Abstract
Among parasitic helminths, biological features of Echinococcus granulosus have been a focus of particular interest in biology and medicine. The determinants and underlying molecular mechanisms of Echinococcus development in different host settings is largely unknown. The phenomenal bi-directional development of E. granulosus protoscoleces into multi-proglottid and/or microcysts, is a fascinating feature of the parasite cultivation. Calmodulin (CaM) is the major intracellular Ca2+ binding protein in plant and animal organisms. Many Ca2+-related processes in the physiology of eukaryotic organisms are CaM-dependent, however little is known on the role of CaM in platyhelminths growth and development. Small interfering (si) RNA-induced manipulations of the genes involving in the parasite development is an opportunity to explore novel approaches for cystic echinococcosis (CE) prevention and management. Regarding the fundamental role of CaM in cellular function of the parasites, in this study, we investigated the molecular and morphological changes induced by siRNA on CaM in different in vitro stages of E. granulosus. Three developmental stages of the tapeworm, protoscoleces, microcysts and strobilated worms, were cultivated in vitro in mono- and di-phasic media and three delivery methods, i.e. electroporation, soaking and electro-soaking, were used for RNA interference. The level of mRNA suppression as well as the phenotypic changes of the parasites were measured. Following RNA interference, EgCaM mRNA suppressions of 65–99% were recorded in different stages of the tapeworm as compared to untreated/unrelated siRNA controls. Lower viability, growth retardation, morphological abnormalities as well as EgCaM expression suppression were documented in the parasite implying potential of siRNA technology for the prevention and management of CE.
Similar content being viewed by others
Introduction
E. granulosus is a zoonotic platyhelminth causing cystic echinococcosis (CE) in human and livestock. The life cycle of E. granulosus is complex and required two mammalian hosts, leading to many morphological, biochemical, and physiological alterations in the parasite. Echinococcus metacestode called hydatid cyst, develops in the viscera of herbivorous intermediate hosts (livestock and humans) and can infect carnivorous final host (most commonly dogs) via feeding with infected organs. This parasite has a worldwide distribution and it has been estimated to infect 2–3 million people across the globe1.
The gravid worm shed eggs in dog small intestine which are disseminated in the environment via defecation. Infection of the intermediate host occurred through ingestion of infective eggs, followed by the release of oncospheres in the small intestine. The oncosphere subsequently migrates via the portal system to various organs and tissues, mainly liver and lungs, where it develops into a unilocular, fluid-filled cyst comprised of an inner germinal layer which responsible for production of the infective stage, a structure called protoscolex (PSC), an extracellular matrix in the middle called laminated layer that is unique to the genus Echinococcus, and an external host-derived adventitial layer formed in response to the parasite2. As a model organism Echinococcus granulosus has been demonstrated as a valuable biological entity influencing advancements in developmental biology of flatworms3.
Following Echinococcus cultivation in appropriate diphasic and/or monophasic media the phenomenon of bi-directional development of E. granulosus protoscoleces into multi-proglottid and/or microcysts, is a fascinating feature of Echinococcus biology. This provides us an opportunity to study genes involving in the parasite development and to manipulate the parasite for disease management and prevention4,5,6. Understanding the determinants and molecular mechanisms in the parasite development in different host settings offers suitable tools for prevention and control of CE2.
Calmodulin (CaM), is a multifunctional intermediate calcium sensor protein expressed in all eukaryotic organisms. The functions of CaM include Ca2+ binding and alteration of calcium signal transduction pathway to regulate a multitude of biological processes, such as cytoskeletal assembly/reorganization, activation of phosphorylase kinase, abiotic stress responses, neurotransmission, smooth muscle contraction, metabolism and cell motility7,8. Wang et al. have used Immunohistochemical localizations on different stages of the parasite, suggesting the expression of E. granulosus CaM (EgCaM) in the tegument tissues, protoscoleces parenchymal region, germinal layer as well as in the adult stage. The study showed physiologically active regions for EgCaM expression and suggested the crucial role of Ca2+ signaling pathway in the growth and development of the helminth and its function between E. granulosus and its host9.
In recent decade, there has been increasing interest in RNA interference (RNAi) technology in the field of biology and development. Our understanding of the genetic basis of development in invertebrates is limited. Small interfering RNAs (siRNAs) provides us a valuable tool to improve our knowledge on the developmental biology of Platyhelminthes10. Molecular methods based on RNAi or Post-Transcriptional Gene Silencing (PTGS) are the new millennium tools that provide information on gene function and characterization of the genes involving in regeneration and development. RNA interference mediated by siRNA with perfect homology to their target can cause silencing of specific genes for experimental and therapeutic purposes11,12.
