Abstract
Animal studies using chronic social defeat stress (CSDS) in mice showed that brain-derived neurotrophic factor (BDNF) signaling in the mesolimbic dopamine (DA) circuit is important for the development of social aversion. However, the downstream molecular targets after BDNF release from ventral tegmental area (VTA) DA terminals are unknown. Here, we show that depressive-like behaviors induced by CSDS are mediated in part by Gadd45b downstream of BDNF signaling in the nucleus accumbens (NAc). We show that Gadd45b mRNA levels are increased in susceptible but not resilient mice. Intra-NAc infusion of BDNF or optical stimulation of VTA DA terminals in NAc enhanced Gadd45b expression levels in the NAc. Importantly, Gadd45b downregulation reversed social avoidance in susceptible mice. Together, these data suggest that Gadd45b in NAc contributes to susceptibility to social stress. In addition, we investigated the function of Gadd45b in demethylating CpG islands of representative gene targets, which have been associated with a depressive phenotype in humans and animal models. We found that Gadd45b downregulation changes DNA methylation levels in a phenotype-, gene-, and locus-specific fashion. Together, these results highlight the contribution of Gadd45b and changes in DNA methylation in mediating the effects of social stress in the mesolimbic DA circuit.
Similar content being viewed by others
Introduction
Animal studies using chronic social defeat stress (CSDS) in mice, an ethologically validated model of aspects of depression in mice1,2, previously showed that the mesolimbic dopamine (DA) circuit is critically involved in the development of social aversion and other behavioral abnormalities3,4. Indeed, CSDS in mice increases the activity of dopamine (DA) neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc)3,5. Furthermore, optogenetic stimulation of this VTA to NAc pathway increases susceptibility to CSDS via a mechanism involving release of brain-derived neurotrophic factor (BDNF) from VTA DA neuron terminals rather than dopaminergic signaling4. BDNF signaling in NAc promotes stress susceptibility through its tyrosine kinase receptor, TrkB, however, the molecular mechanisms underlying these effects remain unknown.
Growing evidence implicates transcriptional alterations induced by CSDS in several limbic brain regions including the NAc in stress susceptibility6, and these alterations in stressed mice have been paralleled by similar transcriptional investigations in the post-mortem brains of patients with major depression7. While the molecular mechanisms underlying these transcriptional changes are a matter of intense investigation, recent findings suggest a causal link between epigenetic mechanisms, including DNA methylation, histone modifications, and chromatin remodeling, and changes in gene expression (reviewed in8,9). Indeed, besides a global reorganization of chromatin complexes, changes in DNA methylation and hydroxymethylation in the NAc have been associated with the effects of CSDS10,11,12. Similarly, genome-wide assessments of DNA methylation changes in human brain previously revealed global reorganization of DNA methylation profiles, associated with psychiatric disorders including major depression, psychosis, bipolar disorder, post-traumatic stress disorder (PTSD), and child abuse13,14,15,16,17.
Gadd45b, a member of the growth arrest and DNA damage (Gadd45) gene family, is a DNA demethylating candidate. In brain, Gadd45b has been shown to be critically involved in activity-induced neurogenesis by decreasing site-specific DNA methylation in the regulatory regions of the Bdnf and fibroblast growth factor 1 (Fgf1) genes18. Subsequent reports showed that, by regulating DNA methylation levels at precise gene loci, Gadd45b regulates juvenile behavior and pro-inflammatory cytokine production19, while influencing long-term memory formation and synaptic plasticity20,21. Furthermore, analyses of human post-mortem tissue revealed increased Gadd45b mRNA and protein expression in the parietal cortex of psychotic patients22. Together, these findings raise the possibility that, by changing DNA methylation levels at specific gene loci, Gadd45b might modulate the molecular cascades regulating stress susceptibility.
In the present study, we assessed the involvement of Gadd45b in mediating the molecular and behavioral effects of CSDS. Our findings suggest that alteration of Gadd45b expression in the NAc, downstream of BDNF signaling, is involved in mediating the stress susceptibility in mice by interfering with the establishment of DNA methylation patterns at specific gene loci in this brain region.
