Abstract
Vigna unguiculata, one of the most important legumes, mainly in underdeveloped countries, is susceptible to post-harvest losses in storage by Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Chrysomelidae). The work evaluated the toxicity, inhibition of oviposition, instantaneous rate of population growth (ri) and the development of fumigated C. maculatus with the essential oil of Vanillosmopsis arborea and its major constituent, α-bisabolol. The experimental units consisted of 0.8 L flasks treated with concentrations of 1.2–11.2 μL L−1of air of the essential oil of V. arborea or its major constituent applied to disks of filter paper. α-Bisabolol was quantified as 409.33 mL L−1 of the essential oil. The development rate of C. maculatus was evaluated by daily adult counts. Oviposition was evaluated at lethal concentrations (LC50, LC25, LC10 and LC1). The LC50 and LC95 of the essential oil of V. arborea and α-bisabolol were 5.23 and 12.97 μL L−1 of air and 2.47 and 8.82 μL L−1 of air, respectively. At some concentrations, the α-bisabolol was more toxic to males than to females of the insect. Increased concentrations of the essential oil reduced the ri, rate of development, oviposition, and number of eggs of C. maculatus and therefore have potential for pest control.
Similar content being viewed by others
Introduction
Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Chrysomelidae), the main cowpea insect pest1, shows cosmopolitan habit, is found on stored legumes and its biology and ecology have been studied2. Seed destruction by this insect is often so great that the grains become unfit for human consumption and nonviable for replanting or commercialization2 after a few months of storage3. Adequate storage of agricultural products aims to reduce losses due to insect damage using mainly chemical control with pyrethroids, organophosphates or fumigants such as phosphine (with aluminum phosphide being its precursor)4. Overuse of synthetic insecticides causes risks, toxicity to human health and environmental contamination5. Insecticides of plant origin have been investigated to manage insects in stored grains6,7,8. Natural products derived from plants with biologically active compounds9 were the first preservatives used by man, originally in their natural state and later on, as oils obtained through distillation in water10.
Essential oil of Vanillosmopsis arborea, a medicinal plant native to the Araripe National Forest, Ceará, Brazil, is rich in α-bisabolol with antibacterial, antifungal and anti-inflammatory activity11. α-Bisabolol, used in dermatological products12, is a sesquiterpene with antiseptic and mutagenic/antimutagenic properties13, and antimicrobial activity against Escherichia coli and Staphylococcus aureus14.
The objective was to evaluate the toxicity, oviposition inhibition, instantaneous population growth rate (ri) and the development of C. maculatus treated with fumigation using Vanillosmopsis arborea essential oil and its major component α-bisabolol.
Results
Essential oil constitution
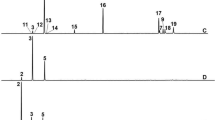
The α-bisabolol (C15H26O) was the major component of the essential oil (Fig. 1) and it was quantified by the retention time of 4.2 min at a concentration of 409.33 mL L−1.
Lethal concentrations
The lethal concentrations (LC) required to kill C. maculatus adults differed between the essential oil and α-bisabolol. The LC50 of 2.47 μL L−1 and the LC95 of 8.82 μL L−1 of air of α-bisabolol were more lethal than those of the V. arborea essential oil with LC50 of 5.23 μL L−1 of air and LC95 of 12.97 μL L−1 of air. The concentration-mortality curve slope for the α-bisabolol was lower (2.97 ± 0.71) than for the V. arborea essential oil (4.17 ± 0.39). The high chi-squared (χ2) and low P (<0.05) values indicate adequacy of the data to the PROBIT model to estimate time-mortality curves (Table 1).
