Abstract
Ocean acidification (OA) is predicted to enhance photosynthesis in many marine taxa. However, photophysiology has multiple components that OA may affect differently, especially under different light environments, with potentially contrasting consequences for photosynthetic performance. Furthermore, because photosynthesis affects energetic budgets and internal acid-base dynamics, changes in it due to OA or light could mediate the sensitivity of other biological processes to OA (e.g. respiration and calcification). To better understand these effects, we conducted experiments on Porolithon onkodes, a common crustose coralline alga in Pacific coral reefs, crossing pCO2 and light treatments. Results indicate OA inhibited some aspects of photophysiology (maximum photochemical efficiency), facilitated others (α, the responsiveness of photosynthesis to sub-saturating light), and had no effect on others (maximum gross photosynthesis), with the first two effects depending on treatment light level. Light also exacerbated the increase in dark-adapted respiration under OA, but did not alter the decline in calcification. Light-adapted respiration did not respond to OA, potentially due to indirect effects of photosynthesis. Combined, results indicate OA will interact with light to alter energetic budgets and potentially resource allocation among photosynthetic processes in P. onkodes, likely shifting its light tolerance, and constraining it to a narrower range of light environments.
Similar content being viewed by others
Introduction
Ocean acidification (OA), which decreases seawater pH and shifts its carbonate chemistry, has significant consequences for the physiology of calcifying photosynthetic taxa1,2,3. OA generally decreases calcification rates3, primarily due to the increased difficulty of building and maintaining calcium carbonate structures under these conditions. OA also affects photophysiology, although the direction and magnitude of these effects vary across taxa3,4 and the aspect of photophysiology being considered, leading to different consequences for photosynthetic performance under different environmental contexts.
For example, photosynthesis may increase under OA in some species as carbon acquisition becomes easier for them due to the increased availability of dissolved inorganic carbon (DIC)5,6,7. However, this effect would be expected only under conditions when photosynthesis is not limited by other factors such as light. Additionally, for species that use carbon-concentrating mechanisms (CCMs) to reduce carbon limitation, OA could increase the energetic efficiency of carbon acquisition, by allowing these species to down-regulate energetically expensive carbon-concentrating enzymes8. In this case, these taxa might not see an increase in their rate of photosynthesis with OA (depending on their type of CCM and their degree of carbon limitation in contemporary environments)4, although energetic savings associated with easier carbon acquisition under OA could facilitate other biological processes.
In contrast to these potential benefits to carbon acquisition, OA may negatively affect other aspects of photophysiology. Specifically, the expression of specific proteins and/or maintenance of enzymes related to photophysiology may be altered by OA, which has been shown to alter protein metabolism and accumulation in a variety of photosynthetic9 and non-photosynthetic taxa10. These effects may not influence metabolic rates like photosynthesis, but could have significant consequences at the cellular level, affecting resource allocation among biological processes and potentially leading to shifts in organismal performance under certain environmental conditions. In the case of photosynthesis, protein turnover is particularly important for repairing damage in Photosystem II (PSII) caused by light11,12. This damage can reduce the photochemical efficiency of PSII, eventually causing photoinhibition (i.e. a reduction in photosynthetic capacity associated with light stress) when damage is large enough in magnitude and duration13,14. Thus, modified or impaired protein metabolism could influence enzymes related PSII function, with increasingly detrimental effects on photochemical efficiency as light increases (and damage to PSII is elevated). Additionally, under low light conditions, photoacclimation processes that facilitate light harvesting also require protein production and maintenance. Therefore, changes in protein metabolism due to OA could enhance negative effects of both high and low light on photophysiology.
Increases in either the rate of photosynthesis or in its efficiency (either due to the effects of OA or other environmental factors such as light) could reduce the sensitivity of other physiological processes like calcification and respiration to OA. For example, enhanced photosynthesis (either in terms of the energetic efficiency of carbon fixation or in its overall rate) could provide additional energy to perform metabolic work to compensate for the effects of OA on these processes. Additionally, hydroxide ion production by photosynthesis15,16 may facilitate calcification by increasing calcification site pH15,17, as well pH within the diffusion boundary layer surrounding the organism, (the latter of which likely reduces dissolution of calcified structure during the daytime)16,18. These effects on internal acid-base dynamics could also save energy by reducing the need for active processes that maintain pH and charge balance in intracellular spaces. Some of these processes, such as proton export, should be more costly under OA, due to elevated proton concentrations in the surrounding seawater19. Therefore, an organism that photosynthesizes faster could hypothetically see less of a reduction in its calcification rate and/or less of an increase in its respiration under OA relative to one with a lower photosynthetic rate.
One group of calcifying phototrophs that will likely be affected by OA are crustose coralline algae (CCA). CCA are typically sensitive to OA, showing reduced calcification20,21,22 and recruitment23 under acidified conditions. These negative effects may have important consequences in habitats where CCA are found, particularly in coral reef ecosystems, where CCA are important agents of reef accretion, and also influence recruitment of benthic invertebrates like corals24,25. Coralline red algae, including CCA, have carbon-concentrating mechanisms (CCMs)5,16. However, not enough is known about these mechanisms to determine whether or not OA should release CCA from carbon limitation, or if their CCM expression is flexible enough to lead to energetic savings under OA.
