Abstract
Natural selection acts on genetic variants by increasing the frequency of alleles responsible for a cellular function that is favorable in a certain environment. In a previous genome-wide scan for positive selection in contemporary humans, we identified a signal of positive selection in European and Asians at the genetic variant rs10180970. The variant is located in the second intron of the ABCA12 gene, which is implicated in the lipid barrier formation and down-regulated by UVB radiation. We studied the signal of selection in the genomic region surrounding rs10180970 in a larger dataset that includes DNA sequences from ancient samples. We also investigated the functional consequences of gene expression of the alleles of rs10180970 and another genetic variant in its proximity in healthy volunteers exposed to similar UV radiation. We confirmed the selection signal and refine its location that extends over 35 kb and includes the first intron, the first two exons and the transcription starting site of ABCA12. We found no obvious effect of rs10180970 alleles on ABCA12 gene expression. We reconstructed the trajectory of the T allele over the last 80,000 years to discover that it was specific to H. sapiens and present in non-Africans 45,000 years ago.
Similar content being viewed by others
Introduction
ATP binding cassette (ABC) transporters are trans-membrane ubiquitous proteins, that translocate natural substrates across plasma membranes. In humans, there are 49 genes coding for ABC transporters, arranged in eight subfamilies extensively studied because at least 11 of 49 genes are known to cause severe inherited diseases1. The ATP-binding cassette, sub-family A member 12 (ABCA12) gene was discovered from cDNA of human placenta2. ABCA12 is 207 kb long with fifty-three exons and two very long introns at its beginning (26.5 kb and 47.3 kb, respectively, Fig. 1a). ABCA12 is a keratinocyte transmembrane transporter that binds and hydrolyzes ATP to transport lipids in the lamellar granules3. This activity is required to form the extracellular lipid barrier in the outermost layer of the skin, the stratum corneum of the epidermis3. The lipid barrier is composed of three lipid classes (cholesterol, free fatty acids, and ceramides) and acts as a primary barrier between the body and the environment to prevent excessive water loss and to avoid penetration of pathogens4. ABCA12 has also a role in immunity: in macrophages it regulates the cellular cholesterol metabolism via an LXRb-dependent post-transcriptional mechanism5.
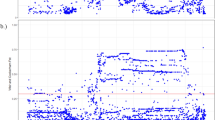
Positive selection at ABCA12. (a) The ABCA12 gene has is 55 exons and two long introns at its beginning. The black rectangle indicates the 70 kb region surrounding rs10180970 considered in this project. (b) rs10180970 is the most differentiated variant between Africans and non-Africans as shown by the absolute difference of the derived allele frequency (ΔDAF), however, other variants seem to be also extremely differentiated. (c) The Cross Population Extended Haplotype Homozogysity statistic (XP-EHH), measured between pairs of continental populations, shows a signal of positive selection in non-Africans over 35 kb downstream rs10180970, especially in East-Asians. (d) The signal is confirmed by the Integrated Haplotype Score (iHS) within continental populations. In panels b–d the dashed line indicates the genomic position of rs10180970.
UV-radiation has a major effect on skin and keratinocytes and it is one of the most studied environmental stressors of the epidermal homeostasis. In keratinocytes, UV-radiation induces mutagenesis, apoptosis, proliferation, and metabolic changes. UV-radiation has a major role in determining skin pigmentation6,7, but it also can have a detrimental effect on the lipid barrier reducing the intercellular lipid cohesion8. Earlier studies suggest that UVB radiation down-regulates epidermal ABCA12 gene expression9 and in general UV light influences other ABC transporters activity in lymphocytes10.
The always growing availability of ancient DNA information allows us to directly reconstruct the history of genetic mutations and understand if consequences of past adaptations are relevant for contemporary humans11. Past events of positive selection can be tested as detection of a major shift in allele frequencies12,13,14.
In a genome-wide scan for positive selection in contemporary humans, we identified a signal of positive selection in Europeans and Asians at rs10180970 C/T, located in the second intron of ABCA1215. In the study, we demonstrated that the derived allele T is highly frequent in Asians and Europeans, compared to Africans, where the ancestral C variant prevails. This evidence, together with the identification of patterns typical of positive selection in the genomic region of rs10180970 let us speculate that ancestor of current Europeans and Asians should have had an advantage in carrying the T allele and that it would be interesting to determine the functional consequences and the trajectory of the T allele through time.
Here we present results from investigating the genomic region surrounding rs10180970. We confirm that this region underwent positive selection and investigate one possible selected phenotype. We have extended the set of individuals used to investigate genetic variation and natural selection at rs10180970, including also DNA sequences from ancient human samples. We confirm the signal of positive selection at rs10180970, investigate linkage with genetic variants in its proximity and the effect of the two alleles on ABCA12 gene expression.
