Abstract
Diabetic retinopathy (DR) is a major cause of blindness in working adults in the industrialized world. In addition to vision loss caused by macular edema and pathological angiogenesis, DR patients often exhibit neuronal dysfunction on electrophysiological testing, suggesting that there may be an independent neuronal phase of disease that precedes vascular disease. Given the tremendous metabolic requirements of the retina and photoreceptors in particular, we hypothesized that derangements in metabolic regulation may accelerate retinal dysfunction in diabetes. As such, we induced hyperglycemia with streptozotocin in mice with monoallelic Nampt deletion from rod photoreceptors, mice lacking SIRT3, and mice lacking SIRT5 and tested multiple components of retinal function with electroretinography. None of these mice exhibited accelerated retinal dysfunction after induction of hyperglycemia, consistent with normal-appearing retinal morphology in hyperglycemic Sirt3−/− or Sirt5−/− mice. However, mice lacking both SIRT3 and SIRT5 (Sirt3−/−Sirt5−/− mice) exhibited significant evidence of inner retinal dysfunction after induction of hyperglycemia compared to hyperglycemic littermate controls, although this dysfunction was not accompanied by gross morphological changes in the retina. These results suggest that SIRT3 and SIRT5 may be involved in regulating neuronal dysfunction in DR and provide a foundation for future studies investigating sirtuin-based therapies.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a major cause of blindness in working adults in the industrialized world1. Patients with early-stage disease can exhibit non-proliferative DR, which consists of initial pericyte loss and microaneurysms, followed by capillary wall damage and retinal edema or hemorrhages. If it is left untreated, proliferative DR ensues, leading to hypoxic pathologic neovascularization and further vision loss. Current therapeutic strategies for DR target vascular endothelial growth factor (VEGF)2. While anti-VEGF therapies have greatly improved treatment outcomes, they are not always effective and are sometimes associated with adverse events3,4,5,6,7. Importantly, anti-VEGF therapies do not address the possibility that neuroretinal dysfunction may contribute to vision loss independently from vascular disease. If there is indeed independent neuronal disease, current therapies directed against pathological angiogenesis leave this retinal neuron death completely unabated. Some groups have also suggested that prolonged VEGF blockade in the retina may affect the neuroprotective functions of VEGF that are important in retinal physiology8,9, although not all studies are in agreement10.
In support of the possibility of an independent neuronal component of disease, there have been numerous reports that DR patients exhibit early deficits on electrophysiological testing, such as delayed implicit times and decreased oscillatory potential amplitudes, observed prior to any evidence of vascular dysfunction11,12,13,14,15. Moreover, other studies have found that various rodent models of diabetes, including high-fat diet-induced metabolic dysfunction and streptozotocin (STZ)-induced hyperglycemia, also exhibit electrophysiological changes that are indeed suggestive of retinal neuron dysfunction independent from vascular disease14,16,17. Therefore, there is a great need not only to characterize the neuroretinal dysfunction associated with DR but also to identify the underlying pathogenic mechanisms to facilitate the discovery of novel approaches for targeted interventions.
Past studies have shown that, in the STZ-induced model of diabetes, prolonged systemic hyperglycemia leads to retinal oxidative stress and retinal mitochondrial damage, which ultimately cause retinal neuron death18,19,20. Therefore, we hypothesized that impaired mitochondrial function broadly contributes to retinal neurodegeneration in DR. This hypothesis is consistent with the fact that the retina and photoreceptors in particular have high bioenergetic demands not only to maintain a constant dark current but also to meet their anabolic needs due to constant membrane turnover, which limit their capacity to tolerate metabolic perturbations21,22.
Since we recently demonstrated that NAMPT-mediated NAD+ biosynthesis, SIRT3, and SIRT5 all play important roles in maintaining retinal energetic homeostasis23, we hypothesized that these pathways may also play neuroprotective roles in DR. In this study, we investigated whether partially disrupting NAMPT-mediated NAD+ biosynthesis or deleting SIRT3/SIRT5 would accelerate early neuroretinal dysfunction in the STZ-induced mouse model of type 1 diabetes. In contrast with our hypothesis, we found that abrogating these pathways individually did not accelerate neuroretinal dysfunction in mice based on any of the electrophysiological parameters that we tested. On the other hand, mice lacking both SIRT3 and SIRT5 exhibited significantly more inner retinal dysfunction following induction of hyperglycemia compared to hyperglycemic littermate controls. Together, these findings suggest that SIRT3 and SIRT5 may have potential redundant neuroprotective roles in a mouse model of type 1 DR.