To gain a better understanding of how OA affects photophysiology in CCA under different light environments, and potential cascading effects on the responses of calcification and respiration to OA, we conducted two laboratory experiments in Moorea, French Polynesia, on Porolithon onkodes, a widespread tropical Pacific species of CCA. However, due to issues with equipment and logistical limitations, the first experiment became a pilot study that only tested a subset of our research questions. Because of this, we focus here on the second experiment, but include the pilot study in Supplementary Material S1 to demonstrate its qualitatively similar results. In both experiments, we crossed two pCO2 and three light treatments, and measured a variety of physiological responses to test the following hypotheses: 1.) OA affects the rate of photosynthesis, but also influences PSII function, and these effects are modulated by light. Specifically, stimulation of photosynthesis can occur under OA due to increased carbon availability, but only at light levels that are saturating for photosynthesis. Additionally, OA negatively affects photochemical efficiency of PSII, and these effects are magnified under high light conditions. 2.) Photosynthesis can mitigate some of the effects of OA on respiration and calcification, and this effect is modulated by light, due to the influence of light on the rate of photosynthesis. Thus, we predicted that P. onkodes in higher light conditions would have more rapid photosynthesis, and would show less of a reduction in calcification under OA, and less of an increase in respiration, particularly if this increase in photosynthesis was greatest in the high light, high pCO2 (i.e. OA) treatment.
Results
Physical Parameters of the Experiment
Mean light and pCO2 treatments were maintained at relatively consistent values within the experiment, as were other physical parameters such as temperature and total alkalinity (summarized in Supplementary Table S3). pCO2 was 406.1 ± 2.3 SEM µatm in the ambient treatment and 995.2 ± 15.0 SEM µatm in the elevated treatment. Mean light levels were 26, 67, and 413 μmol photons m−2 s−1 in the low, medium, and high light treatments respectively. (See Supplementary Table S3 for means with error by light x pCO2 group.) The high light treatment was above the light saturation point for photosynthesis in this species (See the P-E curve results below).
Photosynthesis
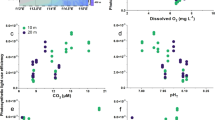
Gross photosynthesis increased significantly with light (p = 0.02), increasing on average by 27% between the low and high light treatments. However, pCO2 did not affect gross photosynthesis, nor did the interaction between pCO2 and light (Fig. 1a). All test statistics and p-values are summarized in Supplementary Table S4.
Photophysiological responses of CCA to experimental OA and light treatments. Points represent group means ± SEM. The x-axis indicates experimental light treatments, with the mean photon flux density (PFD; µmol photons m-2 s-1) for each treatment in parentheses. (a) Area-normalized gross photosynthesis rates, measured at approximately the same PFD that the CCA were kept under in their experimental light treatments (n = 16). Gross photosynthesis for each CCA sample was calculated as the sum of its rate of O2 evolution through net photosynthesis, plus its rate of O2 consumption through light-adapted respiration. Photosynthesis increased significantly with treatment light level (p = 0.016), but did not respond to pCO2. There was no significant light x pCO2 interaction. (b) Maximum photochemical efficiency (Fv/Fm) of dark-adapted samples (n = 32). There was a significant interaction between light and pCO2 (p = 0.016).
In the photosynthesis-light response (P-E) curves constructed from a subset of CCA from each light x pCO2 treatment, photosynthesis increased rapidly with light, and reached light saturation before 200 µmol photons m−2 s−1 (Table 1), indicating the high light treatment was saturating for photosynthesis (Fig. 2). The maximum rate of gross photosynthesis, Pmax, was not affected significantly by pCO2 or by light, or their interaction, and across all treatments had a mean of 0.60 μmol O2 cm−2 hr−1 ± 0.01 SE. However, the initial slope of the P-E curves (α) demonstrated a significant interaction between pCO2 and light (p = 0.05), such that the decrease in α as treatment light level increased did not occur under elevated pCO2 (Fig. 2). Due to this change, α was on average 56% lower under ambient pCO2 compared to elevated in the high light treatment (Table 1). In the medium and low light treatments, α was similar across pCO2 levels, averaging 0.014 μmol O2 cm−2 hr−1/μmol photons m−2 s−1.
Photosynthesis-light response (P-E) curves, measured using the change in O2 concentrations at six photon flux densities (PFDs, µmol photons m−2 s−1). Points represent mean gross photosynthesis rates (net photosynthesis + dark-adapted respiration) for each pCO2 and light treatment group (n = 8) at a given PFD. Error bars represent SEM, with data fitted by a hyperbolic tangent function. Shapes represent the light treatments that samples were kept under in their experimental tanks, with the mean PFD for each treatment indicated in parentheses in the figure legend.
Photochemical efficiency
Overall, maximum photochemical efficiency (Fv/Fm) of dark-adapted samples declined as the treatment light level increased (from 0.49 to 0.33 under ambient pCO2, and 0.48 to 0.27 under elevated pCO2). However, there was a significant interactive effect of light and OA (p = 0.016), such that OA had a stronger negative effect on Fv/Fm in higher light treatments (Fig. 1b). In the highest light treatment, this interaction resulted in an average Fv/Fm that was 17% lower in the OA treatment compared to ambient.