RNAi has been successfully applied to numerous helminth organisms to date. Silencing of the gene encoding Fasciola hepatica leucine aminopeptidase (LAP) with siRNAs as well as double-stranded RNAs (dsRNA), suppressed mRNA and protein expression for up to 3 weeks in the newly excysted juveniles13. RNAi has been used to suppress a number of E. multilocularis endogenous genes expression in protoscoleces14. Protoscoleces electroporation of genes encoding the 14–3–3 and elp with siRNA resulted in reduced expression of up to 21.8 and 35.5% of the genes respectively, compared to untreated control. In addition, the target proteins have been significantly reduced on day 1514.
CaM-specific RNAi technology has been utilized to determine the functional importance of calmodulin in several helminth parasites, however there are very few data on the role of CaM function in E. granulosus. There are reports for schistosomes15,16, the liver flukes Fasciola gigantica and F. hepatica17,18 and Caenorhabditis elegans19. Suppression of calmodulin mRNA by feeding Schistosoma mansoni with dsRNA resulted in a phenotype characterized by waves of contraction in adult worms but not in schistosomula16. RNAi has also been used to determine the biological function of F. hepatica calmodulin in vitro. Stunted growth and lower viability were documented after treating the in vitro cultured flukes with dsRNAs and siRNA20. The purpose of the present study was to determine the effect of RNA interference on EgCaM expression in different developmental stages of E. granulosus in vitro.
Results
In vitro cultivation of protoscoleces
The parasite was characterized using PCR-sequencing and identified as the G1 genotype of E. granulosus (Accession Number MG832791). E. granulosus protoscoleces were successfully cultivated in both monophasic and diphasic media. The protoscoleces started to develop proglottization in diphasic culture and the first proglottids were observed on day 28. However, the full grown three-proglottid strobilated forms were developed after 55 days of cultivation. Egg production in the terminal proglottid was not observed under the culture conditions (Fig. 1). In monophasic condition the protoscoleces were developed into conspicuous microcysts after 45 days. The culture was characterized by wide proliferation of parasite vesicles with no development of new protoscoleces.
The effects of suppressed calmodulin on the protoscoleces
Previous reports had demonstrated the activity of experimental RNAi in protoscoleces, either by electroporation or soaking14,21. Initially, we focused on uptake of fluorescently labeled siRNA in protoscoleces by fluorescent microscope. Fluorescently labeled siRNA was detected in treated parasites comparing to no or very low level of autofluorescence in untreated parasites (Fig. 2a).
Representative images of different in vitro stages of Echinococcus granulosus showing siRNA uptake of the parasites under light and fluorescent microscopy. (a–c) siRNA uptake by protoscoleces (a), microcyst (b) and strobilated worm (c) in comparison to the corresponding Negative Control siRNA images (d–f). Scale bar = 200 μm.
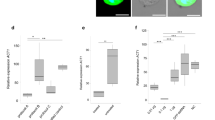
Following RNA interference, 69 to 99% EgCaM mRNA suppression was recorded as compared to untreated controls (Fig. 3a). We found that electro-soaking method exerted the strongest transcript level suppression among three delivery methods (Table 1). Hence the gene expression data for negative control siRNA (siR-Ctrl) were only demonstrated for electro-soaking method. There was no significant difference in gene silencing between the negative control siRNA and no-treatment control groups.
Molecular (a–c) and biological (d–f) changes induced by Echinococcus granulosus Calmodulin (EgCaM)-specific siRNA using three delivery methods in different developmental stages in vitro. (a–c) EgCaM expression profile in different in vitro stages: protoscoleces (PSC) 3 and 8 days after electroporation (EP), soaking (SK) and electro-soaking (ES); microcysts (MC) and strobilated worms (SW). (d) Viability changes of protoscoleces treated with EgCaM-specific siRNA. (e) Size changes of 30 protoscoleces treated with EgCaM-specific siRNA. (f) Changes in body contractions per minute in the strobilated worms treated with EgCaM-specific siRNA. Data was compared to the controls: control protoscoleces (Ctrl) and negative siRNA control (siR-Ctrl). The gene expression data for siR-Ctrl were only demonstrated for electro-soaking method. Bars show the mean ± standard deviation (SD) derived from duplicates experiments. (**P < 0.01, ***P < 0.001, ****P < 0.0001).
The effect of EgCaM suppression on viability and size changes in protoscoleces was evaluated after siRNA treatment. Our results indicated the remarkable effect of EgCaM suppression on the viability of protoscoleces on day 8 post treatment. As shown in Fig. 3d, the viability of the parasites was decreased to 3–49% depending on different delivery methods. The protoscoleces were most affected by RNA interference using electro-soaking so that no protoscoleces were viable after three days post treatment.