Mortality
C. maculatus mortality from V. arborea essential oil was lower than that of its major component α-bisabolol in almost all concentrations except for 1.2, 1.6, and 11.2 μL L−1 air, which were similar to each other. The α-bisabolol was more efficient and caused higher C. maculatus mortality in five of the eight concentrations tested (2.0 to 8.4 μL L−1 of air) (Table 2). When analyzing the mortality of male and females of C. maculatus, it was noticed that the mortality of males at concentrations of 1.2, 1.6, and 8.4 μL L−1 air was similar between V. arborea essential oil and its major constituent. α-Bisabolol was more toxic to male at the other concentrations. On the other hand, the female mortality with V. arborea essential oil and α-bisabolol was similar in low (1.2 and 1.6 μL L−1 of air) and high concentrations (5.6, 8.4, and 11.2 μL L−1 air). α-Bisabolol were more toxic to female at the other concentrations (Table 3).
Oviposition
The oviposition of C. maculatus exposed to V. arborea essential oil and α-bisabolol was lower than in the control with hexane (Table 4). The decrease in C. maculatus oviposition was proportional to the concentration increase, with α-bisabolol at LC50 responsible for the highest reduction in egg numbers. This confirms the impact of V. arborea essential oil and its α-bisabolol component on C. maculatus oviposition.
Population growth rate
The instantaneous C. maculatus population growth rate (ri) decreased with increasing V. arborea essential oil concentrations (r2 = 0.81, F1.27 = 113.5, P < 0.001) (Fig. 2). The daily emergence of C. maculatus adults exposed to V. arborea essential oil was maximal between four and six days after emergence onset. The normal Log model was the best fit to describe C. maculatus daily emergence exposed to V. arborea essential oil. C. maculatus daily emergence with V. arborea essential oil was higher between the fourth and fifth days after application. The number of adults emerged was higher in the LC1 (0.5106 μL L−1 of air) and LC10 (2.5784 μL L−1 of air) of this oil. The minimum C. maculatus daily emergence was recorded between 12 and 15 days, with lower values for insects exposed to LC50 (5.2318 μL L−1 of air). The maximum daily emergence of the insects in the control was between the fourth and sixth days, similar to that of other treatments (Fig. 3).
Daily emergence of Callosobruchus maculatus (Coleoptera: Chrysomelidae) exposed to the fumigant effect of Vanillosmopsis arborea essential oil, to solvent hexane at concentrations LC1 (1.4481 μL L−1 of air), LC10 (2.5784 μL L−1 of air), LC25 (3.6051 μL L−1 of air) and LC50 (5.2318 μL L−1 of air) and to the oil or solvent (control) for 45 days.
Discussion
The chromatographic analysis of V. arborea essential oil showed that α-bisabolol (C15H26O) is its main component, as reported for this compound making up to 80 and 80.43% of this essential oil15,16. However, factors such as altitude, cultivation, drying conditions, storage, sunshine, temperature, and soil type influence the essential oil composition17, explaining the variation in the amount of its compounds.
The LC50, 2.47 μL L−1 of air to 5.23 μL L−1 of air values of α-bisabolol and of V. arborea essential oil, respectively, are low in relation to those of Cymbopogon winterianus, Eucalyptus camaldulensis, E. citriodora and E. staigeriana, LC50 of 2.58 to 56.7 μL L−1 of air18 and E. globulus essential oil, LC50 of 4 μL L−1 of air19 for C. maculatus. The Heracleum persicum essential oil presented 219.4 μL L−1 of air, for LC50 in C. maculatus with 12 h exposure20 and that of V. arborea was lethal for adults of this insect at low concentrations. This oil is a rich source of biodegradable non-toxic bioactive compounds and is potentially suitable against stored grain pests21.
The C. maculatus adult mortality with these essential oils is due to their volatile compounds such as terpenoids22. The effectiveness of these oils depends on factors such as application surface, composition, ecological conditions, dose or concentration, method of application and extraction, penetration pathway, plant parts, season, and insect species23. The toxicity of V. arborea essential oil for C. maculatus adults agrees with its larvicidal effect for Aedes aegypti (Diptera: Culicidade)24. Gaseous contact of essential oils is neurotoxic, acting on acetylcholine (ACh) inhibition33, and it also affects the lipid bilayer cell membrane, the respiratory system, and energy production16 increasing its toxicity for insects.