Respiration
Light-adapted respiration increased significantly with light (p < 0.01), increasing by 43% between the low and high light treatments (averaged across pCO2 treatments). However, it was not affected by pCO2, nor was there a significant interaction between pCO2 and light. In contrast, dark-adapted respiration was affected by the interaction between pCO2 and light (p = 0.04), such that rates were higher under elevated pCO2 compared to ambient. The magnitude of this difference increased with treatment light level (Fig. 3).
Light vs. dark-adapted respiration. Points represent treatment group means ± SEM. Rates are in terms of O2 consumed, normalized to the surface area of the algal samples (n = 16 for light-adapted respiration, and n = 8 for dark-adapted respiration). The x-axis indicates experimental light treatments, with the mean photon flux density (µmol photons m−2 s−1) for each treatment in parentheses. Light-adapted respiration increased with treatment light level (p = 0.007). pCO2 did not affect light-adapted respiration. Dark-adapted respiration had a significant light x pCO2 interaction (p = 0.039).
Calcification
Net calcification was positively correlated with light (p = 0.001), increasing by 22% in the high light treatment relative to the low light treatment. In contrast, calcification significantly decreased under elevated pCO2 (p = 0.046), declining by 15% relative to ambient (Fig. 4). There was no significant interaction between pCO2 and light (Supplementary Table S4).
Area-normalized net calcification rates of P. onkodes. Points represent treatment group means ± SEM (n = 32). The x-axis indicates experimental light treatments, with the mean photon flux density (µmol photons m−2 s−1) for each treatment in parentheses. Calcification declined significantly under elevated pCO2 (p = 0.046), and increased with light (p = 0.001), but there was no light x pCO2 interaction.
Discussion
Our results indicate increased carbon availability under OA does not directly stimulate photosynthesis in this species: neither Pmax, nor gross photosynthesis in the highest light treatment (which were at or above the light saturation point (Ek) for P. onkodes26,27, Table 1) increased in the elevated pCO2 treatment. This result suggests that this species has a CCM with high affinity for DIC, preventing carbon limitation under ambient conditions. However, another (non-mutually exclusive) explanation for this result is that any potential benefit to photosynthesis caused by increased carbon availability is counterbalanced by additional effects of OA on other aspects of physiology in this species.
The results for maximum photochemical efficiency (Fv/Fm) support this latter hypothesis. Fv/Fm is commonly used to estimate stress within PSII14, and a change in it under OA would imply that OA altered cellular and/or biochemical processes related to PSII function. Like we observed in our study, Fv/Fm typically declines for organisms found in higher light environments, which is an acclimatization response that protects their photosynthetic machinery from light damage28,29. Such a decline does not necessarily result in a decreased rate of gross photosynthesis, unless the decline is large enough in magnitude and duration13,14. However, the increasingly negative effect of OA on Fv/Fm as treatment light level increased indicates that OA exacerbates its decline with light, potentially by reducing the repair rate of light-damaged proteins (e.g.11,12). Studies on phytoplankton have found similar synergistic effects of light and OA on other aspects of PSII function, including non-photochemical quenching, another indicator of light stress30,31,32. Therefore, effects of OA on PSII function may be an important mechanism through which it alters photophysiology across multiple taxa.
Another indicator that OA has important indirect effects on photophysiology was the interactive effect of light and OA on α, the responsiveness of photosynthesis to sub-saturating light. Generally, α was negatively correlated with treatment light level, another photoacclimation phenomenon that allows phototrophs to optimize their energy accrual. However, in the highest (saturating) light treatment, α was over two times higher under OA conditions (Table 1). This result implies that in P. onkodes acclimatized to saturating light regimes, OA causes photosynthesis to increase more rapidly as light increases (until the light saturation point). Potentially tradeoffs between carbon acquisition and light harvesting, which has been observed indirectly in other red algae33, could explain this result. Specifically, reduced carbon capture and assimilation costs under OA could make more energy available for processes associated with light harvesting, such as the production of antennae pigments (components of the photosynthetic light harvesting complex attached to the chlorophyll reaction center), which could lead to an increase in α34. The interactive effect of light and OA on α may occur because both light and OA influence the production and activity of CCMs in algae—generally, CCMs are down-regulated under lower light levels as well as under OA7,35. Therefore, CCA in the low and medium light treatments potentially had low CCM production, and thus in these light treatments there was negligible down-regulation of CCM activity in the OA treatment relative to ambient seawater conditions. In contrast, in the high light treatment, reduced CCM activity associated with the OA treatment could free up resources for increased pigment production, thereby enhancing photosynthesis at sub-saturating light levels (and leading to a higher α).