Results
Natural selection signal at the ABCA12 gene extends 10 kb downstream rs10180970
We identified rs10180970 as a possible candidate for positive selection in Europeans and Asians during a genome-wide scan for positive selection conducted on populations from Phase I 1000 Genomes Project15. In this study, we considered a 70 kb region surrounding rs10180970 including the first two exons, the first and most of the second introns, and the transcription starting site of ABCA12 (Fig. 1a). Compared to Phase I, the 1000 Genomes Phase III dataset16 includes 1,412 more samples belonging to 12 more populations (Supplementary Table S1) and in the region under study includes 1,612 variants for which the ancestral state is known and that we used to calculate statistics suggestive of population differentiation and positive selection.
Between pairs of continental populations, we evaluated the absolute difference of the derived allele frequency (ΔDAF) and the Cross Population Extended Haplotype Homozygosity (XP-EHH17). ΔDAF approaches 1 when the derived allele prevails in one population and is almost absent in the other. According to ΔDAF, rs10180970 is still the most differentiated variant in comparisons of Africans and non-Africans in 70 kb surrounding rs10180970 (Fig. 1b). In Phase III the T/T genotype frequency in the combined sample of Europeans and Asians is significantly higher than Africans (Table 1, Fisher exact test p-value < 2.2e-16). Similarly, in the Complete Genomics dataset (CG)18, the T/T genotype is significantly more frequent among Asians and Europeans compared to Africans. This is true both when considering samples partially overlapping with Phase I, and samples unique to CG (Table 1, Fisher exact test p-value < 2.2e-16), suggesting that the observed pattern of higher derived allele frequencies in Europeans and Asians compared to Africans was not due to the specific set of sample used but is reproducible. XP-EHH detects recent positive selection highlighting selective sweeps in which the selected allele has approached or achieved fixation in one population but remains polymorphic in the overall population. In the 1000 Genomes Phase III dataset, we observe a stretch of negative XP-EHH outside the expected variance range (i.e., -1,1) in pairs of Africans with non-Africans. The signal is located approximately at 10 kb downstream rs10180970, suggesting recent positive selection in non-Africans in this region (Fig. 1c), and it is particularly strong in East-Asians. Within continental populations we calculated the Integrated Haplotype Score (iHS19). iHS detects the classic signal of strong directional selection through the identification of unusually long haplotypes with low diversity surrounding core SNPs. As for XP-EHH, also for iHS we observe peaks of unusually high iHS 10 kb downstream rs10180970 in non-African populations and especially in East-Asians (Fig. 1d).
Altogether these results show that while rs10180970 is the most differentiated variant in the ABCA12 gene, haplotypes downstream rs10180970 differ between Africans and non-Africans. Haplotypes carrying the T allele of rs10180970 are very common in Europeans and Asians and tend to be longer and less diverse in these two populations compared to the others, suggesting that this region might have been affected by recent positive selection in these two populations.
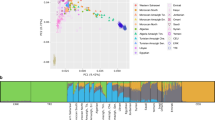
As linkage between variants in a long haplotype can be responsible for spurious signals, we computed linkage disequilibrium (LD) in continental populations between rs10180970 and 2,034 genetic variants in 70 kb surrounding rs10180970. For all populations but Africans, we observe LD within 10 kb downstream rs10180970 (Fig. 2a). In East-Asians and Europeans LD extends over 35 kb downstream rs10180970, in a region inclusive of the first intron and the first exon. Within this block, LD is high in East-Asians (r2 around 0.75) and moderate in Europeans (r2 around 0.50). The genomic region in high LD contains functional features such as chromatin regulation (H3K4Me1 and H3K27Ac), enhancers and a promoter located in the proximity of the first intron and includes SNPs with ΔDAF > 0.70 (Fig. 2b). Therefore we conclude that other SNPs are in high LD with rs10180970 in East-Asians and part of a long haplotype that is located in a genomic region with regulatory features.
Linkage disequilibrium between rs10180970 and variants within 70 kb. (a) Vertical dotted line indicates the genomic position of rs10180970. Circles represent other variants at their genomic position on the x-axis and the r2 measures the non-random association of the alleles of each variant with rs10180970 on the y-axis. Crosses distinguish pairs in which the genetic variants have ΔDAF > 0.70 as shown in Fig. 1. (b) Screen-shot form Genome Browser showing chromatin modification and state segmentation for several cell types in the genomic region under investigation.