Morphometric data are indicative of growth inhibition in siRNA-treated protoscoleces. A significant growth retardation was observed after day 8 in the protoscoleces exposed to siRNA compared to the control parasites (Fig. 3e). The protoscoleces size in the control groups were increased towards microcyst development (mean size of protoscoleces: control = 389 ± 14.5 μM; negative siRNA = 375 ± 17.4 μM) while the growth and development of siRNA-treated parasites were inhibited (mean size = 224 ± 10.07 μM).
The effects of suppressed calmodulin on the microcyst
Figure 2b shows the fluorescence produced by siRNA incorporation into the microcyst wall. Relative expression of EgCaM eight days after siRNA delivery was reduced by 99% (Fig. 3b, Table 1).
Some morphological changes were noticed in siRNA-treated microcysts compared to the control groups. As shown in Fig. 4a the treated microcysts were noticeably darker with irregular outer layer compared to the controls with transparent microcysts and smooth surface. The electro-soaked microcysts were the most affected group when compared with microcysts treated by soaking or electroporation.
Morphological changes in Echinococcus granulosus treated with Calmodulin (EgCaM)-specific siRNA in vitro. Effect of EgCaM suppression on microcysts (a) and strobilated worms (b) using three delivery methods, electroporation (EP), soaking (SK), electro-soaking (ES) compared to the negative siRNA control (siR-Ctrl). Note the morphological changes (arrowheads) in the microcysts (outer layer irregularities and shrinkage, malformations and darkened edges) and the strobilated worms (morphological abnormalities and body swelling) compared to the controls. Scale bar = 200 μm.
The effects of suppressed calmodulin on the strobilated forms
There was successful uptake of fluorescently labeled target siRNA by strobilated forms cultured in vitro. This was visible throughout the helminth body. In comparison to the metacestode stages we observed high levels of autofluorescence in the strobilated control groups (no-treatment and unrelated negative siRNA controls), however the fluorescence pattern was distinct and readily distinguishable in the parasites tegument treated with siRNA. (Fig. 2c).
siRNA treatment of the strobilated forms induced a range of 65 to 89% suppression of mRNA for EgCaM as assessed after 8 days post treatment (Fig. 3c, Table 1). As shown in Fig. 4b EgCaM silencing led to severe morphological abnormalities and reduced activity compared to the both controls. Seven days after EgCaM silencing the helminths were severely damaged after electroporation and electro-soaking.
As shown in Fig. 3f significant decrease in body contractions in the strobilated worms was noted, indicating the contribution of EgCaM silencing by siRNA. In the electro-soaking group while on day 2, on average 20 contractions per minute were recorded, on day 5 no body contractions were found, compared to the untreated control and negative control siRNA with an average of 47 and 46 contractions per minute respectively.
Discussion
RNA-dependent gene silencing has been increasingly utilized as a reverse-genetics approach in a variety of helminth organisms to generate functional gene knockdowns22. RNAi provides us with a powerful tool for predicting potential roles of genes involved in host-parasite interactions and vital processes essential for survival in several helminths such as Brugia malayi23,24,25 Ascaris suum26,27 C. elegans19,28, Schistosoma species29,30,31 and F. hepatica13,32. Although RNAi has been widely used for silencing genes in nematodes and trematodes, there are few studies applying RNAi on cestode parasites e.g. adult Moniezia expansa33, and protoscoleces of E. multilocularis14 and E. granulosus21. In the present study RNA interference technology was applied to suppress CaM in E. granulosus. Although CaM has been extensively studied and well characterized in several parasitic and free-living helminths7,16,19, there are very few data on the function and biological properties of CaM in E. granulosus. In the current investigation, we used siRNA sequences for EgCaM suppression to observe effects on different developmental stages of E. granulosus in vitro. All developmental stages of E. granulosus delivered with EgCaM-specific siRNA showed a robust suppression in EgCaM gene expression leading to obvious phenotypic changes (Figs 3 and 4). These results suggested a probable role for EgCaM in the growth and development ofE. granulosus.
Suppression in CaM gene expression by siRNA and dsRNA has been investigated in a number of platyhelminthes including S. mansoni16, F. hepatica7,20 as well as the free-living nematode, C. elegans19. The results indicate CaM, as a transporter of Ca2+, plays an important role in the helminths growth, motility and metabolism.
Using different siRNA delivery methods produced a consistent suppression at mRNA level. The strongest effect (at least 92% suppression) was observed following electro-soaking in all developmental stages of E. granulosus (Table 1). It has been shown that electroporation produces pores in some biological membranes, favoring entrance of siRNA in the transfection buffer13.