This hypothesized tradeoff between CCM production and antennae pigment production under different light and pCO2 environments is consistent with our finding that OA had an increasingly negative effect on Fv/Fm as treatment light level increased. Larger antenna size is believed to increase the susceptibility of PSII to photoinhibition36, and this susceptibility is frequently inferred from declines in Fv/Fm37,38. Thus, increased antennae size could have facilitated photosynthesis at sub-saturating light levels in the P-E curve measurements, leading to an increase in α, while also increasing susceptibility to photoinhibition (and decreasing Fv/Fm) in the saturating light treatment. Furthermore, internal CCMs have been implicated as a method for the dissipation of excess excitation energy39,40,41, which could help reduce photoinhibition at high light levels. Thus, decreased production of CCMs in high light conditions under OA may lead to an increased susceptibility of PSII to photoinhibition, which again is consistent with our findings for Fv/Fm. These potential linkages between carbon concentrating mechanisms, light harvesting pigments, and the effects of OA on photochemical efficiency and the photosynthesis-light relationship require further investigation. However, if accurate, they imply that the indirect effects of OA – including shifts in the expression or function of specific proteins related to photophysiology – may be just as important in determining the responses of marine phototrophs to OA as its direct effects on their carbon capture efficiency and rates of photosynthesis.
Regardless of the mechanisms, results from this study indicate that OA inhibits some aspects of photophysiology in P. onkodes (Fv/Fm), but also has neutral (Pmax) and facilitative effects on other aspects (e.g., α), and these effects are modulated by the light environment that the organism is acclimatized to. These contrasting results emphasize that it is not appropriate to assume that the response of one metric of photosynthetic performance will be representative of the response of another to OA. Furthermore, important patterns might be obscured if multiple metrics are lumped together for synthetic analyses of the effects of OA on photosynthesis (as has been done in previous meta-analyses and reviews).
In addition to these mixed responses by different components of photophysiology to OA and light, we found that dark-adapted and light-adapted respiration also showed different patterns from one another (Fig. 3). Dark-adapted respiration was higher in the OA treatment, with this difference increasing with treatment light level. Several studies on phytoplankton31,42 and photosynthetic foraminifera43 have found similar increases in dark-adapted respiration under OA, indicating a common effect across multiple photosynthetic taxa. However, light-adapted respiration did not show an effect of OA (consistent with findings in other studies on calcifying macroalgae20). This contrast with dark-adapted respiration suggests that changes in biochemical or physiological processes in the light vs. dark, such as hydroxide production by photosynthesis15 or proton pumping16, could modulate the response of respiration to OA. However, the magnitudes of the effects of these processes are not necessarily determined by the rate of photosynthesis (which increased with treatment light level, Fig. 1a). More work will be necessary to determine the mechanisms behind these differing responses. However, our results indicate that estimates of the effect of OA on respiration integrated over a daily cycle of light and dark could be biased in studies where it is measured under a single set of conditions (light or dark-adapted).
Similar to the light-adapted respiration results, the effect of OA on net calcification was not modified by light environment. Rather, in both experiments calcification increased with treatment light level, but consistently declined under OA and (Fig. 4), which agrees with previous findings in CCA44,45. Thus, we can infer that increased gross photosynthesis under higher light treatments was not sufficient to reduce the negative effects of OA on calcification. Potentially an ameliorative effect of photosynthesis would have been possible if there had been a greater relative increase in it under the high light-high pCO2 treatment. However, work in CCA has described sigmoidal, saturating relationships between calcification and photosynthesis46, and thus there simply may be limits to the ability of photosynthesis to stimulate calcification in this species. Furthermore, since light has an asymptotic relationship with calcification as well as photosynthesis47, there are also likely limits to its effects as well.
In other calcifying phototrophs such as corals, beneficial effects of light are possible when light is above the saturation point for calcification48. However, negative effects of high light have also been observed49, and might occur when light is high enough to induce photoinhibition and/or bleaching, which can reduce the amount of tissue actively calcifying. OA-induced bleaching occurs in P. onkodes under higher photon flux densities (700–1000 µmol photons m−2 s−1) than those used in this experiment21,50. This evidence, along with our finding that the negative effect of OA on photochemical efficiency increases with light (Fig. 1b), suggests that photoinhibition or bleaching could occur under OA in P. onkodes populations found in very shallow, high light habitats (which can reach 1500–2000 µmol photons m−2 s−1 26,51. These effects could further reduce calcification, especially as these shallow environments also receive ultraviolet radiation, which amplifies the negative effects of OA on photosynthesis and calcification in coralline algae52.
Overall, our results indicate that OA will likely alter energetic budgets and potentially resource allocation in P. onkodes, and that these effects will vary across different light environments. Increases in respiration at night will increase the minimum amount of light that P. onkodes requires over a day to maintain a positive energetic balance, while increases in α for individuals found in brighter light environments may help offset increased respiration costs. Additionally, alterations to energy allocation between carbon capture and light harvesting may alter other facets of this organism’s biology such as its PSII function, potentially reducing the upper end of its light tolerance. Combined, these results indicate OA could constrain P. onkodes to a narrower range of depths and light microhabitats—a goldilocks area that is not too bright, due to the enhanced negative effects of high light on photochemical efficiency, and not too dark, due to the increased energetic needs associated with increased rates of respiration at night. Since P. onkodes often forms shallow ridges along reef crests53 that protect inner reefs and near-shore human populations, and is also a competitively dominant CCA species in shallow Indo Pacific reefs54, a shift in its distribution across reef habitats, particularly out of shallow, high light areas, could lead to cascading changes in the physical and community structure of many coral reef ecosystems.