No significant association between ABCA12 expression and genotype at rs10180970
Having confirmed the signal of natural selection at rs10180970 we next asked if the two alleles have a different effect on gene expression. Furthermore, in the hypothesis that another variant in linkage with rs10180970 can be responsible for the functional changes, we decided to include also rs2970968 in the analysis. Besides being in linkage disequilibrium with rs10180970 in East-Asians and having high ΔDAF like other variants, rs2970968 has two further features that make it an interesting variant. First, we predicted the functional consequences of the variants with high ΔDAF and in linkage disequilibrium with rs10180970 in East-Asians using Funseq20 (Supplementary Table S2). We confirmed that rs2970968 (leftmost dark red cross in Fig. 2a) is located in a region predicted to be an enhancer21 in lymphoblastoid, HeLa and HUVEC cell lines and that it is located in transcription factor binding peak of several transcription factors, although not in transcription factor motifs within peaks (Supplementary Fig. S1). One of the transcription factors is implicated in mammalian skin tumors (ATF222,23) and three others are regulated by UVB radiation (STAT3, GATA3, and POU2F2)24,25,26. Secondly, rs2970968 is the closest SNP to a region that has shown to be essential for the functioning of the promoter of ABCA1227 (Supplementary Fig. S1).
We investigated ABCA12 expression in relation to the three genotypes of rs10180970 (C/C, C/T, T/T) and rs2970968 (A/A, A/G, G/G). Human skin would be the ideal tissue to test allele-specific gene expression, however sampling technique is invasive. It is instead possible to quantify ABCA12 expression in total mRNA extracted from pulled hairs without the need for invasive skin biopsies28. Because ABCA12 expression is influenced by sun exposure29, to discriminate between the genetic and the environmental effects, we performed the experiment in a group of individuals of different geographical origin but all resident in the same city. We determined the genotypes at rs10180970 and rs2970968 through DNA sequencing and measured ABCA12 expression through quantitative PCR of reverse transcribed total mRNA extracted from pulled hair roots. We considered as reference the average ABCA12 gene expression of genotypes of the ancestral alleles (C/C for rs10180970, and AA for rs2970968), which are also the allele prevalent in Africans, and calculated the fold change of the other observed genotypes. After quality controls, we had data on both genotype and ABCA12 expression for 36 and 39 samples at rs10180970 and rs2970968, respectively. As shown in Fig. 3, there is no significant correlation between ABCA12 gene expression and genotypes at both loci (One-way non-parametric ANOVA p-values > 0.9 for both sites), suggesting no effect of rs10180970 or rs2970968 genotypes on ABCA12 expression in hair roots. Therefore, we conclude that the effect of the selection at these two loci on gene expression is not obvious when individuals are exposed to similar UV radiation.
While it is not possible to model the gene effect in mouse because there is no homologous region in mouse, we found that ABCA12 is well expressed in HaCaT cells, a spontaneously transformed aneuploid immortal keratinocyte cell line from adult human skin30. We determined by Sanger sequencing that HaCaT cells are heterozygous at rs10180970 and therefore can be used for allele-specific expression assessment at this locus. As a preliminary work, we silenced ABCA12 in HaCatT cells using small interfering RNAs and confirmed that ABCA12 expression is reduced after UVB radiation (20 mJ and 30 mJ), with downregulation remaining sustained up to 18 hours after treatment (Fig. 4).
ABCA12 expression in HaCaT cells and UVB irradiation (a) ABCA12 is expressed in HaCaT cells and can be silenced using small interfering RNA. (b) ABCA12 expression is reduced after Ultraviolet B (UVB) radiation and cells do not recover even after 18 hours. T6 and T18 indicate 6 and 18 hours after UVB radiation; mJ = milliJoule.
The T variant of rs10180970 is specific to H. sapiens and rapidly increased in frequency in non-Africans
We determined the pattern of genetic variation at rs10180970 in time and space in 88 ancient and 2,764 contemporary samples ranging the last 80,000 years with the aim to understand when the T allele first appeared and how its frequency evolved through time. Among contemporary samples, 41 were collected for this study and are from 9 geographically diverse populations (Supplementary Table S1). Their genotype at rs10180970 was determined by Sanger sequencing a 600 bp region containing the variant. For the rest of the contemporary samples genotypes at rs10180970 were publicly available from the 1000 Genomes Phase III16 and a study on West Africans31. For ancient samples, we used publicly available raw sequencing data (Supplementary Table S3) to perform variant calling. Beside H. sapiens, ancient samples include also one Denisova sample and two Neanderthals.