Results
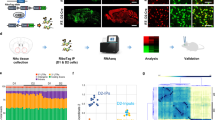
Gadd45b is an activity-induced immediate early gene in mature hippocampal neurons18. As chronic social stress is known to alter transcriptional profiles in several brain regions including the NAc23, we first tested whether Gadd45b expression is altered following chronic social stress. For this experiments, c57bl/6 mice were subjected to social defeat stress for 10 days and then assessed for social interaction with a social target (Fig. 1a). Ten days of CSDS induced a strong social avoidance phenotype (Fig. 1b, Supplementary Fig. 1a,b) in the susceptible versus resilient and control mice. Our results show that Gadd45b expression levels were significantly increased in the NAc of susceptible mice compared to control (Fig. 1c). Importantly, this effect is specific to susceptibility as we found no significant change in Gadd45b expression in the NAc of resilient mice. Interestingly, this is in accordance with previous findings showing the involvement of Gadd45b in hippocampus in fear conditioning and memory consolidation in mice21 and in parietal cortex of humans with psychosis22, thus expanding the involvement of Gadd45b in NAc in the context of chronic social stress.
Chronic social defeat stress (CSDS) induces Gadd45b in the nucleus accumbens (NAc) of susceptible mice. (a) Schematic diagram depicting the experimental procedure for CSDS. (b) Repeated CSDS induces social avoidance in susceptible but not resilient mice [One-way analysis of variance (ANOVA), F(2,27) = 38.22, p < 0.001, Control n = 10, Susceptible n = 10, Resilient n = 10]. Social avoidance was expressed as social interaction ratio (SI ratio) calculated by time spent in the interaction zone with a social target/time in the interaction zone without a social target. c, CSDS induces Gadd45b mRNA levels in NAc of susceptible but not resilient mice (F(2,27) = 3.365, p < 0.05, Control n = 10, Susceptible n = 10, Resilient n = 10). mRNA expression levels were expressed as fold change (FC) compared to control group. (d,e) Phasic stimulation of the VTA-NAc DA pathway (d, Student’s t-test, t(15) = 2.405, *p < 0.05, EYFP n = 8, ChR2 n = 9), or intra-NAc BDNF infusion (e, Student’s t-test, t(17) = 2.281, *p < 0.05, PBS n = 9, BDNF n = 10), induces Gadd45b expression in the NAc. *p < 0.05, ***p < 0.001. Bar graphs show mean ± SEM.
Previous data showed that the phasic optogenetic activation of the VTA to NAc pathway in mice—stimulation either of VTA DA neuron cells bodies or of their nerve terminals in NAc—increases susceptibility to social stress3,4,5. We found that the phasic optogenetic activation of VTA terminals in NAc significantly increased Gadd45b expression in the NAc (Fig. 1d). Furthermore, the effects of phasic activation of this mesolimbic circuit on susceptibility have been shown to be mediated via the release of BDNF, not DA, from VTA projections in the NAc4. Thus, we tested whether the elevated Gadd45b expression found in the NAc of susceptible mice is mediated via similar mechanisms. Indeed, intra-NAc infusion of BDNF significantly increased Gadd45b expression (Fig. 1e). Together, these data suggest that Gadd45b in the NAc is involved in the expression of stress susceptibility through the activation of the mesolimbic circuit and release of BDNF.
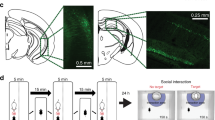
We next tested the hypothesis that reducing Gadd45b in the NAc would rescue the social avoidance phenotype induced by 10 days of CSDS in mice using a viral-mediated gene transfer approach (Fig. 2a). We created a viral vector expressing a miRNA that reduces only Gadd45b but not Gadd45a and Gadd45r expression in the NAc (Fig. 2c, Supplementary Fig. 2, see Methods Section). We carried out CSDS on a cohort of mice and identified susceptible and resilient individuals. Twenty-four hrs after the assessment of social interaction (SI-1), we performed intra-NAc infusion of HSV-Gadd45b miR or HSV-GFP in control, susceptible, and resilient mice (Fig. 2b) and then reassessed social interaction 4 days post-surgeries (SI-2), a period coinciding with peak expression of HSV vectors (Fig. 2a). Our analysis showed that the viral downregulation of Gadd45b expression in the NAc did not affect social interaction in control mice (Fig. 2d). However, in susceptible mice, Gadd45b KD (HSV-Gadd45b miR) significantly reversed the social avoidance phenotype initially expressed before surgery (Fig. 2e). Post-hoc analyses showed that susceptible mice treated with HSV-Gadd45b miR spent more time in the interaction zone in the presence of a social target compared to their pre-surgery levels and to susceptible mice treated with HSV-GFP. In resilient mice, HSV-GFP treatment decreased social interaction scores. However, Gadd45b KD (HSV-Gadd45b miR) in the resilient group successfully normalized social interaction behavior to control conditions (Fig. 2f). We re-analyzed social interaction changes at pre- (SI-1) and post-surgery (SI-2) with social interaction time in each group. We observed the same patterns in the time spent in interaction zone in the presence of a target as in the social interaction ratio (Supplementary Fig. 3). Taken together, these findings suggest that Gadd45b in the NAc has a pro-depressant effect on social behaviors following chronic social defeat stress.