Methods
Experimental setup
A laboratory experiment was conducted at the Richard B. Gump South Pacific Research Station on the island of Moorea, French Polynesia during June-July in 2014. Individual Porolithon onkodes crusts without obvious epibionts were collected using a hammer and chisel at a depth of 1–2.5 m, approximately 100–200 m from reef crest on the back reef on the North shore of Moorea, near Cook’s Bay (17°28′40.51″S, 149°50′22.80″W). See Supplementary Material S1 for more information on CCA species identification. Although some individuals of this species were found in semi-cryptic reef environments, experimental specimens were collected from open reef substrates, which had a mean photon flux density of 832 ± 134 (SD) µmol photons m−2 s−1 at daily peak solar irradiance (within an hour of local apparent noon; methods in Supplementary Material S1). CCA crusts (n = 192) were brought to the lab and trimmed to similar sizes (~ 9 cm2), and any visible bioeroding organisms (e.g., sponges, worms, etc.) were removed from their undersides before the exposed calcium carbonate skeleton was sealed using marine epoxy (Z-SPAR, Splash Zone). Following this procedure, samples were placed in a high flow acclimation tank for 9 days to heal and photoacclimate to the treatment light levels (26, 67, 413 µmol photons m−2 s−1). Previous work has found that photoacclimation processes generally occur within this time frame in red algae55,56. After the acclimation period, samples were placed into one of eight 150-L tanks under their randomly assigned pCO2-light treatments. Within each tank there were three light treatments constructed from acrylic frames topped with neutral-density filters, which manipulated light levels to the desired photon flux density. Photosynthesis exhibits a nonlinear relationship with light47, increasing up to a saturating light level, after which it becomes limited by other factors. Thus, if OA directly stimulated photosynthesis by releasing it from carbon limitation, we would expect to see photosynthesis increase after this light saturation point. We chose three treatment light levels to represent different points along the photosynthesis-light response (P-E) curve for P. onkodes, based on previous work on this species conducted on individuals from a similar range of depths and light environments as in those used in this study26,27. Low and medium light treatments (26 and 67 µmol photons m−2 s−1) represented points along the light-limited portion of the curve for this species, whereas the highest light treatment (413 µmol photons m−2 s−1) was chosen to fall above the light-saturation point of the curve, which was confirmed during the P-E curve measurements. To minimize any positioning effects within the tanks, samples were rotated underneath their light treatments every fourth day, and were brushed gently with a soft-bristle toothbrush to prevent turf algae or biofilm from establishing on their surface.
Four of the tanks were kept at ambient pCO2 (~406 µatm), and four were maintained at an elevated pCO2 (~995 µatm), by bubbling CO2-enriched air into them using a solenoid-controlled gas regulation system (Model A352, Qubit Systems). The high pCO2 treatment value was chosen to approximate predictions for the year 2100 under the IPCC’s Representative Concentration Pathway (RCP), Scenario 8.557. Water in the tanks was replaced using filtered seawater from Cook’s Bay at a mean rate of 6.6 L hr−1, and tank temperature was maintained at 27 °C, a typical temperature for the Moorea backreef where the CCA were collected during the season in which the experiments were conducted58. The tanks were illuminated with LED lights (Aqua Illumination, Sol), which were kept on a 12-hour ramping light/dark cycle. PFDs were measured under light treatments in each tank once each week throughout the experiment during the period of peak irradiance using a 2π PAR sensor attached to a diving-PAM (Walz, Germany), calibrated to a LiCOR 2π PAR sensor (LI-1400).
Seawater chemistry
Temperature was measured twice daily (07:30 and 17:30 hr) using a portable temperature probe (Fisher-Scientific, USA), and tank pH (total scale) was measured daily at 07:30 hr from water samples collected from each tank, using a titrator pH probe (Mettler-Toledo, DGi115-SC) that was calibrated every other day using a TRIS buffer solution. Total alkalinity (TA) was measured for these water samples every other day using an automatic open-cell potentiometric titrator (Mettler-Toledo, T50). Accuracy of the titrations was measured by titrating certified reference material from the Dickson lab (University of California, San Diego). Accuracy was ± 9.7 ± 1.3 μmol kg−1 (n = 21). Tank salinity was measured every other day using a conductivity meter (YSI 3100, YSI, USA). Carbonate chemistry of the tanks then was calculated from their salinity, temperature, pH, and TA values using the seacarb package in R59. Carbonate chemistry and other physical conditions for the treatments are summarized in Supplementary Table S3.
Calcification
Net calcification rates of the CCA over the course of the experiment were calculated from differences in the buoyant weight of each sample measured at the beginning and end of the experiment60. Buoyant weights were converted to skeletal dry weights using the density of calcite (2.71 g cm−3). Although high-Mg calcite is the most common crystalline structure of calcium carbonate produced by CCA61, calculating its exact density requires knowing the mol % of Mg, which can vary due to a variety of factors62. Since the mol % of Mg was not known for these CCA, the density of calcite was used to estimate skeletal dry weight. The change in skeletal dry weight was calculated for each sample, and then was normalized to the duration of the experiment (28 days), as well as the surface area of the sample. Surface area of the living tissue of each sample was estimated using the foil method63.