Results
Monoallelic Nampt deletion in rod photoreceptors does not accelerate retinal dysfunction in a mouse model of type 1 diabetes
Biallelic Nampt deletion in rod or cone photoreceptors leads to rapid retinal degeneration that is complete by 6 weeks of age23. However, monoallelic, rod-specific Nampt deletion does not lead to significant retinal degeneration as late as six months of age24, although this does not rule out the possibility that Nampt becomes essential under conditions of metabolic stress, such as in diabetes. Therefore, we sought to determine whether monoallelic Nampt deletion in rod photoreceptors (Nampt−rod/WT) would render rod photoreceptors more vulnerable to degeneration in a mouse model of type 1 diabetes. Both Nampt−rod/WT mice and their Cre-negative controls (NamptF/WT) were equally susceptible to STZ-induced hyperglycemia (Fig. 1a). We tested the retinal function of these mice with electroretinography (ERG) 11 weeks after STZ induction. We found that there were no significant differences in scotopic a-wave, scotopic b-wave, or photopic b-wave amplitudes (Fig. 1b–d). These findings suggest that monoallelic Nampt deletion in rod photoreceptors does not make mice more susceptible to retinal neurodegeneration in a model of type 1 diabetic retinopathy. Thus, Nampt is haplosufficient in this disease context.
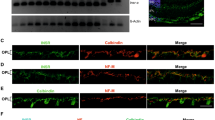
(a) Mice with monoallelic Nampt deletion from rod photoreceptors (Nampt−rod/WT; N = 7) were similarly susceptible to streptozotocin (STZ)-induced hyperglycemia compared to NamptF/WT controls (N = 4; 2-tailed, unpaired t-test). (b–d) At 11 weeks after STZ induction, Nampt−rod/WT mice (N = 6–7) did not exhibit any signs of retinal dysfunction compared to NamptF/WT controls (N = 4) based on their scotopic a-wave, scotopic b-wave, or photopic b-wave amplitudes (2-way mixed ANOVA). (e,f) At 6 weeks after STZ induction in wild-type (WT) mice, there were no changes in retinal SIRT3 or SIRT5 protein levels as measured by immunoblotting shown in (e) with quantifications shown in (f). Open circles depict individual mice (a); graphs depict mean ± S.E.M. (b–d) or mean + S.E.M. (f) Each lane represents an individual mouse; the entire gel is depicted without splicing or cropping (e).
SIRT3 and SIRT5 individually play minimal neuroprotective roles in a mouse model of type 1 diabetes
The fact that monoallelic Nampt deletion did not accelerate retinal neurodegeneration in a mouse model of type 1 diabetes does not completely rule out the possibility that SIRT3 and SIRT5, downstream sensors of NAD+ availability, may still play a role in DR. Because past studies have demonstrated that chronic systemic hyperglycemia induces mitochondrial damage18,19,20, we first evaluated whether there is increased SIRT3 or SIRT5 protein expression in retinas from STZ-induced hyperglycemic, wild-type mice. SIRT3 and SIRT5 were not individually upregulated at the protein level in retinas from hyperglycemic mice compared to those from normoglycemic controls (Fig. 1e,f).
To test whether SIRT3 or SIRT5 exert a neuroprotective role under hyperglycemic conditions, we characterized whether germline deletion of Sirt3 or Sirt5 caused early neuroretinal dysfunction in a mouse model of type 1 diabetes. We found that mice lacking Sirt3 (Sirt3−/−) or Sirt5 (Sirt5−/−) were equally susceptible to STZ-induced hyperglycemia compared to wild-type controls (Fig. 2a–d). Moreover, all mice, regardless of genotype or glycemic status, had similar patterns of weight gain over time (Fig. 2e–h), suggesting a similar systemic burden of disease. When we tested retinal function with ERG, we found that hyperglycemic Sirt3+/+ and Sirt3−/− mice exhibited modest retinal dysfunction compared to their normoglycemic controls in their scotopic a-wave, scotopic b-wave, and photopic b-wave amplitudes (Fig. 3a–f). However, of interest, the magnitude of this difference between STZ- and vehicle-treated mice was similar for both genotypes, suggesting a minimal neuroprotective role for SIRT3. In contrast, hyperglycemic Sirt5+/+ and Sirt5−/− mice did not exhibit retinal dysfunction compared to their normoglycemic controls in their scotopic a-wave, scotopic b-wave, or photopic b-wave amplitudes (Fig. 3g–l).