The lethality of α-bisabolol at lower concentrations (LC50 and LC95) for C. maculatus compared to that of V. arborea essential oil can be explained by its mechanism of action in the activation of K+ATP channels14. The biological activities of α-bisabolol include antibacterial14, scarring25, mutagenic/antimutagenic activity13, inhibition of mast cell granulation26, inhibition of the human P450 system27, and protection against gastric toxicity induced by acetylsalicylic acid28. The α-bisabolol at concentrations of 0.5 to 2 mM increased the permeability of the plasma membrane of Staphylococcus aureus and Escherichia coli14. α-Bisabolol antimicrobial activity seems to be related to the selective inhibition of ergosterol biosynthesis, antifungal in its pure form or the main component to develop antifungal drugs29.
The lower C. maculatus female mortality from V. arborea essential oil than with its main component agrees with findings for females of this insect exposed to Ocimum gratissimum essential oil, being lower than with its main components30. This can be explained by sexual dimorphism, with females having a larger abdomen and, therefore, being more resistant to these components31. The higher lethality of α-bisabolol for C. maculatus females may be due to their greater susceptibility in the first days after their emergence, when C. maculatus adults begin mating and the female suffers lesions in its reproductive tract, from spikes on the male genitalia32. In addition, the period of greatest egg laying occurs one to two days after female emergence, increasing their susceptibility during the first days of adult life to α-bisabolol acting on acetylcholine (ACh) inhibition33.
The proportional reduction of egg numbers per C. maculatus female with increasing concentrations of V. arborea essential oil and α-bisabolol agrees with findings for Melia azedarach L., Sapindus saponaria L., Piper tuberculatum, and Sapindus saponaria L. on C. maculatus oviposition34, but this activity depends on the plant part from which the extracts were obtained34. This behavior may be associated with the secondary substance levels in different plant parts17. However, C. maculatus survival and oviposition were similar to that with Amburana cearensis, Anadenanthera macrocarpa, Aspidosperma pyrifolium, Cleome spinosa, Croton sonderianus, Hyptis suaveolens, Mimosa tenuiflora, Senna occidentalis, and Ziziphus joazeiro powders35. C. maculatus oviposition varied with different doses of the Eucalyptus citriodora essential oil, from 48.40% to 0.5 mg/100 mg thereof 36. Exposure to Eucalyptus camaldulensis and Heracleum persicum oils reduced C. maculatus oviposition20. The reduction in the number of eggs per C. maculatus female with V. arborea essential oil and its component α-bisabolol in the LC50 can be explained by the higher susceptibility of those mated to monoterpenoids37.
Reduction in the instantaneous C. maculatus population growth rate (ri) with increasing concentrations of the V. arborea essential oil agrees with reports for Zabrotes subfasciatus (Boh.) (Coleoptera: Chrysomelidae) on bean grains treated with ethanolic extract of Croton urucurana leaves38. The larvicide effect of V. arborea essential oil against Aedes aegypti (Diptera: Culicidae) was due to the main constituents of this oil acting individually or synergistically with other constituents24 and explains the reduction in the C. maculatus population growth rate (ri). The barrier effect of the essential oil, together with the lack of respiratory activity and accumulation of toxic metabolites, could explain the death of eggs reducing the population growth rate (ri) and development of C. maculatus in cowpea beans39. In addition, penetration of the oil into the insect egg causes a direct toxic effect, delaying adult emergence and causing adverse effects on the progeny40.
V. arborea essential oil is rich in α-bisabolol and may be an alternative for the the management of C. maculatus and other insect-pests in stored products. This oil has a fumigating insecticidal effect on adults reducing female oviposition, population growth rate, and development of this insect. The pure major constituent of V. arborea essential oil, the α-Bisabolol, caused higher mortality of C. maculatus males than V. arborea essential oil with a mortality proportional to the concentration increase.