Photosynthesis and Respiration
Net photosynthesis and respiration rates were measured as the change in dissolved oxygen concentration in closed respirometry chambers (250 mL). This was done for a subset of CCA from each treatment group (n = 16 for photosynthesis and light-adapted respiration, and n = 8 for dark-adapted respiration). Oxygen concentrations were measured using a 2-mm diameter optical oxygen probe (PreSens, DP-PSt3) and logging system (PreSens, Precision Sensing GmbH, Germany). Incubations lasted 25–50 minutes, and were made at the same temperature and pCO2 that the samples experienced in the tanks, and at approximately the same light levels for the photosynthesis measurements (28, 69, and 416 µmol photons m−2 s−1). See Supplementary Material S1 for a full description of methods for incubations. Post-illumination (i.e., light-adapted) respiration was measured immediately after net photosynthesis by fully darkening the incubation chambers and measuring O2 consumption rates. Dark-adapted respiration was measured after the alga had been dark-acclimated for 45–60 minutes to minimize the stimulatory effect of photosynthesis on respiration64. The incubations for dark-adapted respiration took place as part of the P-E curve measurements (described in the following section). Respiration rates of control seawater samples taken from each of the experimental tanks were measured to isolate any signature of microbial activity within the tanks. The control samples all demonstrated a small respiration signal (i.e. a decline in O2 concentrations), so the absolute value of these negative rates were added to the photosynthesis and respiration rates measured for samples from the same tank. Gross photosynthesis was calculated for each CCA from its net photosynthesis rate plus the absolute value of the light-adapted respiration rate. Photosynthesis and respiration rates were normalized to the surface area of the algal sample.
Photosynthesis-light response (P-E) curves
In addition to measuring net photosynthetic rates of the CCA at their treatment light levels, net photosynthesis was measured at six increasing PFDs (0, 36, 98, 235, 415, 610 µmol photons m−2 s−1) for a separate subset of samples (n = 8 per treatment). Rates were measured using the respirometry methods described above (with additional details in Supplementary Material S1). These rates were used to construct photosynthesis-light response (P-E) curves for individual samples. The P-E data were fit with a hyperbolic tangent function47 (Equation 1).
This model uses the incident photon flux density (E) and the measured rate of net photosynthesis at E, to determine α, the initial slope of the light-limited part of the curve (or the responsiveness of photosynthesis to sub-saturating light) and Pmax, the maximum gross photosynthetic rate. P0 is the rate of O2 consumption when E = 0, i.e., the dark-adapted respiration rate.
Photochemical Efficiency
A diving-PAM (pulse-amplitude modulated fluorometer, Walz GmbH, Germany) was used to estimate the maximum photochemical efficiency of PSII13,62 of dark-adapted samples (Fv/Fm, n = 32). See Supplementary Material S1 for details.
Statistical analyses
Because many of the response variables are thought to be strongly correlated with each other, we performed separate analysis of variance (ANOVA) on each variable to test the effects of pCO2 and light. These variables included net calcification, respiration, photosynthesis, and photochemical efficiency, as well as the parameters α and Pmax estimated using the nls() function in R for the individual sample P-E curves. Additionally, since the light and pCO2 treatments were slightly different for the two experiments, we analyzed results separately for each experiment. For each analysis, a mixed-effects model was constructed with light and pCO2 treatments as fixed factors and tank as a random factor, using the R package nlme65. Model residuals were checked graphically for heteroscedasticity, in addition to statistically testing for homogeneity of variances using Levene’s test (using the car package in R66), and all variables met the assumptions for ANOVA. Tukey post hoc tests were conducted on P-E curve parameters that exhibited significant main effects or interactions to determine which groups were different from one another. Tukey adjustments were used to correct p-values for multiple comparisons in the post hoc tests. All statistical analyses were done using R 3.3.267.
Data Availability
The data generated during this study are available in the Moorea Coral Reef LTER dataset repository at http://mcr.lternet.edu/data.
References
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science: Journal du Conseil 65, 414–432 (2008).
Doney, S. C., Balch, W. M., Fabry, V. J. & Feely, R. A. Ocean acidification: A critical emerging problem for the ocean sciences. (2009).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol n/a–n/a, 10.1111/gcb.12179 (2013).
Cornwall, C. E. et al. Inorganic carbon physiology underpinsmacroalgal responses to elevated. Sci. Rep. 1–13, CO2, https://doi.org/10.1038/srep46297 (2017).
Raven, J. A. & Beardall, J. CO2 concentrating mechanisms and environmental change. Aquatic Botany 118, 24–37 (2014).
Raven, J. A. Effects on marine algae of changed seawater chemistry with increasing atmospheric CO2. Biology & Environment: Proceedings of the Royal Irish Academy 111, 1–17 (2011).
Raven, J. A. & Hurd, C. L. Ecophysiology of photosynthesis in macroalgae. Photosynth Res 113, 105–125 (2012).
Raven, J. A., Giordano, M., Beardall, J. & Maberly, S. C. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109, 281–296 (2011).
Edmunds, P. J. & Wall, C. B. Evidence That High pCO2 Affects Protein Metabolism in Tropical Reef Corals. The Biological Bulletin 227, 68–77 (2014).
Pan, T. C. F., Applebaum, S. L. & Manahan, D. T. Experimental ocean acidification alters the allocation of metabolic energy. Proceedings of the National Academy of Sciences 112, 4696–4701 (2015).