(a,b) SIRT3 germline knockout (Sirt3−/−) mice (N = 8–9/group) were similarly susceptible to streptozotocin (STZ)-induced hyperglycemia compared to wild-type (Sirt3+/+) controls (N = 10–12/group). (c,d) SIRT5 germline knockout (Sirt5−/−) mice (N = 7–13/group) were similarly susceptible to STZ-induced hyperglycemia compared to wild-type (Sirt5+/+) controls (N = 5–8/group). (e,f) Sirt3+/+ and Sirt3−/− mice (N = 8–12/group) had similar patterns of weight gain after STZ induction. (g,h) Sirt5+/+ and Sirt5−/− mice (N = 5–13/group) had similar patterns of weight gain after STZ induction. Open circles depict individual mice (a–h).
(a–f) At 11 weeks after streptozotocin (STZ) induction, hyperglycemic SIRT3 germline knockout (Sirt3−/−) mice had significant retinal dysfunction based on their scotopic a-wave, scotopic b-wave, and photopic b-wave amplitudes compared to normoglycemic mice of the same genotype (N = 8–9/group; 2-way mixed ANOVA with Bonferroni post-hoc test). Hyperglycemic wild-type (Sirt3+/+) mice also exhibited similar retinal dysfunction compared to normoglycemic controls of the same genotype (N = 11–12/group; 2-way mixed ANOVA with Bonferroni post-hoc test). (g–l) At 11 weeks after STZ induction, hyperglycemic SIRT5 germline knockout (Sirt5−/−) and wild-type (Sirt5+/+) mice did not exhibit any retinal dysfunction compared to normoglycemic controls of the same genotype (N = 8–13/group; 2-way mixed ANOVA). Graphs depict mean ± S.E.M. (a–l) (*P < 0.05; **P < 0.01; ***P < 0.001; ^P < 0.0001).
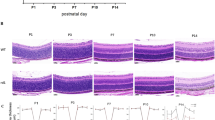
In agreement with this electrophysiological characterization showing similar and only moderate hyperglycemic-induced changes in retinal function between Sirt3+/+ and Sirt3−/− mice, we found no gross differences in retinal morphology between these mice upon histological examination (Fig. 4). Similarly, there were no gross morphological changes between Sirt5+/+ and Sirt5−/− retinas, regardless of whether they were from normo- or hyperglycemic mice (Fig. 4). These data suggest that SIRT3 and SIRT5 individually play only minimal, if any, neuroprotective roles under conditions of hyperglycemia.
Since past reports have suggested that mouse models of DR may exhibit subtle changes in ERG responses, we also assessed whether there were any changes in a- or b-wave implicit times. We did not find any significant differences in scotopic a- or b-wave implicit times in hyperglycemic versus normoglycemic Sirt3+/+ and Sirt3−/− mice (Fig. 5a–d). Photopic b-wave implicit times were mildly reduced when comparing hyperglycemic Sirt3+/+ mice to normoglycemic Sirt3+/+ mice (main effectSTZ: P = 0.0135; Fig. 5e). Although not statistically significant, hyperglycemic versus normoglycemic Sirt3−/− mice showed a similar magnitude of change in photopic b-wave implicit times, arguing for a minimal neuroprotective role for SIRT3 (Fig. 5f). Likewise, except for one pairwise comparison after post-hoc testing, there were no significant differences in scotopic a-wave, scotopic b-wave, or photopic b-wave implicit times in hyperglycemic Sirt5+/+ or Sirt5−/− mice compared to normoglycemic mice of the same genotype (Fig. 6a–f). These data suggest, once again, that SIRT5 plays a minimal neuroprotective role.
(a–d) There were no significant differences in the scotopic a- or b-wave implicit times when comparing hyperglycemic SIRT3 germline knockout (Sirt3−/−) and wild-type control (Sirt3+/+) mice to normoglycemic mice of the same genotype (N = 8–12/group; 2-way mixed ANOVA). (e,f) Hyperglycemic Sirt3+/+ mice had mildly reduced photopic b-wave implicit times compared to normoglycemic Sirt3+/+ mice. Although not statistically significant, there was a similar effect size in hyperglycemic versus normoglycemic Sirt3−/− mice (N = 8–12/group; 2-way mixed ANOVA). Graphs depict mean + S.E.M. (a–f).