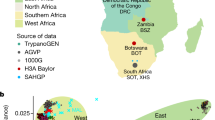
The allele frequency of the derived allele T (fT) through time was determined grouping individuals in bins of 2000 years (Fig. 5). Some pools are made of very few individuals (n = 1 in extreme cases). Individuals were subdivided according to species and within H. sapiens in Africans and non-Africans. In our data, the T allele appeared for the first time around 45 kya in a H. sapiens sample from Russia32 and rapidly fT reached 0.9 in non-Africans remaining stable until the present day.
Prevalence of the T allele of rs10180970 in time and space. (a) T allele trajectory in the last 80,000 years in 88 ancient and 2,764 contemporary samples. The T allele is observed for the first time 45,000 years ago in non-Africans and rapidly rose in frequency. (b) T allele frequency is very low among African populations with the exception of North-Africans (especially Egyptians and Ethiopians) and African American. (c) T allele was probably recently introgressed in African American, as admixture analysis show a shared genetic component of African American with Europeans and East-Asians in the region under investigation. Vertical bars represent single individuals and colors reflect membership to admixture clusters in the most likely hypothesis of five clusters.
Of the 16 African ancient samples available to date, only three have reliable data in the rs10180970 region (Supplementary Table S3). The most ancient African data is from Ethiopia 4.5 kya33, while the other two are from 1.3-2 kya34. All ancient Africans have genotype C/C. In contemporary Africans fT is very low but not completely absent (0.09), while in North-Africans fT is on average 0.4 and T is the major allele in Egyptians, within North-Africans (Fig. 5b). Admixture analysis shows that the Africans and North-Africans with higher fT, in fact, have quite high European and South Asian components probably reflecting recent admixture (Fig. 5c).
Both Neanderthals and the one Denisova sample available are homozygous for the ancestral C allele, suggesting that the T allele might have been specific to H. sapiens, as far as we know about the other two species. In support of this hypothesis, two recent publications find that there is a very little probability of admixture in the region surrounding rs10180970 between H. sapiens and Neanderthals35 (Supplementary Fig. S2) and H. sapiens and Denisova36.
We conclude that the T variant of rs10180970 is specific to H. sapiens and rapidly increased in frequency in non-Africans, while the low prevalence of T in contemporary Africans might be due to recent admixture with Europeans and South-Asians.
Discussion
Humans are exposed to environmental agents through the naked skin, and in fact, strong natural selection has been detected in a number of skin-related phenotypic traits such as hair and eye pigmentation6,37,38, and hair thickness39,40. The lipid barrier is the most external layer of the skin and it acts as an interface with the environment, providing protection against UV, dehydration, and pathogens. The formation of lipid barrier is a complex process and mutations in genes involved in this process result in skin diseases. Nevertheless, to date, to our knowledge, no studies investigated natural selection of genes that contribute to build the lipid barrier such as ABCA12.
The genetic variant rs10180970 located on chromosome 2 in the ABCA12 gene, is predicted to be under positive selection in Europeans and Asians, as we discovered in a genome-wide scan based on population differentiation15. Here we investigated the genomic region containing rs10180970 to ask whether the variant is truly under positive selection and investigated the functional consequences of the selection event.
We confirmed the signal of selection in Europeans and Asians and discovered high linkage disequilibrium in a 35 kb region downstream rs10180970 (Fig. 2). In the 35 kb region the signals of positive selection are as strong or stronger than those initially found at rs10180970 (Fig. 1) and include the promoter of the gene (Fig. 2). By using a larger dataset of DNA sequences and a more fine population subdivision compared to the one used to discover the signal, we observe that the signal is stronger in East-Asians compared to South-Asians, possibly because South-Asians exposure to UV radiation is more similar to that of Africans. Despite confirming the positive selection signal we could not observe obvious allele-specific effect of rs10180970 on ABCA12 gene expression (Fig. 3) in a sample of individuals exposed to the same level of UV radiation. Finally, we traced the history through time of the allele under selection using publicly available ancient human sequences and established that the mutation is specific to H. sapiens (Fig. 5, Supplementary Fig S2). While our findings strongly corroborate the signal of selection they also suggest that a different functional approach should be used to decipher the functional consequences of the event of selection in terms of gene expression.
We suggest that further work is required to fully decipher the effect of selection on ABCA12 and identified the keratinocyte-derived HaCaT cells as a model system for in vitro experiments. We determined that ABCA12 is expressed and can be silenced in the HaCaT cells, and more importantly HaCaT cells are heterozygous at rs10180970, therefore ideal for investigating allele-specific effects. On the other hand, because of the possibility that other sites in linkage with rs10180970 are involved, it would be probably worth while to consider sequence information at other sites as well. More in general, overall indication for future research implicate the study of the non-coding regions of ABCA12, like sequence/haplotype-specific enhancer assay, even if the dimensions of the gene can be a limitation.