Prasil, O., Adir, N. & Ohad, I. Dynamics of photosystem II: mechanism of photoinhibition and recovery process. (Topics in photosynthesis, 1992).
Han, B.-P. A mechanistic model of algal photoinhibition induced by photodamage to Photosystem-II. Journal of Theoretical Biology 214, 519–527 (2012).
Warner, M. E., Lesser, M. P. & Ralph, P. J. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications (ed. Suggett, D. J.) 209–222 (Springer Netherlands, https://doi.org/10.1007/978-90-481-9268-7_10 (2010).
Murchie, E. H. & Lawson, T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany 64, 3983–3998 (2013).
Comeau, S., Carpenter, R. C. & Edmunds, P. J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. P Roy Soc B-Biol Sci 280, 20122374–20122374 (2012).
Hofmann, L. C., Koch, M. & de Beer, D. Biotic Control of Surface pH and Evidence of Light-Induced H+ Pumping and Ca2+−H+ Exchange in a Tropical Crustose Coralline Alga. PLoS ONE 11, e0159057 (2016).
Furla, P., Galgani, I., Durand, I. & Allemand, D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457 (2000).
Cornwall, C. E. et al. Diffusion Boundary Layers Ameliorate the Negative Effects of Ocean Acidification on the Temperate Coralline Macroalga Arthrocardia corymbosa. PLoS ONE 9, e97235 (2014).
Taylor, A. R., Brownlee, C. & Wheeler, G. L. Proton channels in algae: reasons to be excited. Trends in Plant Science 17, 675–684 (2012).
Hofmann, L. C. & Bischof, K. Ocean acidification effects on calcifying macroalgae. Aquat. Biol. 22, 261–279 (2014).
Anthony, K. R. N., Kline, D. I., Diaz-Pulido, G., Dove, S. & Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences 105, 17442–17446 (2008).
Comeau, S., Edmunds, P. J., Spindel, N. B. & Carpenter, R. C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceangr. 58, 388–398 (2013).
Kuffner, I. B., Andersson, A. J., Jokiel, P. L., Rodgers, K. U. S. & Mackenzie, F. T. Decreased abundance of crustose coralline algae due to ocean acidification. Nature Geosci 1, 114–117 (2007).
Negri, A. P., Webster, N. S. & Hill, R. T. Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Marine Ecology Progress … (2001).
Harrington, L., Fabricius, K., De’Ath, G. & Negri, A. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85, 3428–3437 (2004).
Payri, C. E., Maritorena, S., Bizeau, C. & Rodière, M. Photoacclimation in the tropical coralline alga Hydrolithon onkodes (Rhodophyta, Corallinaceae) from a French Polynesian reef. Journal of Phycology 37, 223–234 (2001).
Chisholm, J. R. Primary productivity of reef-building crustose coralline algae. Limnol. Oceangr. 1376–1387 (2003).
Ralph, P. J., Gademann, R., Larkum, A. & Schreiber, U. In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar Ecol-Prog Ser 180, 139–147 (1999).
Gorbunov, M. Y., Kolber, Z. S. & Lesser, M. P. Photosynthesis and photoprotection in symbiotic corals. Limnology and … 1–11 (2001).
Wu, Y., Gao, K. & Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923 (2010).
Gao, K. et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nature Clim. Change 2, 519–523 (2012).
Gao, G., Liu, Y., Li, X., Feng, Z. & Xu, J. An ocean acidification acclimatised green tide alga is robust to changes of seawater carbon chemistry but vulnerable to light stress. PLoS ONE 11, e0169040 (2016).
Kübler, J. E. & Raven, J. A. The interaction between inorganic carbon acquisition and light supply in Pamaria palmata (Rhodophyta). Journal of Phycology 31, 369–375 (1995).
Machalek, K. M., Davison, I. R. & Falkowski, P. G. Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina. Plant Cell Environ 19, 1005–1016 (1996).
Raven, J. A., Beardall, J. & Giordano, M. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res 121, 111–124 (2014).
Neidhardt, J., Benemann, J., Zhang, L. & Melis, A. Photosystem-II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae). Photosynth Res 56, 175–184 (1998).
Maxwell, K. & Johnson, G. N. Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany 51, 659–668 (2000).
Cosgrove, J. & Borowitzka, M. A. Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications, Developments in Applied Phycology, https://doi.org/10.1007/978-90-481-9268-7_1 (2010).
Raven, J. A. The cost of photoinhibition. Physiologia Plantarum 142, 87–104 (2011).
Sobrino, C., Ward, M. L. & Neale, P. J. Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: Effects on growth, photosynthesis, and spectral sensitivity of …. Limnol. Oceangr. 53, 494–505 (2008).
Qiu, B. S. & Liu, J. Y. Utilization of inorganic carbon in the edible cyanobacterium Ge‐Xian‐Mi (Nostoc) and its role in alleviating photo‐inhibition. Plant Cell Environ 27, 1447–1458 (2004).
Goldman, J. A. L., Bender, M. L. & Morel, F. M. M. The effects of pH and pCO2 on photosynthesis and respiration in the diatom Thalassiosira weissflogii. Photosynth Res https://doi.org/10.1007/s11120-016-0330-2 (2017).