Methods
Insects
Adult C. maculatus were obtained from the municipality of Crato, Ceará State, Brazil in 2014. These insects were kept in 1.5 L glass flasks with Vigna unguiculata cv. always-green grains with moisture content of 10.7% wet basis (w.b.). These flasks were kept in an air-conditioned room at a temperature of 27 ± 2 °C, relative humidity of 75 ± 5% and a 12 h photo period.
Essential oil
The Vanillosmopsis arborea essential oil was obtained by hydrodistillation in a five-liter capacity Clevenger in the Laboratory of Product Technology of the Agricultural Sciences Campus of the Federal University of Cariri. The plant material (leaves, branches and wood) was collected at the Araripe National Forest in Ceará, Brazil (7°19′52.0″S, 39°26′15.9″W). The material was placed in a round bottom flask immersed in 1.5 L distilled water. The extraction time was 2 h, time taken for the oil to accumulate in the water in the condenser, subsequently being separated and stored at 4 °C.
Chromatographic analysis
The concentration of α-bisabolol in the V. arborea essential oil was analyzed by Shimadzu GC2010 gas chromatograph (Tokyo, Japan) equipped with flame ionization detector (FID) and DB-5 capillary column (30 m × 0.25 mm × 0.25 μm). The GC configurations were an initial column temperature of 100 °C increasing from 30 °C min−1 until 280 °C was reached, the injector temperature was set at 220 °C, and the detector temperature was set at 300 °C. The separation rate of the samples (1.0 μL) injected was 1:5 with nitrogen as the carrier gas and a flow rate of 1.2 mL min−1. The concentration of α-bisabolol in the essential oil was determined based on the calibration curve constructed from injections of the analytical standard of α-bisabolol purchased from Engetec (São Paulo, Brazil) with purity of 99.9%. The total run time was 6 min.
Fumigation of V. arborea essential oil on C. maculatus adults
The bioassays were carried out in 0.8 L glass flasks (8 cm diameter × 15 cm height) with 20 one to two day-old C. maculatus each in four replications. V. arborea essential oil concentrations ranged from 1.2 to 11.2 μL L−1 of air. The working solutions for the essential oils were prepared with the solvent hexane (Quimex, F. MAIA Ltda., Brazil) and applied with a microsyringe (Hamilton, Reno, NV, USA) on filter paper disks with a 4.4 cm diameter placed in Petri dishes (6.5 cm in diameter). These plates were covered with organza-type fabric and placed in the base of the flasks. The pure solvent (hexane) was used as the control. The flasks were closed with a screw cap and sealed with parafilm (PM996, American, NV, USA), after the insects were distributed, to prevent oil vapor from leaking during the exposure period. The flasks were kept in a climatic chamber (Model 347 CD, Fanem, São Paulo, Brazil) at 27 ± 2 °C for 48 h. After this period, dead and living insects were counted. This procedure was also performed to evaluate the toxicity for C. maculatus males and females with 10 adult insect couples per sample. The average number of dead insects was corrected to adjust their natural mortality and to calculate the efficacy of the essential oil and their respective components by Abbott’s formula41.
Fumigation of α-bisabolol on C. maculatus adults
Pure α-bisabolol was purchased from Engetec (Engenharia das Essências, Brazil). Toxicity assays for LC50 and LC95 were performed at the 1.2 to 11.2 μL L−1 concentrations. Each filter paper disc (4.4 cm) was treated with 25 μL of α-bisabolol solution diluted in hexane in a Petri dish (6.5 cm in diameter) covered with organza and inserted into the base of glass flasks with 0.8 L capacity. Twenty unsexed and 20 sexed C. maculatus adults (toxicity for males and females) were placed per flask to expose the insects to the fumigant activity of the compounds for 48 h. Each treatment had four replications. The control had 25 μL of pure hexane.