We observed a robust suppression in EgCaM gene expression, resulted in phenotypic changes and viability and growth inhibition in siRNA-treated protoscoleces (Fig. 3). Mizukami et al. successfully suppressed 14-3-3 and elp genes expression by 21.8% and 35.5% respectively. Also, in the analysis of viability, 14-3-3, and elp siRNA-treated samples displayed 58.0 ± 23.0, and 55.1 ± 14.6% viability on day 15, respectively, compared to the untreated control14. This is in line with our data on the viability of protoscoleces after siRNA-mediated CaM suppression (Fig. 3d).
Our findings indicate that EgCaM specific siRNA in the protoscoleces induces growth retardation and inhibits protoscoleces transformation to microcysts compared to the controls (Fig. 3e). This imply that the reduction in CaM expression may prevent growth and development processes as shown in previous studies that linked CaM with the growth and development of other helminth species including miracidial transformation to sporocysts in S. mansoni15 and reduced growth in the free-living nematode C. elegans19 and the liver fluke Fasciola hepatica20. The growth retardation induced by RNAi has been demonstrated also in several other genes in S. manson34,35,36. Tegument malformations have been documented after using Eg-TSP1-specific siRNA on the soaked protoscoleces of E. granulosus21.
In the current study RNAi-based interventions were associated with strong suppression of EgCaM in siRNA-treated microcysts, causing shrinkage and darkness in the outer wall of the microcysts (Figs 3b and 4a). Wang et al. showed EgCaM expression in the germinal layer and speculated that an unknown calcium-dependent mechanism occurs in this layer9. More in-depth investigations are needed on the probable role of CaM in the stability of the microcyst wall and germinal layer.
After 8 days of siRNA treatment on the strobilated forms, 65–89% EgCaM expression suppression was observed (Table 1). In a recent study conducted on S. mansoni by Guidi et al., Sm-CaM suppressions ranging from 70 to 95% were documented using dsRNA. Waves of contraction/dilation in the adult worms were also reported in the treated worms16. Another study showed siRNA-mediated knockdown of a 24 kDa calcium-regulated heat-stable protein (CRHSP-24) in juvenile S. japonicum, inducing death or changes in the parasite morphology37.
In the strobilated worms we noted significant phenotype changes including severe morphological abnormalities and reduced body contractions from day 2 onwards (Fig. 4b). It seems the suppression of EgCaM in the strobilated forms of E. granulosus leads to decreased motility and strobilar contractions (Fig. 3f). While parasites in the untreated control and negative siRNA groups were quite active with normal contractions, no body contractions were found in their siRNA-treated counterparts. The association of motility and CaM expression is well-documented in the literature. In 1990s, Ashizawa et al. were first to demonstrate disturbed motility of spermatozoa after experimental disruption of CaM function38. In general, Ca2+-dependent protein kinases like Calmodulin, MAPK and Polo-Like Kinase (PLK) are proved to play a key role in the motility-related functions in invertebrates16,39,40. However, using both RNAi and the CaM inhibitor, Trifluoperazine, McCammick et al. showed a significant increase in motility, migration and movement of the juvenile F. hepatica20.
Generalizations of in vitro data to in vivo experiments is difficult. Therefore, cautions have to be made in the interpretation of culture data at the host level41. As in vitro-reared E. granulosus do not develop as much as they do in the hosts, we could not precisely predict the behavior of the helminth in its natural habitat in the host42. As the dsRNAs are generally induced more persistent effects on target genes, this study would be supplemented by using an EgCaM-specific dsRNA. However, using dsRNAs has its own limitations and challenges43,44. Another prospect for RNAi-induced gene silencing in E. granulosus is to investigate the interference impacts at protein level using western blotting and other protein-based tools as well as further in-depth in vivo studies followed by deep sequencing analyses.
The present study used siRNA for suppressing EgCaM in different developmental stages of E. granulosus. The results indicate the significant effect of siRNA treatments on the phenotype as well as the gene expressions in all development stages of E. granulosus cultured in mono- and di-phasic media using different delivery methods. The study demonstrated that EgCaM is essential for viability, growth and development of the protoscoleces. Different phenotypic changes and transcript knockdowns observed in the present study encourage further investigations towards the development of novel therapeutic agents against cystic echinococcosis.
Methods
In vitro cultivation of protoscoleces
Parasite specimens were obtained by dissection of livers from naturally infected sheep at the municipal abattoir of Kerman, southeastern Iran with consent from animal handlers and the abattoir veterinary officer. Animals were slaughtering as part of the normal daily practice in the abattoir. The study was approved by the University Ethics Review Committee (code 95000288). All procedures were performed according to the University guidelines and regulations. The infected organs were immediately transferred to the Helminthology Lab, Dept of Medical Parasitology, Kerman University of Medical Sciences. After careful examinations of fertility and viability, one single viable cyst was finally selected for further experiments. Genotyping has been carried out using mitochondrial cox1 PCR-sequencing and the sequence was submitted to GenBank. Protoscoleces used for in vitro cultivation, were obtained from a single sheep liver cyst. Therefore, the microcysts and strobilated worms were derived from the cultivation of protoscoleces obtained from the same cyst.