(a–f) There were no significant differences in the scotopic a-wave, scotopic b-wave, or photopic b-wave implicit times when comparing hyperglycemic SIRT5 germline knockout (Sirt5−/−) and wild-type control (Sirt5+/+) mice to normoglycemic mice of the same genotype except for one pairwise comparison at a single flash intensity after post-hoc testing (N = 8–13/group; 2-way mixed ANOVA with Bonferroni post-hoc test). Graphs depict mean + S.E.M. (a–f) (^P < 0.0001).
We next evaluated oscillatory potential (OP) amplitudes, which may reveal inner retinal dysfunction. There were no significant differences of appreciable effect size in scotopic OP amplitudes between hyperglycemic Sirt3+/+ and Sirt3−/− mice and normoglycemic mice of the same genotype, except for one pairwise comparison at a single flash intensity after post-hoc testing (Fig. 7a,b). Although photopic OP amplitudes were significantly increased in hyperglycemic versus normoglycemic Sirt3−/− mice (main effectSTZ: P = 0.0113), we observed a similar pattern in hyperglycemic versus normoglycemic Sirt3+/+ mice (main effectSTZ: P = 0.0051) (Fig. 7c,d). Finally, there were no differences in scotopic or photopic OPs in hyperglycemic Sirt5+/+ and Sirt5−/− mice compared to normoglycemic mice of the same genotype (Fig. 7e–h). Together, these findings further support our assertion that SIRT3 and SIRT5 individually play minimal neuroprotective roles in a mouse model of DR.
(a,b) There were no significant differences in scotopic oscillatory potential (OP) amplitudes between hyperglycemic SIRT3 germline knockout (Sirt3−/−) and wild-type control (Sirt3+/+) mice and normoglycemic mice of the same genotype except for one pairwise comparison at a single flash intensity after post-hoc testing (N = 8–12/group; 2-way mixed ANOVA with Bonferroni post-hoc test). (c,d) Although photopic OP amplitudes were significantly increased in hyperglycemic versus normoglycemic Sirt3−/− mice, we observed a similar pattern in hyperglycemic versus normoglycemic Sirt3+/+ mice (N = 8–12/group; 2-way mixed ANOVA). (e–h) There were no significant differences in scotopic or photopic OP amplitudes in hyperglycemic SIRT5 germline knockout (Sirt5−/−) and wild-type control (Sirt5+/+) mice compared to normoglycemic mice of the same genotype (N = 8–13/group; 2-way mixed ANOVA). Graphs depict mean + S.E.M. (a–h) (RMS: root mean square; **P < 0.01).
Combined SIRT3 and SIRT5 deletion is associated with inner retinal dysfunction in a mouse model of type 1 diabetes
Numerous past studies have suggested that SIRT3 and SIRT5 may have some degree of redundancy since they are both mitochondrial deacylases with many of the same protein targets25,26. Moreover, we have previously reported that although mice lacking both SIRT3 and SIRT5 do not exhibit retinal degeneration at baseline, they are more vulnerable to light-induced degeneration23. Therefore, we sought to determine whether combined deletion of both SIRT3 and SIRT5 may render mice more susceptible to retinal dysfunction in a model of type 1 diabetes. Mice lacking both SIRT3 and SIRT5 (Sirt3−/−Sirt5−/−) were equally susceptible to STZ-induced hyperglycemia compared to control littermates of various genotypes, including Sirt3+/−Sirt5+/−, Sirt3−/−Sirt5+/−, and Sirt3+/−Sirt5−/− mice (Fig. 8a). When tested with ERG, hyperglycemic Sirt3−/−Sirt5−/− mice did not exhibit any retinal dysfunction in scotopic a-wave, scotopic b-wave, or photopic b-wave amplitudes at 6 weeks following STZ induction compared to hyperglycemic littermate controls (Fig. 8b–d), consistent with absence of any gross changes in retinal morphology upon histological examination (Fig. 8e). Moreover, hyperglycemic Sirt3−/−Sirt5−/− mice did not show significant changes in a- or b-wave implicit times (Fig. 8f–h). However, hyperglycemic Sirt3−/−Sirt5−/− mice did exhibit reduced scotopic OP amplitudes compared to hyperglycemic Sirt3+/−Sirt5+/− littermate controls, indicative of inner retinal dysfunction, although photopic OP amplitudes were unchanged (Fig. 8i,j). These results suggest that SIRT3 and SIRT5 may play redundant, neuroprotective roles in the inner retina under hyperglycemic conditions.