Effect of V. arborea/α-bisabolol essential oil on C. maculatus oviposition
The fumigant effect of V. arborea essential oil and its isolated component on C. maculatus oviposition was studied with a lethal concentration and three sub-lethal ones per treatment (LC50, LC25, LC10 and LC1) (Table 4). The control had untreated grains. A total of 25 μL of each oil solution, component and solvent (hexane) was applied on filter paper discs (diameter of 4.4 cm). These discs were placed in Petri dishes (6.5 cm diameter) covered with organza and placed on 100 g of cowpea in glass vials (0.8 L capacity). Ten pairs of C. maculatus, with one-day emergence, were added to each flask. The insects were kept in a climatic chamber at 27 ± 2 °C. After 48 h, the insects were removed and the number of eggs on the beans counted.
Effect of V. arborea essential oil on the instantaneous growth rate (ri) of C. maculatus on cowpea
The assay was organized in a completely randomized design with four replications. Each plot was made up of a glass flask (0.8 L capacity) with 100 g of cowpea grains with a moisture content of 10.7% wet basis (w.b.), free of pests and insecticides. The working solutions of the essential oil were prepared with hexane as solvent and applied with a microsyringe (Hamilton, Reno, NV, USA) onto filter paper discs in Petri dishes (diameter 6.5 cm). The Petri dishes were covered with organza-type fabric to prevent direct contact of the insects with the oil and placed on the beans per flask. In the present study, four lethal and sublethal concentrations of the oil, LC50 (5.231 μL L −1 of air), LC25 (3.605 μL L −1 of air), LC10 (2.578 μL L −1 of air) and LC1 (1.448 μL L−1 of air) were used. The control had untreated grains. The insects were placed in the flasks, which were immediately closed with a screwable metal cap and sealed with parafilm and kept in climatic chambers with a temperature of 30 ± 2 °C and a relative humidity of 70%. After 48 h, the Petri dishes with the essential oil were removed, ending the exposure period for the insects. The metal caps were then removed and the organza flasks returned to the climatic chamber at a temperature of 30 ± 2 °C and relative humidity of 70% for 45 days. The number of live insects and the final weight of the grain mass were evaluated after this period. The instantaneous growth rate of the insects was calculated with the Walthall and Stark equation42 (Eq. 1).
where: Nf = final number of insects; N0 = initial number of insects and Δt = duration (days) of the test.
Effect of V. arborea essential oil on C. maculatus population growth rate on cowpea
Each experimental unit consisted of a glass flask with 0.8 L capacity, containing 100 g of cowpea grains, free of pests and insecticides. The cowpea grains were submitted to treatments in four replications with LC50 (5.231 μL L−1 air), LC25 (3.605 μL L−1 air), LC10 (2.578 μL L−1 air) and LC1 (1.448 μL L−1 of air) of V. arborea essential oil. The control had 25 μL of pure solvent (hexane) and grains without treatment. The solutions of each concentration were applied to the filter paper disks, which were placed in Petri dishes covered with organza, and placed on the grains at the base of the flask. Then, 20 unsexed insects were added to each flask.
The insects were placed in the flasks, which were closed and sealed with parafilm. After 48 h of exposure, the Petri dishes were removed from the flasks, which were covered with organza and packed in a B.O.D. type climatic chamber at 30 ± 2 °C and 70% relative humidity for 15 days. Adult F1 progeny, obtained in the feeding substrate, were counted and removed daily over 45 days from the emergence of the first insect.
Statistical analyses
The toxicity data was submitted to PROBIT analysis with SAS software (SAS Institute, Cary, NC, USA), generating concentration-mortality curves. The instantaneous growth rate (Ri) and population development data were subjected to regression analysis using Sigma Plot 12.0 (Systat Software, San Jose, CA, USA). The effect of essential oil or α-bisabolol on oviposition and mortality efficiency were subjected to ANOVA and Tukey’s test with Statistica 8 software (StatSoft Inc, Tulsa, OK, USA).