Behavioral effects of local deletion of Gadd45b in NAc on social avoidance behaviors. (a) Schematic diagram depicting the experimental procedures for CSDS and intra-NAc infusion of HSV-Gadd45b miR. (b) Immunohistochemistry after HSV-Gadd45b miR infusion into NAc. (c) HSV-Gadd45b miR infused into NAc significantly decreases Gadd45b mRNA levels in NAc (t(15) = 2.405, *p < 0.05, HSV-GFP n = 8, HSV-Gadd45b miR n = 9). (d–f) Local Gadd45b KD in the NAc had no effect on social interaction in stress naïve animals [d, Mixed model two-way ANOVA, time effect: F(1,32) = 1.305, p = 0.262; genetic effect: F(1,32) = 0.142, p = 0.709; time × genetic effect: F(1,32) = 0.028, p = 0.868, HSV-GFP (pre- and post-) n = 9, HSV-Gadd45b miR (pre- and post-) n = 9]. In contrast, local Gadd45b KD in this brain region reverses the CSDS-induced social avoidance behavior in susceptible mice [e, time effect: F(1,44) = 3.25, p = 0.078; genetic effect: F(1,44) = 1.669, p = 0.203; time × genetic effect: F(1,44) = 8.398, p < 0.01, HSV-GFP (pre- and post-) n = 11, HSV-Gadd45b miR (pre- and post-) n = 13]. After HSV-GFP infusion into NAc, resilient mice showed reduction of social interaction, whereas intra-NAc infusion of HSV-Gadd45b miR blocks this effect [f, time effect: F(1,40) = 6.534, p < 0.05; genetic effect: F(1,40) = 6.913, p < 0.05; time × genetic effect: F(1,40) = 4.411, p < 0.05, HSV-GFP (pre- and post-) n = 11, HSV-Gadd45b miR (pre- and post-) n = 11]. Mixed model two-way ANOVA with Fisher’s LSD post-hoc tests, *p < 0.05, **p < 0.01, ***p < 0.001. Bar graphs show mean ± SEM.
The behavioral effects of Gadd45b have been suggested to be mediated via its DNA demethylating properties19,20,21. In our results (Fig. 2e), Gadd45b induced-depressive phenotype effect was only shown in susceptible group. Since Gadd45b can modulate the molecular cascades regulating stress susceptibility by changing DNA methylation levels at specific gene loci18,19,we tested whether the reversal of CSDS-induced susceptibility by Gadd45b KD associates with changes in DNA methylation in a phenotype-, gene-, and locus-specific way in the NAc. We focused our investigation on the Gad1 (glutamate decarboxylase-1), Dlx5 (distal-less homeobox 5), Nrtn (neurturin), and Ntrk2 (TrkB) genes (Table 1), each of which has been shown previously to be associated with the effects of stress in both rodents and humans6,10,12,24.
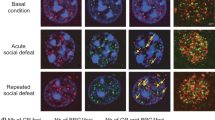
DNA methylation was assessed in the NAc at 23 CpGs within Gad1’s gene promoter region (chr2:70562278- 70562531; Fig. 3a,e). Our analysis shows significant main effects of phenotype, viral KD, and CpG with a significant viral KD by CpG interaction effect. Post-hoc analysis shows that Gadd45b KD significantly increased total DNA methylation levels within the Gad1 promoter region in control and susceptible mice (Fig. 3e). More interestingly, our post-hoc analysis highlighted several CpG sites for which Gadd45b KD changed DNA methylation levels (Fig. 3a). Specifically, our data show a significant upregulation of DNA methylation levels at CpG sites 2–3 and 4–5 and a significant downregulation at CpG sites 10–11. Additional sites of differential methylation are shown in Fig. 3a. Our data indicate that these alterations were associated with a trend toward a significant change in Gad1 expression in the NAc (viral KD main effect; Fig. 3i), although this change was not specific to any particular group.