(a) SIRT3/SIRT5 double knockout mice (3KO5KO) were equally susceptible to streptozotocin (STZ)-induced hyperglycemia compared to littermate controls of various genotypes. (b–d) At 6 weeks after STZ induction, hyperglycemic 3KO5KO mice did not exhibit any retinal dysfunction based on their scotopic a-wave, scotopic b-wave, and photopic b-wave amplitudes compared to hyperglycemic littermate controls (N = 4–7/group; 2-way mixed ANOVA). (e) Representative histological images show that hyperglycemic 3KO5KO mice did not exhibit any changes in retinal morphology compared to hyperglycemic littermate controls. (f–h) Hyperglycemic 3KO5KO mice did not have any significant changes in scotopic a-wave, scotopic b-wave, or photopic b-wave implicit times compared to hyperglycemic littermate controls (N = 4–7/group; 2-way mixed ANOVA). (i,j) Hyperglycemic 3KO5KO mice had significant reductions in scotopic but not photopic oscillatory potential (OP) amplitudes compared to hyperglycemic littermate controls (N = 4–7/group; 2-way mixed ANOVA with Bonferroni post-hoc test). Open circles depict individual mice (a); graphs depict mean ± S.E.M. (b–d, f–j) (RMS: root mean square; **P < 0.01; ***P < 0.001; red asterisks indicate significant differences compared to Sirt3+/−Sirt5+/− mice after post-hoc testing).
Discussion
DR is a major cause of blindness in industrialized nations. Although therapies for DR largely focus on antagonizing VEGF to treat the vascular component of disease, there is increasing evidence that there is independent neuronal disease. Therefore, the motivation behind this study was to test whether there was a neuroprotective role for metabolic regulation via NAMPT-mediated NAD+ biosynthesis, SIRT3, or SIRT5 in a mouse model of type 1 diabetes. We have previously identified that NAMPT-mediated NAD+ biosynthesis is important for retinal energy homeostasis and that both SIRT3 and SIRT5 are important for protecting the retina against light-induced degeneration23,27. In the current study, we found that although these pathways have important roles in regulating some facets of retinal homeostasis, they individually play only minimal neuroprotective roles in retinopathy in a mouse model of type 1 diabetes. Moreover, SIRT3 and SIRT5 may have redundant roles in the inner retina and therefore possess the ability to functionally compensate for one another, as deletion of both was associated with modest, but significant, inner retinal dysfunction in the STZ-induced model of type 1 diabetes.
Although our findings are contrary to our original hypothesis, this study sheds important light on the pathophysiology underlying retinal neuron dysfunction in DR and the role of metabolic regulation in this disease process. NAMPT-mediated NAD+ biosynthesis has been reported to be essential in numerous cell types, including but not limited to rod and cone photoreceptors23, hippocampal and cortical excitatory neurons28, adipocytes29 and skeletal muscle30. Many of these studies have examined animal phenotypes after cell-specific, biallelic Nampt deletion using the Cre-lox system. Although these findings provide important insights that provide a foundation for future research, they also may not be a perfect model for what may be occurring in human disease when NAD+ homeostasis is perturbed, as they involve complete abrogation of an important cellular pathway. As such, we tested whether monoallelic Nampt deletion from rod photoreceptors was sufficient to render rod photoreceptors more vulnerable to dysfunction in a mouse model of diabetes. Our null results indicate that Nampt is haplosufficient in this disease context. These findings suggest that there is likely mitochondrial reserve that enables rod photoreceptors to maintain relative metabolic homeostasis even when challenged by partial disruptions in NAMPT-mediated NAD+ biosynthesis.