References
Heidari, N., Sedaratian-Jahromi, A. & Ghane-Jahromi, M. Possible effects of Ultraviolet ray (UV-C) on biological traits of Callosobruchus maculatus (Col.: Chrysomelidae). J. Stored Prod. Res. 69, 91–98 (2016).
Oliveira, G. B. et al. Variant vicilins from a resistant Vigna unguiculata lineage (IT81D-1053) accumulate in side Callosobruchus aculatus larval midgut epithelium. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 168, 45–52 (2014).
Badii, K. B., Asante, S. K. & Bayorbor, T. B. Susceptibility of some kersting’s groundnut landrace cultivars to infestation by Callosobruchus maculatus (fab.) [Coleoptera: Bruchidae]. J. Sci. Technol. 31, 11–20 (2011).
Sousa, A. H., Faroni, L. R. A., Guedes, R. N. C., Tótola, M. & Urruchi, W. I. Ozone as a management alternative against phosphine-resistant insect pests of stored products. J. Stored Prod. Res. 44, 379–385 (2008).
Wakil, W., Riasat, T. & Lord, J. C. Effects of combined thiamethoxam and diatomaceous earth on mortality and progeny production of four Pakistani populations of Rhyzopertha dominica (Coleoptera: Bostrichidae) on wheat, rice and maize. J. Stored Prod. Res. 52, 28–35 (2013).
Abdelghany, A. Y., Awadalla, S. S., Abdel-Baky, N. F., El-Syrafi, H. A. & Fields, P. G. Stored-product insects in botanical warehouses. J. Stored Prod. Res. 46, 93–97 (2010).
Rajashekar, Y., Kumar, H. V., Ravindra, K. V. & Bakthavatsalam, N. Isolation and characterization of biofumigant from leaves of Lantana camara for control of stored grain insect pests. Ind. Crops Prod. 51, 224–228 (2013).
Suthisut, D., Fields, P. G. & Chandrapatya, A. Contact toxicity, feeding reduction, and repellency of essential oils from three plants from the Ginger Family (Zingiberaceae) and their major components against Sitophilus zeamais and Tribolium castaneum. J. Econ. Entomol. 104, 1445–1454 (2011).
Zebitz, C. P. Effect of some crude and azadirachtin‐enriched neem (Azadirachita indica) seed kernel extracts on larvae of Aedes aegypti. Entomol. Exp. Appl. 35, 11–16 (1984).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475 (2008).
Marco, C. A., Santos, H. R., Feitosa, A. G. S., Feitosa, J. V. & Costa, J. G. M. Contenido, rendimiento y la calidad del aceite esencial de árbol Vanillosmopsis arborea (Gardner) Baker (candeeiro) y su alelopatía. Rev. Cubana Plant. Med. 20, 131–141 (2015).
Oliveira, E. C., Piña-Rodrigues, F. C. M. & Figliolia, M. B. Propostas para a padronização de metodologias em análise de sementes florestais. Rev. Bras. Sementes 11, 1–42 (1996).
Gomes-Carneiro, M. R., Dias, D. M., Oliveira, A. C. & Paumgartten, F. J. Evaluation of mutagenic and antimutagenic activities of α-bisabolol in the Salmonella/microsome assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 585, 105–112 (2005).
Brehm-Stecher, B. F. & Johnson, E. A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 47, 3357–3360 (2003).
Santos, N. K. A., Coutinho, H. D. M., Viana, G. S. B., Rodrigues, F. F. G. & Costa, J. G. M. Chemical characterization and synergistic antibiotic activity of volatile compounds from the essential oil of Vanillosmopsis arborea. Med. Chem. Res. 20, 637–641 (2011).