There are two main findings from our work. First, we reconstructed the demographic history of the T allele using the currently available information to discover that it is specific to H. sapiens and it was apparently very frequent in humans that migrated out of Africa prior to 50 kya. The second main finding is that while rs10180970 might act as a sentinel for the signal of selection, the signal extends over 35 kb and includes the first intron, the first two exons and the transcription starting site of ABCA12. Linkage disequilibrium in this region is high in East-Asians and Europeans, there are several regions predicted to be enhancers and two regions predicted to be sites of chromatin modifications (methylation and acetylation), thus possibly influencing gene expression.
Our study endorses and refines previous studies. Similar to ABCA12, other members of the ABC family have also been identified in previous scans for positive selection41. The genomic region containing ABCA12 was identified as one of the most differentiated between continental populations42, and in fact ABCA12 hosts one of the top ten SNPs in the genome, useful to discriminate continental populations in clustering analyses43. ABCA12 was identified as one of the top candidate genes in a study investigating local adaptation in Asians44 and stands out in a selection scan performed with LD-based statistics on HapMap Phase II data45. However, none of these studies provides an indication of the exact genomic location of the signal within the gene. In this study, we corroborate the signal of selection at rs10180970 that was localized in our previous study15, and provide initial evidence that will help understand why the T allele of rs10180970 was favored in the ancestors of current Asians, in particular, East-Asians, and Europeans. Our work also shows that with the exception of few cases (e.g.46,47) it is surprisingly difficult to identify the selected allele and selected phenotype, even with abundant sequence data and functional tests.
Methods
Samples and genotyping
Forty samples were collected from healthy fully informed, consenting, adult volunteers from 9 countries (Supplementary Table S1). Saliva samples were collected using the Oragene DNA (OG-500) kit. Genomic DNA was phenol–chloroform extracted from saliva48. All experiments were carried out in accordance with relevant guidelines and regulations. The study was approved by the ethics committee of the University of Naples Federico II (protocol number 106/15) and conducted in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants.
Genotypes at rs10180970 and rs2970968 were obtained by Sanger-sequencing a 325 bp and 400 bp regions, respectively, of the ABCA12 gene where the variants are located. The fragments were amplified from genomic DNA by PCR in a final volume of 50 μl using oligos designed with Primer349,50 and the following conditions: 1 cycle 95 °C for 5′; 35 cycles at 95 °C for 1′, 62 °C for 1′, 72 °C for 45″; 1 cycle at 72 °C for 10′. PCR products were purified using PCR purification kit from QIAGEN.
All other contemporary samples in the study are part of public collections and genotype data at rs10180970 was available in form of vcf files16,18,31. For ancient samples we screened publicly available sequence reads data of 400 samples spanning 80,000–200 years before present32,51,52,53,54,55,56,57,58,59,60,61,62,63,64, however filtering for coverage >2 in the region of interest resulted in 89 samples listed in Supplementary Table S3. Variant calling in ancient samples was performed with samtools/bcftools65.
Derived allele frequencies, linkage disequilibrium, admixture, introgression, natural selection analyses
Derived allele frequencies were computed using vcftools66 using the information on derived allele from the 1000 Genomes Project16. Linkage disequilibrium (LD) between rs10180970 and any other SNP in ±2 Mb was computed with PLINK1.967. Clustering analysis in a region of 2 Mb surrounding rs10180970 including 55,683 Single Nucleotide Polymorphisms (SNPs) was done with ADMIXTURE68. ADMIXTURE was run under the hypotheses of 2 to 10 clusters with cross-validation computation.
Data for checking if the region of rs10180970 is among the ones putatively introgressed form Neanderthal were downloaded from http://genetics.med.harvard.edu/reichlab/Reich_Lab/Datasets_-_Neandertal_Introgression.html.
Integrated haplotype score (iHS) and Cross-Population Extended Haplotype Homozygosity (XP-EHH) were calculated with Hapbin69. Parameters for iHS calculation were–minmaf 0.01, –cutoff 0.01. iHS and XP-EHH log-ratio were normalized to have zero mean and unit variance using the standard normalization implemented in Hapbin that groups variants in bins of 2% frequency.
RNA extraction and processing from hair follicles
Hair follicles (5 to 7 bulbs) were grasped as near to the scalp as possible without damaging hair roots. Total RNA was extracted from bulbs on the day of collection, or following storage at 4 °C for 1–5 days in RNA Later and placed into a 1,5 ml microcentrifuge tube containing 1 ml of Trizol Reagent (Fisher Molecular Biology). RNA extraction was then conducted according to manufacturer’s instructions. 500 ng RNA were reverse-transcribed using SuperScript® VILOTM MasterMix (Invitrogen). qPCR was performed on technical triplicates per each sample using Light Cycler 480® II (Roche), on 2 μl of previously diluted cDNA (1:5) template using LightCycler® 480 SYBR Green I Master Mix (Roche), according to the manufacturer instructions. The thermal profile consisted of 1 cycle at 95 °C for 10′; 40 cycles at 95 °C for 10″, 60 °C for 10″, 72 °C for 10″. Fold changes were calculated relative to Hypoxanthine Phospho Ribosyl Transferase (HPRT1) expression as in70.