Effects of CSDS and viral-mediated downregulation of Gadd45b on DNA methylation and on gene expression of stress-related genes in NAc. (a,e) DNA methylation at individual CpGs (a) and total DNA methylation (e) in the Gad1 gene promoter in the NAc of control (CTRL; white), susceptible (SUS; blue), and resilient (RES; green) mice with (dashed lines) and without (full box) Gadd45b viral KD [phenotype (F(2,137) = 4.996, p < 0.01), viral KD (F(1,137) = 17.648, p < 0.001), CpG (F(12,258) = 263.746, p < 0.001), viral KD by CpG interaction (F(12,258) = 4.320, p < 0.001)]. (i) Gad1 relative expression in NAc of CTRL, SUS, and RES mice with and without Gadd45b KD (F(1,34) = 2.101, p = 0.078). (b,f) DNA methylation at individual CpGs (b) and total DNA methylation (f) in the Dlx5 gene promoter in CTRL, SUS, and RES mice with and without Gadd45b KD [viral KD (F(1,237) = 15.421, p < 0.001), CpG site (F(20,414) = 74.633, p < 0.001), phenotype by CpG site interaction (F(40,410) = 2.378, p < 0.001)]. (j) Dlx5 relative expression in NAc of CTRL, SUS, and RES mice with and without Gadd45b KD (F(1,33) = 11.26, p < 0.005). (c,g) DNA methylation at individual CpGs (c) and total DNA methylation (g) in the Nrtn gene promoter in CTRL, SUS, and RES mice with and without Gadd45b KD [CpG site (F(12,268) = 37.918, p < 0.001), phenotype by viral KD (F(24,268) = 1.338, p < 0.05)]. (k) Nrtn relative expression in NAc of CTRL, SUS, and RES mice with and without Gadd45b KD (F(1,33) = 2.359, p = 0.067). (d,h) DNA methylation at individual CpGs (d) and total DNA methylation (h) in the Ntrk2 gene promoter in CTRL, SUS, and RES mice with and without Gadd45b KD [CpG (F(19,352) = 17.453, p < 0.001) and viral KD by CpG interaction (F(19,352) = 4.313, p < 0.001]. (l) Ntrk2 relative expression in NAc of CTRL, SUS, and RES mice with and without Gadd45b KD (F(1,33) = 6.337, p = 0.017). For (a–h) CTRL GFP n = 4, CTRL Gadd45b KD n = 6, SUS GFP n = 9, SUS Gadd45b KD n = 10, RES GFP n = 6, RES Gadd45b KD n = 5. Mixed model two-way ANOVA with Fisher’s LSD post-hoc tests, *p < 0.05, compared to the corresponding GFP group, #p < 0.05, compared to the corresponding Gadd45b KD group. For (i–l) CTRL GFP n = 5, CTRL Gadd45b KD n = 6, SUS GFP n = 8, SUS Gadd45b KD n = 10, RES GFP n = 6, RES Gadd45b KD n = 5. Mixed model two-way ANOVA with Fisher’s LSD post-hoc tests, #p < 0.05, compared to the corresponding Gadd45b KD group. Bar graphs show mean ± SEM.
We found similar effects in the NAc in the promoter region of the Dlx5 gene for which we covered 38 CpGs (Chr6: 6881849-6881515; Fig. 3b,f). Specifically, our analysis revealed significant viral KD and CpG site main effects with significant phenotype by CpG site interaction. Post-hoc analysis shows that Gadd45b KD increased significantly total DNA methylation in susceptible mice compared to GFP-treated susceptible mice (Fig. 3f). Here again, post-hoc analysis highlighted site-specific effects with significantly higher DNA methylation levels at CpG sites 1, 10, and 28 in HSV-Gadd45b miR compared to GFP-treated susceptible mice (Fig. 3b). Additional sites of differential methylation are shown in Fig. 3b. Furthermore, our results show that Gadd45b KD also significantly reduced Dlx5 mRNA expression (viral KD main effect) in the NAc of control, susceptible, and resilient mice compared to their GFP counterparts (Fig. 3j).
DNA methylation of the Nrtn gene promoter was evaluated on 22 CpGs (chr17:56757630- 56757402; Fig. 3c,g). Our analysis revealed significant CpG site and phenotype by viral KD interaction effects (Fig. 3c). Interestingly, our post-hoc analysis showed that Gadd45b KD significantly raised total DNA methylation levels in susceptible mice with no effect in control and resilient individuals (Fig. 3g). Similar to Gad1 and Dlx5, these alterations were associated with a trend toward a significant change in Nrtn gene expression in the NAc (viral KD main effect, Fig. 3k), although this change was not specific to any particular group.
Finally, we quantified DNA methylation levels in the NAc at 34 CpGs within the Ntrk2 gene promoter (Ntrk2: chr13:58806389-58806757). Our analysis highlighted CpG and viral KD by CpG interaction effects (Fig. 3d,h). In Ntrk2 gene promoter, our analysis revealed significantly lower DNA methylation levels in HSV-Gadd45b miR-treated susceptible mice compared to GFP susceptible mice at CpG sites 1 and 28 (Fig. 3d). Furthermore, our results show that Gadd45b KD significantly reduced Ntrk2 expression in the NAc of susceptible mice compared to GFP susceptible, control, and resilient animals (Fig. 3l).