Under sterile conditions the hydatid fluid containing protoscoleces was aspirated with a 50 ml syringe and then the laminated/germinal layer were removed. The aspirate as well as the cyst layers were carefully washed five times with PBS containing 100 U/ml penicillin and 100 μg/ml streptomycin (PBS-PS). The number of protoscoleces per ml was adjusted to 1 × 104 protoscoleces in 0.9% NaCl solution with a viability rate of at least 95%. Before cultivation the viability of the protoscoleces was checked by 0.1% aqueous eosin under a light microscope44,45. Two layers of sterile gauze were used to release protoscoleces from the brood capsules.
The protoscoleces were used for in vitro culture in diphasic and monophasic media to reach the strobilated as well as the microcyst forms, respectively, according to the method described by Smyth et al.5,45. The diphasic medium was S.10E.H, consisted of two phases: (i) the liquid phase, containing 260 mL of CMRL 1066 medium (Gibco, Grand Island, NY,), 100 mL of heat-inactivated fetal calf serum (FCS, Gibco-BRL, Gaithersburg, MD), 36 mL of 5% yeast extract (Sigma-Aldrich, St. Louis, MO) in CMRL 1066, 5.6 mL of 30% glucose (Sigma-Aldrich) in distilled water, 1.4 mL of 5% dog bile in PBS, 20 mM HEPES (Sigma-Aldrich), 10 mM NaHCO3 supplemented with penicillin (100 IU/mL), streptomycin (100 mg/mL), and (ii) the solid phase that is bovine serum coagulated at 76 °C for 20–30 min42,46. The medium was changed every 7 days and viability and morphological development of the parasite was observed under an invert microscope (TCM 400, Labomed Inc., CA).
For monophasic culture the protoscoleces were cultivated in Dulbecco’s minimal essential medium (DMEM) (Gibco, Grand Island, NY) containing 10% heat-inactivated FBS (Gibco), 2 mM glutamine (Sigma-Aldrich), penicillin (100 IU/ml), and streptomycin (100 mg/ml) at the bottom of 25 cm2 flasks incubated at 37 °C with 5% CO2. The medium was changed every 8 days. The flasks containing the protoscoleces were monitored weekly under an optical microscope to check the growth status of the microcysts5,42,47,48.
Designing and Synthesis of small interfering RNA
We used several online softwares for siRNA design i.e. siDirect (sidirect2.rnai.jp/), siRNA Design (Integrated DNA Technologies, IDT), BLOCK-iT RNAi Designer (www. invitrogen.com/rnai), and RNA wizard (https://www.invivogen.com/sirnawizard). After prediction, all siRNAs were manually re-checked relative to their position, target site, length of siRNA, nucleotide content and specificity of siRNA (off targets), and finally the best was chosen. The siRNA was synthesized commercially by TAG Copenhagen A/S (Copenhagen, Denmark). The sequences of the EgCaM siRNA were as follows: sense 5′UCGUUAAAGUCAAUAACACCC3′-fluorescein and antisense 5′GUGUUAUUGACUUUAACGAAU3′ –fluorescein. Another negative (non-silencing) control siRNA was purchased as an unrelated siRNA (Qiagen, Germany).
siRNA delivery and phenotype studies
Five sets of experiments were designed for each of three developmental stages of E. granulosus: (1) no-treatment group without any RNAi intervention, (2) unrelated/irrelevant Negative Control siRNA (siR-Ctrl) group that was transfected into the parasites using three transfection techniques i.e. soaking, electroporation and electro-soaking, (3) EgCaM-specific siRNA group delivered by soaking, (4) EgCaM-specific siRNA group delivered by electroporation and (5) EgCaM-specific siRNA group delivered by electro-soaking13,14,21,49. EgCaM-specific and Negative Control siRNAs were delivered into the parasites of corresponding treatment groups using three transfection techniques. No interventions were made on the parasites in no-treatment groups. For siRNA delivery, in 6-well plates different stages of the parasite with three separate batches of 5000 protoscoleces, 15 microcysts and 15 strobilated worms were treated by soaking, electroporation and electro-soaking in the presence of 50 nM/ml of fluorescent siRNA50. For soaking, protoscoleces were cultured in 2.5 ml DMEM supplemented with siRNAs and a lab-made transfection reagent was then added to each well and maintained for 6 hours51. For electroporation, protoscoleces were removed from the culture, resuspended in 200 μl of electroporation buffer containing the fluorescently labeled siRNA to give a final concentration of 50 nM, transferred to a 2 mm gap cuvette, and were subjected to time constant protocol (125 V, 20 ms) using Gene Pulser II (Bio-Rad, CA)13,16,31. For electro-soaking following electroporation, the parasites were maintained in a culture media containing siRNAs for 6 hours. After treatment, the parasites were rinsed thoroughly in PBS to remove unincorporated siRNA molecules. A fluorescent microscope (HB-10101AF, Nikon, Japan) was used to examine siRNA delivery. The parasites viability, as revealed by flame cell activity, were recorded daily for eight days and any phenotypic changes e.g. motility, transparency and tegumental alterations were examined. In addition, the size of the protoscoleces was measured using a calibrated microscope. The motility of the strobilated worms from each group of target siRNA and controls were measured as the number of body contractions per minute (see the Supplementary Fig. S1).