HaCat cells culturing, genotyping, silencing and UV radiation
HaCat cells were cultured in 75 cm2 tissue culture flasks in Dulbecco’s Modified Eagle’s Medium, containing 10% fetal bovine serum and 1% penicillin and streptomycin. Cells were maintained in a humidified atmosphere (5% CO2) at 37 °C. Genomic DNA was extracted from HaCat cells71 and genotype at rs10180970 was determined by Sanger sequencing.
For silencing experiments, HaCaT cells were transfected with a ABCA12-targeting siRna (Sigma Aldrich). Transfection was performed with Oligofectamine following the manufacturer’s protocol. Cells were collected 72 hours after transfection. Silencing efficiency was evaluated by Real Time Quantitative PCR (RT-qPCR) following total RNA extraction using RNeasy Mini Kit-QIAGEN and 1 μg and reverse-transcription using SuperScript® VILOTM MasterMix (Invitrogen).
For UVB radiation HaCaT cells were plated in 60 mm plates and grown to 70–90% confluence. Subsequently, cells were irradiated with 20 and 30 mJ/cm2 using three lamps (Philips Ultraviolet 8 TL 20 W/01 RS lamps; Philips, Eindhoven, Netherlands) generating UVB light in the range of 290–320 nm with an emission peak at 312 nm. The intensity of UVB irradiation was measured using a phototherapy radiometer (International Light, Newburyport, MA) of UVB. After irradiation cells were incubated for 0, 6 and 18 hours and ABCA12 expression was assessed by Real Time PCR (RT-PCR). Fold changes were calculated relative to Ribosomal Protein Large P0 (RPLP0) expression as in70,72.
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author on request.
References
Vasiliou, V., Vasiliou, K. & Nebert, D. W. Human atp-binding cassette (abc) transporter family. Human genomics 3, 281 (2009).
Annilo, T. et al. Identification and characterization of a novel abca subfamily member, abca12, located in the lamellar ichthyosis region on 2q34. Cytogenetic and genome research 98, 169–176 (2002).
Akiyama, M. The roles of abca12 in epidermal lipid barrier formation and keratinocyte differentiation. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1841, 435–440 (2014).
van Smeden, J. & Bouwstra, J. A. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. In Skin Barrier Function. 49, 8–26 (Karger Publishers (2016).
Fu, Y. et al. Abca12 regulates abca1-dependent cholesterol efflux from macrophages and the development of atherosclerosis. Cell metabolism 18, 225–238 (2013).
Jablonski, N. G. & Chaplin, G. The colours of humanity: the evolution of pigmentation in the human lineage. Phil. Trans. R. Soc. B 372, 20160349 (2017).
Jablonski, N. G. & Chaplin, G. Human skin pigmentation as an adaptation to uv radiation. Proceedings of the National Academy of Sciences 107, 8962–8968 (2010).
Biniek, K., Levi, K. & Dauskardt, R. H. Solar uv radiation reduces the barrier function of human skin. Proceedings of the National Academy of Sciences 109, 17111–17116 (2012).
Markó, L. et al. Keratinocyte atp binding cassette transporter expression is regulated by ultraviolet light. Journal of Photochemistry and Photobiology B: Biology 116, 79–88 (2012).
Dumitriu, I. et al. Uv irradiation inhibits abc transporters via generation of adp-ribose by concerted action of poly (adp-ribose) polymerase-1 and glycohydrolase. Cell death and differentiation 11, 314 (2004).
Marciniak, S. & Perry, G. H. Harnessing ancient genomes to study the history of human adaptation. Nature Reviews Genetics 18, nrg–2017 (2017).
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient eurasians. Nature 528, 499 (2015).
Ye, K., Gao, F., Wang, D., Bar-Yosef, O. & Keinan, A. Dietary adaptation of fads genes in europe varied across time and geography. Nature ecology & evolution 1, 0167 (2017).
Gelabert, P., Olalde, I., de Dios, T., Civit, S. & Lalueza-Fox, C. Malaria was a weak selective force in ancient europeans. Scientific reports 7, 1377 (2017).
Colonna, V. et al. Human genomic regions with exceptionally high levels of population differentiation identified from 911 whole-genome sequences. Genome biology 15, R88 (2014).