Uthicke, S. & Fabricius, K. E. Productivity gains do not compensate for reduced calcification under near-future ocean acidification in the photosynthetic benthic foraminifer species Marginopora vertebralis. Glob Change Biol 18, 2781–2791 (2012).
Russell, B. D., Passarelli, C. A. & Connell, S. D. Forecasted CO2 modifies the influence of light in shaping subtidal habitat. Journal of Phycology 47, 744–752 (2011).
Comeau, S., Carpenter, R. C. & Edmunds, P. J. Effects of irradiance on the response of the coral Acropora pulchra and the calcifying alga Hydrolithon reinboldii to temperature elevation and ocean acidification. Journal of Experimental Marine Biology and Ecology 453, 28–35 (2014).
Vásquez-Elizondo, R. M. & Enríquez, S. Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci. Rep. 1–14, https://doi.org/10.1038/srep19030 (2015).
Chalker, B. E. Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar Biol 63, 135–141 (1981).
Suggett, D. J. et al. Light availability determines susceptibility of reef building corals to ocean acidification. Coral Reefs 32, 327–337 (2013).
Marubini, F., Barnett, H., Langdon, C. & Atkinson, M. J. Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa. Mar Ecol-Prog Ser 220, 153–162 (2001).
Diaz-Pulido, G., Anthony, K. R. N., Kline, D. I., Dove, S. & Hoegh-Guldberg, O. Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. Journal of Phycology 47, 32–39 (2011).
Chisholm, J. R. Calcification by crustose coralline algae on the northern Great Barrier Reef, Australia. Limnol. Oceangr. 45, 1476–1484 (2000).
Gao, K. & Zheng, Y. Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob Change Biol 16, 2388–2398 (2010).
Littler, M. M. Standing stock measurements of crustose coralline algae (Rhodophyta) and other saxicolous organisms. Journal of Experimental Marine Biology and Ecology (1971).
Briggs, A. A. Effects of light, temperature, and ocean acidification on the physiology and ecology of tropical crustose coralline algae. 1–136 (Master’s Thesis, 2016).
Hamel, K. Comparative time course of photoacclimation in Hawaiian endemic and invasive species of Gracilaria (Rhodophyta). 1–121 (University of Hawai’i, 2012).
Ritz, M., Thomas, J. C., Spilar, A. & Etienne, A. L. Kinetics of photoacclimation in response to a shift to high light of the red alga Rhodella violacea adapted to low irradiance. Plant Physiology 123, 1415–1426 (2000).
Meehl, G. A. et al. Global climate projections. Climate change 747–845 (2007).
Edmunds, P. J., Leichter, J. J. & Adjeroud, M. Landscape-scale variation in coral recruitment in Moorea, French Polynesia. Mar Ecol-Prog Ser 414, 75–89 (2010).
Lavigne, H. & Gattuso, J. P. seacarb: seawater carbonate chemistry with R. R package version 2.3.5 (2010).
Davies, P. S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol (1989).
Bischoff, W. D., Mackenzie, F. T. & Bishop, F. C. Stabilities of synthetic magnesian calcites in aqueous solution: Comparison with biogenic materials. Geochimica et Cosmochimica Acta 51, 1413–1423 (1987).
Andersson, A. J., Mackenzie, F. T. & Bates, N. R. Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol-Prog Ser 373, 265–273 (2008).
Marsh, J. A. Jr., Primary productivity of reef-building calcareous red algae. Ecology (1970).
Edmunds, P. J. & Davies, P. S. Post-illumination stimulation of respiration rate in the coral Porites porites. Coral Reefs 7, 7–9 (1988).
Pinheiro, J., Bates, D., Debroy, S., Sarkar, D. & Team, R. C. nlme: linear and nonlinear mixed effects models. R package version 31–128 (2016).
Fox, J. & Weisberg, S. An R companion to applied regression. (Sage Publications, 2011).
R Core Team. R: a language and environment for statistical computing. Available at, https://www.R-project.org/. (Accessed: 19 May 2018) (2016).
Acknowledgements
We would like to acknowledge the Moorea Coral Reef (MCR) LTER and the Richard B. Gump Research Station staff for logistic support of this research, as well as H. Hillard, L. Valentino, M. Ho, K. Dao, J. Ohde, and M. Ospina for assistance in the lab and field. We would also like to thank S. Dudgeon and P.J. Edmunds for their feedback on methods and ideas relevant to this research. This research was completed under permits issued by the Haut-Commissariat de la République en Polynésie Francaise (DRRT) (Protocole d’Accueil 2013-2014). Funding for this work came from NSF Grants OCE-1236905 to the MCR-LTER program and OCE-1041270 to RCC and PJE, and grants through the College of Science and Mathematics at California State University, Northridge. This is contribution No. 283 from the CSUN Marine Biology Program.
Author information
Authors and Affiliations
Contributions
A.A.B. and R.C.C. conceived the ideas and designed methodology for this research. A.A.B. collected and analyzed the data, and wrote the manuscript; R.C.C. provided edits. All authors contributed to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Briggs, A.A., Carpenter, R.C. Contrasting responses of photosynthesis and photochemical efficiency to ocean acidification under different light environments in a calcifying alga. Sci Rep 9, 3986 (2019). https://doi.org/10.1038/s41598-019-40620-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40620-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.