Discussion
In the current study, we present results on the molecular mechanism underlying the expression of susceptibility to chronic social stress in mice. Our findings show that CSDS in mice induces the expression of the DNA demethylase, Gadd45b. Importantly, both the optogenetic stimulation of the VTA to NAc pathway and the local infusion of BDNF in the NAc, both of which induce stress susceptibility in mice3,4,5, induced the expression of Gadd45b in the NAc. Using a viral approach, we showed that downregulation of Gadd45b in the NAc reverses the susceptibility phenotype in chronically stressed mice, suggesting that elevated expression of Gadd45b is required for the maintenance of stress susceptibility. We showed further that this reversal (from susceptible to resilient) is associated with DNA methylation changes in the regulatory regions of several genes previously implicated in the effect of stress in both mice and humans. Overall, our findings suggest that alteration of Gadd45b expression in the NAc, resulting from dysregulation of the VTA to NAc pathway, is involved in mediating the behavioral effects of stress susceptibility in mice by interfering with the establishment of DNA methylation patterns at specific gene loci in the NAc.
CSDS in mice increases the activity of the VTA to NAc DA circuit in susceptible but not resilient mice3,23,25. Optical stimulation of dopaminergic projections from the VTA to the NAc induces stress susceptibility, as measured by reduced social interaction and other behavioral abnormalities3,4, through a BDNF-dependant signaling mechanism1,4,5,23. Indeed, we previously showed that the behavioral impact of VTA-NAc pathway stimulation is blocked by the intra-NAc administration of a Trkb antagonist but not DA receptor antagonists4. Together, this suggests that BDNF signaling in the NAc is a downstream mediator of the deleterious effects of sustained activation of VTA to NAc DA neurons induced by chronic stress. Our present results suggest that Gadd45b is one downstream molecular target of BDNF. Gadd45b expression was significantly upregulated in the NAc of susceptible mice following CSDS, an effect that was reproduced following either: 1) prolonged optical activation of VTA to NAc DA projections or 2) the intra-NAc infusion of BDNF, two procedures known to increase stress susceptibility and to reproduce the circuit alterations induced by CSDS. These findings thus establish Gadd45b as a novel downstream molecular target of BDNF in the NAc, which contributes to the behavioral effects of CSDS.
Previous studies highlighted the role of Gadd45b in juvenile behavior and pro-inflammatory cytokine production19, synaptic plasticity, learning and memory and fear conditioning20,21, and psychosis in human brain22. Here, we provide results supporting the pro-depressant role of Gadd45b following CSDS. Interestingly, Gadd45b KD also reversed the social avoidance observed in our susceptible group following intra NAc viral infusions. The delayed effects of stress have been observed before in other mouse models26,27,28,29,30 and could be explained by the delayed induction of a stress phenotype exacerbated by an intermittent re-exposure to an aggressor or by surgeries and their recovery. While unexpected, these effects were still reversed by Gadd45b KD supporting its role on mediating susceptibility to social stress. Importantly, we also provide mechanistic insight into the epigenetic mechanisms by which Gadd45b might mediate its behavioral effects. Consistent with the findings presented here, previous studies reported changes in DNA methylation within the Gad1 and Ntrk2 gene promoters in rodent models of stress and humans suffering from schizophrenia, bipolar disorder, or depression24,31,32. Dlx5 and Nrtn also came out as potential targets in DNA methylation and gene expression genome-wide profiling studies6,10,12. Altered methylation of these gene promoters could affect their transcriptional activity by regulating the binding of transcription factors and other transcriptional regulatory proteins. More work will be required to investigate these and other possible mechanisms downstream of the DNA methylation alterations found in our analyses.
The Gadd45b targets identified in this study include a GABAergic gene (Gad1), the BDNF TrkB receptor (Ntrk2), a gene important in VTA development (Nrtn), and Dlx5 which is involved in several facets of brain development, each of which has been linked to stress phenotypes in previous investigations24,31,32. However, these findings remain preliminary, as global genome-wide assessment of DNA methylation and gene expression following Gadd45b KD will provide a more complete picture of the downstream targets of Gadd45b in the NAc in the context of stress susceptibility. In this sense, DNMT3A overexpression in the NAc, altering genome-wide epigenetic patterns, has previously been shown to increase susceptibility to CSDS and subchronic variable stress (CVS) in males, while its downregulation promotes resilience in females10,12. Consistently, loci specific33 and genome-wide reorganization13,14,16 of DNA methylation profiles has been described in the brain of suicide completers and in the blood of PTSD patients17,34. However, while DNA methylation is cell-type specific, it is likely that Gadd45b downstream targets might vary in a cell-type specific fashion as well. In the NAc, this would ultimately interfere with the activity of D1-type or D2-type medium spiny neuron populations differently as the two populations express distinct transcriptional profiles35 and are known to be affected by stress differently4,36,37,38. Future work should address these issues.