Consortium, G. P. et al. A global reference for human genetic variation. Nature 526, 68 (2015).
Sabeti, P. C. et al. Detecting recent positive selection in the human genome from haplotype structure. Nature 419, 832 (2002).
Drmanac, R. et al. Human genome sequencing using unchained base reads on self-assembling dna nanoarrays. Science 327, 78–81 (2010).
Voight, B. F., Kudaravalli, S., Wen, X. & Pritchard, J. K. A map of recent positive selection in the human genome. Plos biology 4, e72 (2006).
Khurana, E. et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science 342, 1235587 (2013).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43 (2011).
Leslie, M. C. & Bar-Eli, M. Regulation of gene expression in melanoma: new approaches for treatment. Journal of cellular biochemistry 94, 25–38 (2005).
Papassava, P. et al. Overexpression of activating transcription factor-2 is required for tumor growth and progression in mouse skin tumors. Cancer research 64, 8573–8584 (2004).
Bito, T. et al. Ultraviolet light induces stat3 activation in human keratinocytes and fibroblasts through reactive oxygen species and dna damage. Experimental dermatology 19, 654–660 (2010).
Rácz, E. et al. Gata3 expression is decreased in psoriasis and during epidermal regeneration; induction by narrow-band uvb and il-4. Plos One 6, e19806 (2011).
Heinloth, A. N. et al. Identification of distinct and common gene expression changes after oxidative stress and gamma and ultraviolet radiation. Molecular carcinogenesis 37, 65–82 (2003).
Shimizu, Y. et al. A palindromic motif in the- 2084 to- 2078 upstream region is essential for abca12 promoter function in cultured human keratinocytes. Scientific reports 4, 6737 (2014).
Takeichi, T., Sugiura, K., Matsuda, K., Kono, M. & Akiyama, M. Novel abca12 splice site deletion mutation and abca12 mrna analysis of pulled hair samples in harlequin ichthyosis. Journal of dermatological science 69, 259–261 (2013).
Lonsdale, J. et al. The genotype-tissue expression (gtex) project. Nature genetics 45, 580 (2013).
Boukamp, P. et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. The Journal of cell biology 106, 761–771 (1988).
Pagani, L. et al. Tracing the route of modern humans out of africa by using 225 human genome sequences from ethiopians and egyptians. The American Journal of Human Genetics 96, 986–991 (2015).
Fu, Q. et al. Genome sequence of a 45,000-year-old modern human from western siberia. Nature 514, 445–449 (2014).
Llorente, M. G. et al. Ancient ethiopian genome reveals extensive eurasian admixture in eastern africa. Science 350, 820–822 (2015).
Skoglund, P. et al. Reconstructing prehistoric african population structure. Cell 171, 59–71 (2017).
Sankararaman, S. et al. The genomic landscape of neanderthal ancestry in present-day humans. Nature 507, 354–357 (2014).
Sankararaman, S., Mallick, S., Patterson, N. & Reich, D. The combined landscape of denisovan and neanderthal ancestry in present-day humans. Current Biology 26, 1241–1247 (2016).
Wilde, S. et al. Direct evidence for positive selection of skin, hair, and eye pigmentation in europeans during the last 5,000 y. Proceedings of the National Academy of Sciences 111, 4832–4837 (2014).
Crawford, N. G. et al. Loci associated with skin pigmentation identified in african populations. Science (2017).
Sabeti, P. C. et al. Genome-wide detection and characterization of positive selection in human populations. Nature 449, 913 (2007).
Grossman, S. R. et al. A composite of multiple signals distinguishes causal variants in regions of positive selection. Science 327, 883–886 (2010).
Pickrell, J. K. et al. Signals of recent positive selection in a worldwide sample of human populations. Genome research 19, 826–837 (2009).
Tennessen, J. A. & Akey, J. M. Parallel adaptive divergence among geographically diverse human populations. PLoS genetics 7, e1002127 (2011).
Lao, O., van Duijn, K., Kersbergen, P., de Knijff, P. & Kayser, M. Proportioning whole-genome single-nucleotide–polymorphism diversity for the identification of geographic population structure and genetic ancestry. The American Journal of Human Genetics 78, 680–690 (2006).
Qian, W., Deng, L., Lu, D. & Xu, S. Genome-wide landscapes of human local adaptation in asia. PLoS One 8, e54224 (2013).
Jacobs, G. S., Sluckin, T. J. & Kivisild, T. Refining the use of linkage disequilibrium as a robust signature of selective sweeps. Genetics 203, 1807–1825 (2016).