Santos, N. K. A., Rodrigues, F. F. G., Coutinho, H. D. M., Viana, G. S. B. & Costa, J. G. M. Isolation of alpha-bisabolol from the essential oil of Vanillosmopsis arborea baker and modulation of antibiotic activity using gaseous contact. J. Essent. Oil Bear. Pl. 16, 826–831 (2013).
Souza, A. P. & Vendramim, J. D. Atividade inseticida de extratos aquosos de meliáceas sobre a mosca-branca Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotrop. Entomol. 30, 133–137 (2001).
Gusmão, N. M. S. et al. Contact and fumigant toxicity and repellency of Eucalyptus citriodora Hook., Eucalyptus staigeriana F., Cymbopogon winterianus Jowitt and Foeniculum vulgare Mill. essential oils in the management of Callosobruchus maculatus (FABR.)(Coleoptera: Chrysomelidae, Bruchinae). J. Stored Prod. Res. 54, 41–47 (2013).
Toudert-Taleb, K., Hedjal-Chebheb, M., Hami, H., Debras, J. F. & Kellouche, A. Composition of essential oils extracted from six aromatic plants of Kabylian origin (Algeria) and evaluation of their bioactivity on Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae). Afr. Entomol. 22, 417–427 (2014).
Izakmehri, K., Saber, M., Mehrvar, A., Hassanpouraghdam, M. B. & Vojoudi, S. Lethal and sublethal effects of essential oils from Eucalyptus camaldulensis and Heracleum persicum against the adults of Callosobruchus maculatus. J. Insect Sci. 13, 1–10 (2013).
Nenaah, G. Individual and synergistic toxicity of solanaceous glycoalkaloids against two coleopteran stored-product insects. J. Pest Sci. 84, 77–86 (2011).
Pereira, A. C. R. L., Oliveira, J. V., Gondim Junior, M. G. C. & Câmara, C. A. G. Influência do período de armazenamento de caupi [Vigna unguiculata (L.) Walp.] tratado com óleo essencial e fixos, no controle de Callosobruchus maculatus (Fabricius, 775) (Coleoptera Chrysomelidae: Bruchinae). Ciênc. Agrotec. 23, 319–325 (2009).
Lee, S. E. et al. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag. Sci. 57, 548–553 (2001).
Furtado, R. F., Lima, M. G. A., Neto, M. A., Bezerras, J. N. S. & Silva, M. G. V. Atividade larvicida de óleos essenciais contra Aedes aegypti L.(Diptera: Culicidae). Neotrop. Entomol. 34, 843–847 (2005).
Andersen, F. et al. The hairless guinea‐pig as a model for treatment of acute irritation in humans. Skin Res. Technol. 12, 183–189 (2006).
Miller, T., Wittstock, U., Lindequist, U. & Teuscher, E. Effects of some components of the essential oil of chamomile, Chamomilla recutita, on histamine release from rat mast cells. Planta Med. 62, 60–61 (1996).
Ganzera, M., Schneider, P. & Stuppner, H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 78, 856–861 (2006).
Torrado, S., Agis, A., Jimenez, M. E. & Cadorniga, R. Effect of dissolution profile and (-)-alpha-bisabolol on the gastrotoxicity of acetylsalicylic acid. Pharmazie 50, 141–143 (1995).
Pauli, A. Natural principles for growth inhibition of microorganisms. Int. J. Aromather. 13, 143–146 (2003).
Kéita, S. M., Vincent, C., Schmit, J.-P., Arnason, J. T. & Bélanger, A. Efficacy of essential oil of Ocimum basilicum L. and O. gratissimum L. applied as an insecticidal fumigant and powder to control Callosobruchus maculatus (Fab.) [Coleoptera: Bruchidae]. J. Stored Prod. Res. 37, 339–349 (2001).
