña, R. Life at the dry edge: Microorganisms of the Atacama Desert. FEBS Letters 586, 2939–2945 (2012).
Ivelic-Sáez, J., Radic, S., Domínguez, E. & Salinas, C. Background of Hieracium pilosella L. control for their application in the Chilean Magallanes and Antarctic Region. Agro Sur 45, 53–62 (2017).
Mukhtar, S. et al. Comparison of microbial communities associated with halophyte (Salsola stocksii) and non-halophyte (Triticum aestivum) using culture-independent approaches. Pol. J. Microbiol. 66, 353–364 (2017).
Hong, Y. et al. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Can. J. Microbiol. 61, 723–33 (2015).
Jin, H. et al. Characterization of rhizosphere and endophytic bacterial communities from leaves, stems and roots of medicinal Stellera chamaejasme L. Syst. Appl. Microbiol. 37, 376–385 (2014).
Mackenzie, R., Pedrós-Alió, C. & Díez, B. Bacterial composition of microbial mats in hot springs in Northern Patagonia: variations with seasons and temperature. Extremophiles 17, 123–136 (2013).
Patzelt, D. J., Hoda, L. & Fried, T. Biodiversity of soil cyanobacteria in the hyper-arid Atacama Desert, Chile. J. Phycol. 50, 698–710 (2014).
Parro, V. et al. A microbial oasis in the hypersaline Atacama subsurface discovered by a life detector chip: implications for the search for life on Mars. Astrobiology 11, 969–996 (2011).
Azua-Bustos, A. et al. Unprecedented rains decimate surface microbial communities in the hyperarid core of the Atacama Desert. Sci. Rep. 8, 16706 (2018).
Cope-Selby, N. et al. Endophytic bacteria in Miscanthus seed: implications forgermination, vertical inheritance of endophytes, plantevolution and breeding. GCB Bioenergy 9, 57–77 (2017).
Navarro-Torre, S. et al. Kushneria phyllosphaerae sp. nov. and Kushneria endophytica sp. nov., plant growth promoting endophytes isolated from the halophyte plant Arthrocnemum macrostachyum. Int. J. Syst. Evol. Microbiol. 6, 2800–2806 (2018).
Zhao, G. Y. et al. Salinicola tamaricis sp. nov., a heavy-metal-tolerant, endophytic bacterium isolated from the halophyte Tamarix chinensis Lour. Int. J. Syst. Evol. Microbiol. 67, 1813–1819 (2017).
Zhao, Y. et al. D2. Endosphere microbiome comparison between symptomatic and asymptomatic roots of Brassica napus infected with Plasmodiophora brassicae. PLoS One 12, e0185907 (2017).
Kumar, M. et al. Plants assemble species specific bacterial communities from common core taxa in three arcto-alpine climate zones. Front. Microbiol. 8, 12 (2017).
Gadhave, K. R., Devlin, P. F., Ebertz, A., Ross, A. & Gange, A. C. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb. Ecol. 76, 741–750 (2018).
Naylor, D., DeGraaf, S., Purdom, E. & Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 11, 2691–2704 (2017).
Acknowledgements
The authors would like to thank Dr. Ruben Araya from Antofagasta University for his assistance during sampling in AD. The authors also acknowledge to the Editor and anonymous reviewers, whose comments have helped to refine and improve the quality the manuscript. This study was funded, in part, by Fondecyt projects No. 1160302 (to M.A.J.) and 1181050 (to M.L.M.), by SATREPS-MACH by JST/JICA Japan (to M.A.J.), and by the Minnesota Corn Research and Promotion Council (to M.J.S. and Q.Z). J.J.A. also acknowledges to Initiation Fondecyt project No. 11160112 and S.R. thanks to FIC-R 2017 No. 30488841.
Author information
Authors and Affiliations
Contributions
Q.Z., M.J.S. and M.A.J. wrote the main manuscript, prepared the figures and tables, and analyzed and interpreted the data. N.G.I. analyzed qPCR data. J.J.A., M.L.M. and S.R. made critical revisions of the main manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Acuña, J.J., Inostroza, N.G. et al. Endophytic Bacterial Communities Associated with Roots and Leaves of Plants Growing in Chilean Extreme Environments. Sci Rep 9, 4950 (2019). https://doi.org/10.1038/s41598-019-41160-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41160-x
This article is cited by
-
Diversity of endophytic bacteria isolated from leguminous agroforestry trees in western Kenya
AMB Express (2024)
-
Variovorax sp. strain P1R9 applied individually or as part of bacterial consortia enhances wheat germination under salt stress conditions
Scientific Reports (2024)
-
Deciphering the Role of WWTPs in Cold Environments as Hotspots for the Dissemination of Antibiotic Resistance Genes
Microbial Ecology (2024)
-
Endophytic bacterial communities in ungerminated and germinated seeds of commercial vegetables
Scientific Reports (2023)
-
Contribution of Leaf-Associated Microorganisms from Native Andean Ericaceae against Botrytis cinerea in Vaccinium corymbosum Cultivars
Journal of Soil Science and Plant Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.