Fumagalli, M. et al. Greenlandic inuit show genetic signatures of diet and climate adaptation. Science 349, 1343–1347 (2015).
Gerbault, P., Moret, C., Currat, M. & Sanchez-Mazas, A. Impact of selection and demography on the diffusion of lactase persistence. PLoS One 4, e6369 (2009).
Sweet, D., Lorente, M., Valenzuela, A., Lorente, J. & Alvarez, J. C. Increasing dna extraction yield from saliva stains with a modified chelex method. Forensic science international 83, 167–177 (1996).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic acids research 40, e115–e115 (2012).
Koressaar, T. & Remm, M. Enhancements and modifications of primer design program primer3. Bioinformatics 23, 1289–1291 (2007).
Prüfer, K. et al. The complete genome sequence of a neandertal from the altai mountains. Nature 505, 43 (2014).
Meyer, M. et al. A high-coverage genome sequence from an archaic denisovan individual. Science 338, 222–226 (2012).
Günther, T. et al. Ancient genomes link early farmers from atapuerca in spain to modern-day basques. Proceedings of the National Academy of Sciences 112, 11917–11922 (2015).
Seguin-Orlando, A. et al. Genomic structure in europeans dating back at least 36,200 years. Science 346, 1113–1118 (2014).
Raghavan, M. et al. The genetic prehistory of the new world arctic. Science 345, 1255832 (2014).
Jones, E. R. et al. Upper palaeolithic genomes reveal deep roots of modern eurasians. Nature communications 6 (2015).
Rasmussen, M. et al. The genome of a late pleistocene human from a clovis burial site in western montana. Nature 506, 225–229 (2014).
Lazaridis, I. et al. Ancient human genomes suggest three ancestral populations for present-day europeans. Nature 513, 409 (2014).
Haak, W. et al. Massive migration from the steppe was a source for indo-european languages in europe. Nature 522, 207 (2015).
Skoglund, P. et al. Genomic diversity and admixture differs for stone-age scandinavian foragers and farmers. Science 344, 747–750 (2014).
Allentoft, M. E. et al. Population genomics of bronze age eurasia. Nature 522, 167–172 (2015).
Rasmussen, M. et al. Ancient human genome sequence of an extinct palaeo-eskimo. Nature 463, 757 (2010).
Malaspinas, A.-S. et al. Two ancient human genomes reveal polynesian ancestry among the indigenous botocudos of brazil. Current Biology 24, R1035–R1037 (2014).
Raghavan, M. et al. Genomic evidence for the pleistocene and recent population history of native americans. Science 349, aab3884 (2015).
Li, H. et al. The sequence alignment/map format and samtools. Bioinformatics 25, 2078–2079 (2009).
Danecek, P. et al. The variant call format and vcftools. Bioinformatics 27, 2156–2158 (2011).
Chang, C. C. et al. Second-generation plink: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome research 19, 1655–1664 (2009).
Maclean, C. A., Chue Hong, N. P. & Prendergast, J. G. hapbin: An efficient program for performing haplotype-based scans for positive selection in large genomic datasets. Molecular biology and evolution 32, 3027–3029 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative pcr and the 2-δδct method. methods 25, 402–408 (2001).
Laird, P. W. et al. Simplified mammalian dna isolation procedure. Nucleic acids research 19, 4293 (1991).
Sirica, R. Analysis of a genomic site in the ABCA12 gene under positive selection in Europeans and Asians. Ph.D. thesis, Universita della Campania Luigi Vanvitelli (2017).
Acknowledgements
We would like to thank all the anonymous volunteers that participated in the project. We also thank Cristina D’Aniello for helping with DNA extraction protocol and Erik Garrison for assisting sampling. A special thanks to Lucia De Martino for helping to submit the project to the ethical committee. C.T.S., Q.A., and Y.X. were supported by the Wellcome Trust grant 098051. C.M. and D.A. were supported by Telethon grant GGP16235. This article is based on chapters of the doctoral thesis of the first author.
Author information
Authors and Affiliations
Contributions
V.C., C.M., Q.A., C.T.S., M.S. and G.D.A. conceived the experiment(s). V.C., R.S., M.B., V.P., L.S., D.T., D.A., O.G., G.A. and H.K. conducted the experiments. V.C., R.S., M.S. and G.D.A., analysed the results. V.C., R.S., M.B., Y.X., Q.A., C.T.S., M.S. and G.D.A. worked at the interpretation of the results. V.C., M.S. and G.D.A. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sirica, R., Buonaiuto, M., Petrella, V. et al. Positive selection in Europeans and East-Asians at the ABCA12 gene. Sci Rep 9, 4843 (2019). https://doi.org/10.1038/s41598-019-40360-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40360-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.