To conclude, our findings support the idea of a dynamic, activity-dependent process regulating behavioral susceptibility via changes in the epigenetic regulation of gene expression in the NAc of stressed mice. By being a highly plastic, but relatively stable epigenetic mark, DNA methylation exerts a dynamic control over gene expression in response to neuronal activity. While several processes are likely involved, our results suggest that Gadd45b, by being a downstream target of BDNF in the NAc, is involved in mediating these effects. Further explication of this novel pathway will contribute to improving our understanding of the molecular mechanisms underlying stress susceptibility.
Methods
Experimental subjects
Male 7–12-week-old C57BL/6 J mice (25–30 g, Jackson) and 4–6 month old CD1 retired breeders (35–45 g, Charles River) were used. Mice were fed ad libitum at 22~25 °C on a 12-hr light/dark cycle. CD1 mice were singly housed except during social defeats. All C57BL/6J mice were group housed before social defeats and singly housed after social defeats.
Social defeat stress paradigm
CSDS was conducted as described previously4,23. Twenty-four hr after the last defeat, a social interaction test was performed. Based on social interaction ratios (time in interaction zone with social target/time in interaction zone without social target), mice were designated as susceptible or resilient: susceptible ratio <1; resilient ratio ≥1. For the effect of Gadd45b downregulation on social behaviors, HSV-Gadd45b miRNA (miR) or its control vector (HSV-GFP) were infused into the NAc 24 hr after the first social interaction test. Four days after the HSV infusion, the 2nd social interaction test was performed. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Icahn School of Medicine at Mount Sinai (# LA12-00051) and at the Korea Brain Research Institute (# IACUC-15-00026). All experiments were performed in accordance with the guidelines of the IACUC at the Icahn School of medicine at Mount Sinai and at the Korea Brain Research Institute. All efforts were made to minimize animal suffering and to reduce the number of animals used.
HSV vectors
Knockdown (KD) constructs designed to target Gadd45b mRNA were cloned using BLOCK-iT Pol II miR RNAi kit (Invitrogen). Briefly, four artificial miRNA oligonucleotides were designed using Invitrogen’s RNAi Designer (www.invitrogen.com/rnai) to KD Gadd45b and cloned into pcDNA6.2-GW. Mouse neuroblastoma (N2a) cells were transfected using lipofectamine 2000 (Invitrogen) with the different plasmids designed to target Gadd45b or LacZ as control. The level of Gadd45b mRNA KD was assessed using qRT-PCR 24 hr after transfection. The miRNA causing the most efficient downregulation was further Gateway cloned (Invitrogen) into the p1005 + HSV vector.
Stereotaxic surgeries
Stereotaxic surgeries were performed as described previously39. Mice were anesthetized with a mixture of ketamine (100 mg/kg/10 ml) and xylazine (10 mg/kg/10 ml) (Henry Schein) in sterile saline. HSVs (-Gadd45b miR or -GFP) or BDNF (0.25 µg/side, recombinant human BDNF, R&D Systems) were bilaterally infused into the NAc (AP = 1.5, ML = ±1.5, and DV = −4.4 mm; 10° angle), while an AAV2 vector expressing ChR2, fused with enhanced yellow fluorescent protein (AAV2-EYFP-ChR2, purchased from University of North Carolina Vector Core) or its control (AAV2-EYFP), was infused into the VTA (AP = −3.2, ML = ±1.0, and DV = −4.6 mm; 7° angle). An infusion volume of 0.5 µl was delivered using 5 μL Hamilton syringe (Hamilton Company) over the course of 5 min (at a rate of 0.1 μl/min). Mice were allowed to recover for 4 days following the HSV infusion or for 7 days following the BDNF infusion before going through behavioral assessment. For the optogenetic stimulation of the VTA-NAc pathway, optic fibers were bilaterally implanted into the NAc (AP = 1.5; ML = ±1.3; DV = −3.9; 0° angle), three weeks after AAV2-EYFP-ChR2 or AAV2-EYFP infusion into VTA. Mice were allowed to recover for seven days following the cannulation, and then stimulated in home cages.