Furthermore, we found that germline deletion of neither SIRT3 nor SIRT5 individually affected the progression of retinal dysfunction in the STZ-induced model of type 1 diabetes, while combined germline deletion of both SIRT3 and SIRT5 was associated with inner retinal dysfunction under hyperglycemic conditions. In contrast, single SIRT3 germline knockout mice themselves are more vulnerable to light-induced degeneration compared to wild-type controls27 and SIRT3/SIRT5 double knockout mice are even more vulnerable compared to either single knockout23. These contrasting findings confirm that although there are some similarities in the underlying pathophysiology modeled by light-induced degeneration and STZ-induced hyperglycemia, there are also differences in the cell stress and death pathways that are activated by photopic stimuli versus hyperglycemic stress. Although further studies are necessary to confirm our findings and to provide additional mechanistic insight, our study provides evidence that SIRT3 and SIRT5 may have redundant neuroprotective roles in the inner retina. These findings provide a foundation for future investigation aimed at developing sirtuin-based neuroprotection strategies for DR and other retinal neurodegenerative diseases.
Methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington University in St. Louis and were performed in accordance with all relevant guidelines and regulations. We obtained Namptflox/flox mice from Dr. Shin-ichiro Imai31 and Rhodopsin-iCre75 transgenic mice from Dr. Ching-Kang Jason Chen32. We obtained Sirt3−/− and Sirt5−/− mice from Jackson Laboratories (Bar Harbor, ME) and bred them with the appropriate strain-matched, wild-type mice from Jackson Laboratories (129S1/SvImJ for Sirt3−/− and B6129SF2/J for Sirt5−/−). To induce systemic hyperglycemia, we injected mice daily for five days with 65 mg/kg body weight streptozotocin (STZ; Sigma, St. Louis, MO) freshly prepared in citrate buffer (0.10 M; pH 4.5) and compared hyperglycemic mice to vehicle-treated mice receiving citrate buffer alone. We measured blood glucose levels with the GLUCOCARD Vital (Arkray, Edina, MN) and weighed mice regularly, humanely euthanizing any mice exhibiting signs of diabetic ketoacidosis or other distress.
Electroretinography
We performed electroretinography (ERG) with the UTAS-E3000 Visual Electrodiagnostic System running EM for Windows (LKC Technologies, Gaithersburg, MD), as described previously23. We extracted quantitative measurements, such as a- and b-wave amplitudes and implicit times, from the ERG waveforms using a Microsoft Excel macro that defines the peak of the a-wave as the most negative point of the average trace and the peak of the b-wave as the most positive point without subtracting oscillatory potentials. To isolate oscillatory potentials, we used a custom script in MATLAB (Mathworks, Natick, MA) to digitally process the ERG waveforms with a 25 Hz high-pass filter and calculated the root-mean-square (RMS) of the oscillatory potentials after normalizing to b-wave amplitudes.
Immunoblotting
We compared SIRT3 and SIRT5 protein levels in STZ-induced hyperglycemic versus normoglycemic, wild-type control mice by Western blot. We loaded retinal lysate containing 15 μg protein into individual lanes, separated proteins using SDS-polyacrylamide gel electrophoresis, and then transferred them to a nitrocellulose membrane (0.2 μm, BIO-RAD, Hercules, CA). We then blocked membranes with 2.5% milk in 1X PBS. Next, we incubated membranes at 4 °C overnight with a cocktail of primary antibodies containing 0.1% Tween-20 (v/v); a mouse monoclonal antibody against COX IV (Cell Signaling Technology #11967S, dilution 1:1,000); and either a rabbit monoclonal antibody against SIRT3 (Cell Signaling Technology #5490S, dilution 1:1,000) or a rabbit monoclonal antibody against SIRT5 (Cell Signaling Technology #8782, dilution 1:500). We then incubated membranes with a secondary antibody cocktail containing 0.1% Tween-20 (v/v); goat anti-mouse secondary antibody (IRDye 680CW, LI-COR, dilution 1:5,000); and goat anti-rabbit secondary antibody (IRDye 800RD, LI-COR, dilution: 1:5,000). We visualized blots using an Odyssey CLx Imaging System and quantified protein bands of interest using Image Studio 4.0, normalizing to COX IV band intensity as a loading control. Protein expression of SIRT3 and SIRT5 in hyperglycemic, wild-type mice is provided as a ratio relative to expression in normoglycemic, wild-type mice.
Histology
After euthanizing the mice, we enucleated the eyes and fixed them in 4% glutaraldehyde for 2 hours and then in 4% paraformaldehyde for 24 hours. Next, we embedded the eyes in methacrylate and prepared six sections of 6–8 μm thickness cut through the optic nerve. We stained slides with H&E and acquired bright-field images with an Olympus BX51 microscope.