Gene expression assay
Total RNA was extracted from different developmental stages of the parasite (protoscoleces, microcysts and three or more proglottids) using a commercial kit (RNeasy Mini Kit, Qiagen, Germany). Furthermore, RNA was quantified by measuring the absorbance ratios at 260 and 280 nm by spectrophotometry (Nano Drop ND-1000, Nano Drop Technologies, Wilmington, DE). cDNA was then synthesized from 100 ng RNA in a total volume of 20 μl using miscript®II Reverse Transcriptase Kit (Qiagen, Germany) according to the manufacturer’s instructions.
Real-time qPCR (RT-qPCR) was carried out in a Rotor-Gene Q System (QIAGEN, Hilden, Germany). The primers specifically designed for EgCaM RT-qPCR were CalF (5′-GAAGGA TAC CGA TAG TGA GGA AGA-3′ and CalR 5′-ATC ATT TCG TCA ACC TCC TCG TC-3′). The primers of the ACTB (encoding β-actin) were ACTBF (5′-ATG GTT GGT ATG GGA CAA AAG G-3′ and ACTBR 5′- TTC GTC ACA ATA CCG TGC TC-3′). Relative quantification of gene expression levels was carried out by using SYBR green PCR Master Mix with 5 µl 2X QuantiNova SYBR Green PCR, 0.4 µM primer, 2 µl 0.5X diluted Template cDNA and 2 µl RNase-free water with a final volume of 10 µl. All samples were run in duplicate and underwent 40 cycles of 95 °C, 2 min for initiation, 94 °C, 5 sec for denaturation, and 60 °C, 10 sec for annealing.
For relative quantification, 2−ΔΔCT method was employed, using β-actin as the reference gene for each sample. Results obtained from the parasites treated with the unrelated Negative Control siRNA were used as calibrators. Negative Control siRNA target gene was unknown, as it is the proprietary information of Qiagen®. According to the manufacturer, the unrelated siRNA has been proven to have no significant effect on cell proliferation, viability, or morphology.
Statistical analysis
Differences between and within groups were assessed for statistical significance using one-way or two-way ANOVA test using GraphPad Prism 7.0 Software (www.graphpad.com). P values of less than 0.05 were considered significant.
Data Availability
The data of the present study will be available online.
References
Craig, P. S. et al. Prevention and control of cystic echinococcosis. The Lancet infectious diseases 7, 385–394 (2007).
Thompson, R. Biology and systematics of Echinococcus. In Advances in parasitology, Vol. 95 65–109 (Elsevier, 2017).
Thompson, R. & Jenkins, D. Echinococcus as a model system: biology and epidemiology. International journal for parasitology 44, 865–877 (2014).
Smyth, J., Howkins, A. B. & Barton, M. Factors controlling the differentiation of the hydatid organism, Echinococcus granulosus, into cystic or strobilar stages in vitro. Nature 211, 1374–7 (1966).
Smyth, J. Studies on tapeworm physiology: XI. In vitro cultivation of Echinococcus granulosus from the protoscolex to the strobilate stage. Parasitology 57, 111–133 (1967).
Koziol, U. & Brehm, K. Recent advances in Echinococcus genomics and stem cell research. Veterinary parasitology 213, 92–102 (2015).
Russell, S. L., McFerran, N. V., Hoey, E. M., Trudgett, A. & Timson, D. J. Characterisation of two calmodulin-like proteins from the liver fluke, Fasciola hepatica. Biological chemistry 388, 593–599 (2007).
Berchtold, M. W. & Villalobo, A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1843, 398–435 (2014).
Wang, N. et al. Molecular and biochemical characterization of calmodulin from Echinococcus granulosus. Parasites & vectors 10, 597 (2017).
Kalinna, B. H. & Brindley, P. J. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends in parasitology 23, 197–204 (2007).
Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Lochmatter, D. & Mullis, P.-E. RNA interference in mammalian cell systems. Hormone research in paediatrics 75, 63–69 (2011).
Dell’Oca, N. et al. RNA interference in Fasciola hepatica newly excysted juveniles: Long dsRNA induces more persistent silencing than siRNA. Molecular and biochemical parasitology 197, 28–35 (2014).
Mizukami, C. et al. Gene silencing in Echinococcus multilocularis protoscoleces using RNA interference. Parasitology international 59, 647–652 (2010).
Taft, A. S. & Yoshino, T. P. Cloning and functional characterization of two calmodulin genes during larval development in the parasitic flatworm Schistosoma mansoni. The Journal of parasitology 97, 72–81 (2011).
Guidi, A. et al. Application of RNAi to genomic drug target validation in schistosomes. PLoS neglected tropical diseases 9, e0003801 (2015).
McVeigh, P. et al. RNAi dynamics in juvenile Fasciola spp. liver flukes reveals the persistence of gene silencing in vitro. PLoS neglected tropical diseases 8, e3185 (2014).
Anandanarayanan, A. et al. RNA interference in Fasciola gigantica: Establishing and optimization of experimental RNAi in the newly excysted juveniles of the fluke. PLoS neglected tropical diseases 11, e0006109 (2017).
Karabinos, A. et al. Functional analysis of the single calmodulin gene in the nematode Caenorhabditis elegans by RNA interference and 4-D microscopy. European journal of cell biology 82, 557–563 (2003).
McCammick, E. M. et al. Calmodulin disruption impacts growth and motility in juvenile liver fluke. Parasites & vectors 9, 46 (2016).
Hu, D. et al. Molecular insights into a tetraspanin in the hydatid tapeworm Echinococcus granulosus. Parasites & vectors 8, 311 (2015).
Liu, Q. & Paroo, Z. Biochemical principles of small RNA pathways. Annual review of biochemistry 79, 295–319 (2010).
Aboobaker, A. A. & Blaxter, M. L. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Molecular and Biochemical Parasitology 129, 41–51 (2003).
Singh, M., Singh, P. K. & Misra-Bhattacharya, S. RNAi mediated silencing of ATPase RNA helicase gene in adult filarial parasite Brugia malayi impairs in vitro microfilaria release and adult parasite viability. Journal of biotechnology 157, 351–358 (2012).
Misra, S., Gupta, J. & Misra-Bhattacharya, S. RNA interference mediated knockdown of Brugia malayi UDP-Galactopyranose mutase severely affects parasite viability, embryogenesis and in vivo development of infective larvae. Parasites & vectors 10, 34 (2017).
Islam, M. K., Miyoshi, T., Yamada, M. & Tsuji, N. Pyrophosphatase of the roundworm Ascaris suum plays an essential role in the worm’s molting and development. Infection and immunity 73, 1995–2004 (2005).
Chen, N. et al. Ascaris suum: RNAi mediated silencing of enolase gene expression in infective larvae. Experimental parasitology 127, 142–146 (2011).
Zhuang, J. J. & Hunter, C. P. RNA interference in Caenorhabditis elegans: uptake, mechanism, and regulation. Parasitology 139, 560–573 (2012).
Cheng, G.-F. et al. Dose-dependent inhibition of gynecophoral canal protein gene expression in vitro in the schistosome (Schistosoma japonicum) by RNA interference. Acta biochimica et biophysica Sinica 37, 386–390 (2005).
Bhardwaj, R., Krautz-Peterson, G. & Skelly, P. J. Using RNA interference in Schistosoma mansoni. in Therapeutic Oligonucleotides 223–239 (Springer, 2011).
Rinaldi, G. et al. Genetic manipulation of Schistosoma haematobium, the neglected schistosome. PLoS neglected tropical diseases 5, e1348 (2011).
McGonigle, L. et al. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. International journal for parasitology 38, 149–155 (2008).
Pierson, L. et al. RNA interference in a cestode reveals specific silencing of selected highly expressed gene transcripts. Int J Parasitol 40, 605–15 (2010).
Correnti, J. M., Brindley, P. J. & Pearce, E. J. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Molecular and biochemical parasitology 143, 209–215 (2005).
Dinguirard, N. & Yoshino, T. P. Potential role of a CD36-like class B scavenger receptor in the binding of modified low-density lipoprotein (acLDL) to the tegumental surface of Schistosoma mansoni sporocysts. Molecular and biochemical parasitology 146, 219–230 (2006).
de Moraes Mourão, M., Dinguirard, N., Franco, G. R. & Yoshino, T. P. Phenotypic screen of early-developing larvae of the blood fluke, Schistosoma mansoni, using RNA interference. PLoS Neglected Tropical Diseases 3, e502 (2009).