Immunohistochemistry
Mice were anesthetized with a lethal dose of chloral hydrate and intracardially perfused with 0.1 M phosphate-buffered saline (PBS) and 4% (wt/vol) PBS-buffered paraformaldehyde 24 hr post social interaction test. Post-fixed brains were incubated overnight in 30% sucrose at room temperature before being sliced on a microtome at 40 μm on a microtome. Free-floating sections were washed with PBS and then blocked in 3% bovine serum albumin (BSA) and 0.3% Triton-X for 1 hr. For GFP labeling of localized HSV infusion, brain sections were incubated in 1:1000 of chicken anti-GFP (GFP-1020, Aves) in block solution overnight at 4 °C. The next day, sections were rinsed in PBS then incubated in 1:500 of donkey anti-chicken Cy2 (Immuno Research) in PBS for 1 hr then subsequently rinsed in PBS. All sections were counterstained and mounted with antifade solution, including DAPI then subsequently imaged on a LSM 710 confocal microscope.
DNA methylation - MassARRAY EpiTYPER assays
Genomic DNA from the NAc was extracted using the triple prep kit from QIAgene according to the manufacturer’s instruction. DNA was sent to the Innovation Center of Genome Quebec where it was treated with sodium bisulfite (Na-BIS) using the Epitech Bisulfite kit (QIAgen) and where Epityper40 was performed.
RNA Extraction and qRT-PCR
For RNA isolation, 14-gauge bilateral NAc punches were processed according to published protocol39. Primers were designed to amplify regions of 85–125 bp located within the target gene (Table 2). SYBR Green qRT-PCR was run in triplicate on the ABI7900HT Real-Time Cycler and analyzed using the ΔΔCt method as previously described41 with Gapdh as a normalization control. Gapdh mRNA expression was not altered among the groups in all experiments.
Data analysis
Data were analyzed with Prism 6.0 (GraphPad) and SPSS. Phenotype and Gadd45b expression following CSDS was assessed using one-way ANOVA followed by Fisher’s LSD post-hoc tests. The effect of BDNF infusion and optogenetic stimulation on Gadd45b expression was assessed using independent sample t-test. The impact of Gadd45b KD on social interaction was tested using a mixed model two-way ANOVA with phenotype and viral infusion as main factors followed by Fisher’s PLSD post-hoc tests. MassARRAY EpiTYPER data were analyzed using mixed models ANOVAs with phenotype, viral infection and CpG as main factors followed by Fisher’s LSD post-hoc tests. Gene expression following Gadd45b KD was assessed using mixed model two-way ANOVAs with phenotype and viral infusion as main factors followed by Fisher’s LSD post-hoc tests. All p values of < 0.05 were considered to be statistically significant. All data are expressed as mean ± SEM.
Ethics statement
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Icahn School of Medicine at Mount Sinai (# LA12-00051) and at the Korea Brain Research Institute (# IACUC-15-00026). All experiments were performed in accordance with the guidelines of the IACUC at the Icahn School of medicine at Mount Sinai and at the Korea Brain Research Institute. All efforts were made to minimize animal suffering and to reduce the number of animals used.
References
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Krishnan, V. & Nestler, E. J. Animal Models of Depression: Molecular Perspectives. Curr Top Behav Neurosci (2011).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Koo, J. W. et al. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol Psychiatry 80, 469–478 (2016).
Walsh, J. J. et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17, 27–29 (2014).
Bagot, R. C. et al. Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron 90, 969–983 (2016).
Labonte, B. et al. Sex-specific transcriptional signatures in human depression. Nat Med (2017).
Bagot, R. C., Labonte, B., Pena, C. J. & Nestler, E. J. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues in clinical neuroscience 16, 281–295 (2014).
Russo, S. J. & Nestler, E. J. The brain reward circuitry in mood disorders. Nat Rev Neurosci 14, 609–625 (2013).
Hodes, G. E. et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 35, 16362–16376 (2015).
Feng, J. et al. Tet1 in Nucleus Accumbens Opposes Depression- and Anxiety-Like Behaviors. Neuropsychopharmacology (2017).
LaPlant, Q. et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13, 1137–1143 (2010).
Labonte, B. et al. Genome-Wide Methylation Changes in the Suicide Brain. American Journal of Psychiatry Accepted (2012).
Labonte, B. et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry 69, 722–731 (2012).
Mill, J. et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 82, 696–711 (2008).
Nagy, C. et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20, 320–328 (2015).
Uddin, M. et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA 107, 9470–9475 (2010).
Ma, D. K. et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077 (2009).
Kigar, S. L., Chang, L. & Auger, A. P. Gadd45b is an epigenetic regulator of juvenile social behavior and alters local pro-inflammatory cytokine production in the rodent amygdala. Brain Behav Immun 46, 60–69 (2015).