Statistics
We performed statistical analysis with Prism 5 (GraphPad, San Diego, CA), using the appropriate test for each data set. We considered P < 0.05 to be statistically significant. All data generated or analyzed during this study are included in this published article.
References
Antonetti, D. A., Klein, R. & Gardner, T. W. Diabetic retinopathy. The New England journal of medicine 366, 1227–1239, https://doi.org/10.1056/NEJMra1005073 (2012).
Aiello, L. P. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England journal of medicine 331, 1480–1487, https://doi.org/10.1056/nejm199412013312203 (1994).
Bressler, N. M. et al. Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA ophthalmology, https://doi.org/10.1001/jamaophthalmol.2017.6565 (2018).
Do, D. V. et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA ophthalmology 131, 139–145, https://doi.org/10.1001/2013.jamaophthalmol.91 (2013).
Apte, R. S. Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Does Flavor Matter? JAMA ophthalmology, https://doi.org/10.1001/jamaophthalmol.2017.6559 (2018).
Apte, R. S., Gordon, M. & Kass, M. A. Anti-VEGF Injections and Glaucoma Surgery. JAMA ophthalmology 135, 368–369, https://doi.org/10.1001/jamaophthalmol.2017.0148 (2017).
Apte, R. S. What Is Chronic or Persistent Diabetic Macular Edema and How Should It Be Treated? JAMA ophthalmology 134, 285–286, https://doi.org/10.1001/jamaophthalmol.2015.5469 (2016).
Saint-Geniez, M. et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PloS one 3, e3554, https://doi.org/10.1371/journal.pone.0003554 (2008).
Nishijima, K. et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. The American journal of pathology 171, 53–67, https://doi.org/10.2353/ajpath.2007.061237 (2007).
Miki, A. et al. Prolonged blockade of VEGF receptors does not damage retinal photoreceptors or ganglion cells. Journal of cellular physiology 224, 262–272, https://doi.org/10.1002/jcp.22129 (2010).
Satoh, S., Iijima, H., Imai, M., Abe, K. & Shibuya, T. Photopic electroretinogram implicit time in diabetic retinopathy. Japanese journal of ophthalmology 38, 178–184 (1994).
Bresnick, G. H., Korth, K., Groo, A. & Palta, M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Archives of ophthalmology (Chicago, Ill.: 1960) 102, 1307–1311 (1984).
Tzekov, R. & Arden, G. B. The electroretinogram in diabetic retinopathy. Survey of ophthalmology 44, 53–60 (1999).
Pardue, M. T. et al. Rodent Hyperglycemia-Induced Inner Retinal Deficits are Mirrored in Human Diabetes. Translational vision science & technology 3, 6, https://doi.org/10.1167/tvst.3.3.6 (2014).
Bresnick, G. H. & Palta, M. Temporal aspects of the electroretinogram in diabetic retinopathy. Archives of ophthalmology (Chicago, Ill.: 1960) 105, 660–664 (1987).
Rajagopal, R. et al. Functional Deficits Precede Structural Lesions in Mice With High-Fat Diet-Induced Diabetic Retinopathy. Diabetes 65, 1072–1084, https://doi.org/10.2337/db15-1255 (2016).
Chang, R. C. et al. High-Fat Diet-Induced Retinal Dysfunction. Investigative ophthalmology & visual science 56, 2367–2380, https://doi.org/10.1167/iovs.14-16143 (2015).
Du, Y., Veenstra, A., Palczewski, K. & Kern, T. S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proceedings of the National Academy of Sciences of the United States of America 110, 16586–16591, https://doi.org/10.1073/pnas.1314575110 (2013).
Li, X., Zhang, M. & Zhou, H. The morphological features and mitochondrial oxidative stress mechanism of the retinal neurons apoptosis in early diabetic rats. Journal of diabetes research 2014, 678123, https://doi.org/10.1155/2014/678123 (2014).
Santos, J. M., Tewari, S. & Kowluru, R. A. A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free radical biology & medicine 53, 1729–1737, https://doi.org/10.1016/j.freeradbiomed.2012.08.588 (2012).