Campos, F. A. P., Xavier-Filho, J., Silva, C. P. & Ary, M. B. Resolution and partial characterization of proteinases and α-amylases from midguts of larvae of the bruchid beetle Callosobruchus maculatus (F.). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 92, 51–57 (1989).
van Lieshout, E., McNamara, K. B. & Simmons, L. W. Why do female Callosobruchus maculatus kick their mates? PLoS One 9, e95747 (2014).
Nurulain, S. et al. Inhibitory actions of bisabolol on α7-nicotinic acetylcholine receptors. Neuroscience 306, 91–99 (2015).
Castro, M. J. P., Silva, P. H. S., Santos, J. R. & Silva, J. A. L. Efeito de pós vegetais sobre a oviposição de Callosobruchus maculatus (Fabr.) (Coleoptera: Bruchidae) em feijão-caupi. BioAssay 5, 3–5 (2010).
Melo, B. A., Molina-Rugama, A. J., Leite, D. T., Godoy, M. S. & Araujo, E. L. Bioatividade de pós de espécies vegetais sobre a reprodução de Callosobruchus maculatus (FABR. 1775) (Coleoptera: Bruchidae). Biosci. J. 30, 346–356 (2014).
Singh, R. Evaluation of some plant products for their oviposition deterrent properties against the Callosobruchus maculatus (F.) on chik pea seeds. J. Agric. Technol. 7, 1363–1367 (2011).
Mbata, G. N. & Payton, M. E. Effect of monoterpenoids on oviposition and mortality of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under hermetic conditions. J. Stored Prod. Res. 53, 43–47 (2013).
Carvalho, G. S. et al. Mortalidade e comprometimento do desenvolvimento de Zabrotes subfasciatus Boh. (Coleoptera: Chrysomelidae), induzido pelo extrato de sangra d’água Croton urucurana Baill (Euphorbiaceae). Com. Sci. 5, 331 (2014).
Credland, P. F. The structure of bruchid eggs may explain the ovicidal effect of oils. J. Stored Prod. Res. 28, 1–9 (1992).
Gurusubramanian, G. & Krishna, S. S. The effects of exposing eggs of four cotton insect pests to volatiles of Allium sativum (Liliaceae). Bull. Entomol. Res. 86, 29–31 (1996).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18, 265–267 (1925).
Walthall, W. K. & Stark, J. D. Comparison of two population‐level ecotoxicological endpoints: The intrinsic (rm) and instantaneous (ri) rates of increase. Environ. Toxicol. Chem. 16, 1068–1073 (1997).
Acknowledgements
To the Brazilian agencies Conselho Nacional e Desenvolvimento Cientifico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Dr. Phillip John Villani (The University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author information
Authors and Affiliations
Contributions
L.R.A.F., J.C.Z. and F.F.H. advised the conduction of the experiments. E.S.M., F.F.H. and L.H.F.P. conducted the experiments and drafted the 1st version of the text. L.R.A.F. and J.C.Z. reviewed and edited the final version of the manuscript. All author reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moura, E.d., Faroni, L.R.D., Zanuncio, J.C. et al. Insecticidal activity of Vanillosmopsis arborea essential oil and of its major constituent α-bisabolol against Callosobruchus maculatus (Coleoptera: Chrysomelidae). Sci Rep 9, 3723 (2019). https://doi.org/10.1038/s41598-019-40148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40148-x
This article is cited by
-
A comprehensive review of effective essential oil components in stored-product pest management
Journal of Plant Diseases and Protection (2023)
-
Potential of cold plasma to control Callosobruchus chinensis (Chrysomelidae: Bruchinae) in chickpea cultivars during four year storage
Scientific Reports (2021)
-
Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: prospects and retrospects
Environmental Science and Pollution Research (2021)
-
Evaluation of the Persistence of Linalool and Estragole in Maize Grains via Headspace Solid-Phase Microextraction and Gas Chromatography
Food Analytical Methods (2021)
-
Callosobruchus embryo struggle to guarantee progeny production
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.