Sultan, F. A., Wang, J., Tront, J., Liebermann, D. A. & Sweatt, J. D. Genetic deletion of Gadd45b, a regulator of active DNA demethylation, enhances long-term memory and synaptic plasticity. J Neurosci 32, 17059–17066 (2012).
Leach, P. T. et al. Gadd45b knockout mice exhibit selective deficits in hippocampus-dependent long-term memory. Learn Mem 19, 319–324 (2012).
Gavin, D. P. et al. Growth arrest and DNA-damage-inducible, beta (GADD45b)-mediated DNA demethylation in major psychosis. Neuropsychopharmacology 37, 531–542 (2012).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Zhang, T. Y. et al. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci 30, 13130–13137 (2010).
Cao, J. L. et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 30, 16453–16458 (2010).
Adamec, R. E., Blundell, J. & Collins, A. Neural plasticity and stress induced changes in defense in the rat. Neurosci Biobehav Rev 25, 721–744 (2001).
Buwalda, B. et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29, 83–97 (2005).
Franklin, T. C. et al. Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior. Biol Psychiatry 83, 50–60 (2018).
Kavushansky, A., Ben-Shachar, D., Richter-Levin, G. & Klein, E. Physical stress differs from psychosocial stress in the pattern and time-course of behavioral responses, serum corticosterone and expression of plasticity-related genes in the rat. Stress 12, 412–425 (2009).
Matuszewich, L. et al. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav 90, 674–681 (2007).
Kundakovic, M., Chen, Y., Costa, E. & Grayson, D. R. DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol 71, 644–653 (2007).
Veldic, M. et al. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA 101, 348–353 (2004).
Labonte, B. et al. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry 72, 41–48 (2012).
Labonte, B., Azoulay, N., Yerko, V., Turecki, G. & Brunet, A. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Transl Psychiatry 4, e368 (2014).
Lobo, M. K., Karsten, S. L., Gray, M., Geschwind, D. H. & Yang, X. W. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci 9, 443–452 (2006).
Heshmati, M. et al. Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc Natl Acad Sci USA 115, 1111–1116 (2018).
Dias, C. et al. beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516, 51–55 (2014).
Francis, T. C. et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77, 212–222 (2015).
Koo, J. W. et al. BDNF is a negative modulator of morphine action. Science 338, 124–128 (2012).
Coolen, M. W., Statham, A. L., Gardiner-Garden, M. & Clark, S. J. Genomic profiling of CpG methylation and allelic specificity using quantitative high-throughput mass spectrometry: critical evaluation and improvements. Nucleic Acids Res 35, e119 (2007).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
We gratefully acknowledge the contributions of Korea Brain Research Institute (KBRI) basic research program through KBRI funded by the Ministry of Science and ICT, South Korea (18-BR-02-03 to J.W.K.) and Basic Science Research Program through the National Research Foundation (NRF) of the Ministry of Education, South Korea (2015R1D1A1A01059602 to J.W.K.). Support was also provided by National Institute of Mental Health, NIH, USA (P50MH096890 to E.J.N.), Hope for Depression Research Foundation, USA (to E.J.N.), Fond de Recherche du Québec en Santé (FRQS) Junior 1 award (to B.L.), and Sentinelle-Nord Research Chair (B.L.) in Neurobiology of Mood Disorders at Laval University, Canada.
Author information
Authors and Affiliations
Contributions
B.L. and J.W.K. conceived and designed research. B.L., Y.H.J., E.P., O.I., M.F., O.E., K.C. and R.N. performed the experiments. B.L., Y.H.J., K.C. and J.W.K carried out the analyses, B.L., Y.H.J., J.W.K., and E.J.N. prepared figures, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Labonté, B., Jeong, Y.H., Parise, E. et al. Gadd45b mediates depressive-like role through DNA demethylation. Sci Rep 9, 4615 (2019). https://doi.org/10.1038/s41598-019-40844-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40844-8
This article is cited by
-
Genome-wide expression analysis reveals different heat shock responses in indigenous (Bos indicus) and crossbred (Bos indicus X Bos taurus) cattle
Genes and Environment (2023)
-
Methylation and expression of glucocorticoid receptor exon-1 variants and FKBP5 in teenage suicide-completers
Translational Psychiatry (2023)
-
Transcriptomic organization of the human brain in post-traumatic stress disorder
Nature Neuroscience (2021)
-
Regulation of dopamine-dependent transcription and cocaine action by Gadd45b
Neuropsychopharmacology (2021)
-
Molecular and neurocircuitry mechanisms of social avoidance
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.