Chinchore, Y., Begaj, T., Wu, D., Drokhlyansky, E. & Cepko, C. L. Glycolytic reliance promotes anabolism in photoreceptors. eLife 6, https://doi.org/10.7554/eLife.25946 (2017).
Kooragayala, K. et al. Quantification of Oxygen Consumption in Retina Ex Vivo Demonstrates Limited Reserve Capacity of Photoreceptor Mitochondria. Investigative ophthalmology & visual science 56, 8428–8436, https://doi.org/10.1167/iovs.15-17901 (2015).
Lin, J. B. et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell reports 17, 69–85, https://doi.org/10.1016/j.celrep.2016.08.073 (2016).
Lin, J. B. & Apte, R. S. NAD(+) and sirtuins in retinal degenerative diseases: A look at future therapies. Progress in retinal and eye research, https://doi.org/10.1016/j.preteyeres.2018.06.002 (2018).
Hebert, A. S. et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Molecular cell 49, 186–199, https://doi.org/10.1016/j.molcel.2012.10.024 (2013).
Park, J. et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Molecular cell 50, 919–930, https://doi.org/10.1016/j.molcel.2013.06.001 (2013).
Ban, N. et al. Neuroprotective role of retinal SIRT3 against acute photo-stress. NPJ aging and mechanisms of disease 3, 19, https://doi.org/10.1038/s41514-017-0017-8 (2017).
Stein, L. R. et al. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. The Journal of neuroscience: the official journal of the Society for Neuroscience 34, 5800–5815, https://doi.org/10.1523/jneurosci.4730-13.2014 (2014).
Stromsdorfer, K. L. et al. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell reports 16, 1851–1860, https://doi.org/10.1016/j.celrep.2016.07.027 (2016).
Frederick, D. W. et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell metabolism 24, 269–282, https://doi.org/10.1016/j.cmet.2016.07.005 (2016).
Revollo, J. R. et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell metabolism 6, 363–375, https://doi.org/10.1016/j.cmet.2007.09.003 (2007).
Li, S. et al. Rhodopsin-iCre transgenic mouse line for Cre-mediated rod-specific gene targeting. Genesis (New York, N.Y.: 2000) 41, 73–80, https://doi.org/10.1002/gene.20097 (2005).
Acknowledgements
This work was supported by NIH Grants R01 EY019287 (R.S.A.) and P30 EY02687 (Vision Core Grant); the Starr Foundation (R.S.A.); the Carl Marshall Reeves and Mildred Almen Reeves Foundation (R.S.A.); the Bill and Emily Kuzma Family Gift for retinal research (R.S.A.); a Physician-Scientist Award and a Nelson Trust Award from Research to Prevent Blindness (R.S.A.); the Jeffrey Fort Innovation Fund (R.S.A.); the Glenn Foundation (R.S.A.); and the Thome Foundation (R.S.A.). Additional funding comes from an unrestricted grant to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness. Jonathan B. Lin was supported by the Washington University in St. Louis Medical Scientist Training Program (NIH Grant T32 GM07200) and the Washington University in St. Louis Institute of Clinical and Translational Sciences (NIH Grants UL1 TR002345, TL1 TR002344). Joseph B. Lin was supported by NIH Grant T32 GM07200. We thank Gregory Bligard for help with ERG analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.S.A. and Jonathan B. Lin, Investigation: Jonathan B. Lin, Joseph B. Lin, H.C.C., T.C. Writing – Original Draft: Jonathan B. Lin, Writing – Review & Editing: Jonathan B. Lin, Joseph B. Lin, R.S.A., Supervision: R.S.A., Jonathan B. Lin, Funding Acquisition: R.S.A.
Corresponding author
Ethics declarations
Competing Interests
R.S.A. is a co-founder of Metro International, which is developing NMN-based therapeutics. Jonathan B. Lin, Joseph B. Lin, H.C.C., and T.C. declare no potential conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, J.B., Lin, J.B., Chen, H.C. et al. Combined SIRT3 and SIRT5 deletion is associated with inner retinal dysfunction in a mouse model of type 1 diabetes. Sci Rep 9, 3799 (2019). https://doi.org/10.1038/s41598-019-40177-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40177-6
This article is cited by
-
Nicotinamide mononucleotide, a potential future treatment in ocular diseases
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Sirt5-mediated desuccinylation of OPTN protects retinal ganglion cells from autophagic flux blockade in diabetic retinopathy
Cell Death Discovery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.