Zou, X. et al. RNAi silencing of calcium-regulated heat-stable protein of 24 kDa in Schistosoma japonicum affects parasite growth. Parasitology research 108, 567–572 (2011).
Ashizawa, K., Tomonaga, H. & Tsuzuki, Y. Regulation of flagellar motility of fowl spermatozoa: evidence for the involvement of intracellular free Ca2+ and calmodulin. Journal of reproduction and fertility 101, 265–272 (1994).
Batters, C. et al. Calmodulin regulates dimerization, motility, and lipid binding of Leishmania myosin XXI. Proceedings of the National Academy of Sciences 111, E227–E236 (2014).
Mukhopadhyay, A. G. & Dey, C. S. Role of calmodulin and calcineurin in regulating flagellar motility and wave polarity in Leishmania. Parasitology research 116, 3221–3228 (2017).
Krautz-Peterson, G. et al. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PLoS pathogens 6, e1000932 (2010).
Dezaki, E. S. et al. Comparison of ex vivo harvested and in vitro cultured materials from Echinococcus granulosus by measuring expression levels of five genes putatively involved in the development and maturation of adult worms. Parasitology research 115, 4405–4416 (2016).
Krautz-Peterson, G., Radwanska, M., Ndegwa, D., Shoemaker, C. B. & Skelly, P. J. Optimizing gene suppression in schistosomes using RNA interference. Molecular and biochemical parasitology 153, 194–202 (2007).
Yu, N. et al. Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect science 20, 4–14 (2013).
Smyth, J. & Davies, Z. In vitro culture of the strobilar stage of Echinococcus granulosus (sheep strain): A review of basic problems and results. International Journal for Parasitology 4, 631–644 (1974).
Dezaki, E. S. et al. Differential Expression of Hox and Notch Genes in Larval and Adult Stages of Echinococcus granulosus. The Korean journal of parasitology 54, 653 (2016).
Hemphill, A. & Gottstein, B. Immunology and morphology studies on the proliferation of in vitro cultivatedEchinococcus multilocularis metacestodes. Parasitology Research 81, 605–614 (1995).
Hemphill, A. et al. Culture of Echinococcus multilocularis metacestodes: an alternative to animal use. Trends in parasitology 18, 445–451 (2002).
Han, Q. et al. Suppression of VAMP2 Alters Morphology of the Tegument and Affects Glucose uptake, Development and Reproduction of Schistosoma japonicum. Scientific Reports 7, 5212 (2017).
Nicholas, P. et al. Serotonin Signaling in Schistosoma mansoni: A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement. Plos pathogen 10, e1003878 (2014).
Afgar, A. et al. MiR-339 and especially miR-766 reactivate the expression of tumor suppressor genes in colorectal cancer cell lines through DNA methyltransferase 3B gene inhibition. Cancer biology & therapy 17, 1126–1138 (2016).
Acknowledgements
The authors wish to thank the veterinary staff of the municipal abbatoir for providing the cyst materials. Technical assistance from Alireza Kayhani is greatly appreciated. This study was financially supported by the Vice-Chancellor for Research and Technology, Kerman University of Medical Sciences, Grant No. 95000288.
Author information
Authors and Affiliations
Contributions
S.M.M., A.A., M.A.M., B.S. and M.F.H. conceived and designed the study. S.M.M., A.A. and S.M. performed the experiments. S.M.M., A.A., S.M. and M.F.H. analyzed the data. S.M.M., A.A., M.A.M., S.M., B.S. and M.F.H. wrote and drafted the manuscript. All authors interpreted and discussed the results.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mousavi, S.M., Afgar, A., Mohammadi, M.A. et al. Calmodulin-specific small interfering RNA induces consistent expression suppression and morphological changes in Echinococcus granulosus. Sci Rep 9, 3894 (2019). https://doi.org/10.1038/s41598-019-40656-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40656-w
This article is cited by
-
Genetic manipulations in helminth parasites
Journal of Parasitic Diseases (2023)
-
Transcriptional effects of electroporation on Echinococcus multilocularis primary cell culture
Parasitology Research (2022)
-
In vitro effects of tropisetron and granisetron against Echinococcus granulosus (s.s.) protoscoleces by involvement of calcineurin and calmodulin
Parasites & Vectors (2021)
-
MicroRNA profile of the strobilated worms of Echinococcus granulosus derived from in vivo and in vitro systems by using high-throughput approach
Parasitology Research (2021)
-
Biological and morphological consequences of dsRNA-induced suppression of tetraspanin mRNA in developmental stages of Echinococcus granulosus
Parasites & Vectors (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.