Abstract
RAG1 or RAG2 mutations are associated with defects in V(D)J recombination activity, causing severe immunodeficiency with a wide spectrum of clinical phenotypes. A R229Q mutation of RAG2 was identified in patients with severe combined immunodeficiency (SCID) or Omenn syndrome (OS). Although some factors determining the clinical features between SCID and OS were not clear, the molecular mechanism of OS was studied in a mouse model in which an EGFP tag is fused to Rag2 with the R229Q mutation. To design the human disease model mimicking severe immunodeficiency, we generated Rag2-R229Q knock-in mice without an epitope tag. Mutant mice showed impaired T and B cell differentiation with reduced V(D)J recombination activity; however, the extent to which the R229Q mutation affects severe immunodeficiency was not severe. While Rag2-R229Q mutation under some conditions may cause severe immunological and clinical phenotypes similar to human SCID or OS, R229Q mutation per se did not cause severe immunodeficiency in mice, suggesting that additional factors other than R229Q mutation are required to induce severe immunodeficiency. Thus, our report implies that the effects of genetic background and/or a tagged protein sequence may alter the mouse immune system, revealing the mechanism of phenotypic heterogeneity arising from an identical mutation.
Similar content being viewed by others
Introduction
The adaptive immune response to a particular pathogen relies on B and T lymphocytes, which possess genetically rearranged and highly diverse antigen receptors1. During the early stage of B and T cell development, they generate a repertoire of immunoglobulins and T cell receptors by recombining variable (V), diversity (D), and joining (J) gene segments of antigen receptor loci2. Each of the V, D, and J gene segments is flanked by recombination signal sequences (RSSs), composed of conserved heptamer and nonamer elements which are separated by a spacer of either 12 or 23 base-pairs long2. The lymphocyte-specific recombination activating gene 1 (RAG1) and 2 (RAG2) initiate the process of V(D)J recombination by introducing site-specific DNA cleavage at the junction between the RSSs and the adjacent coding segment3. Thus, the RAG genes play an essential role in the rearrangement of the genes encoding antigen-specific receptors of B and T lymphocytes, thereby facilitating the diversity of antigen recognition present in the mature lymphocyte population4.
RAG deficiency in humans leads to severe combined immunodeficiency (SCID), associated with a complete absence of mature B and T lymphocytes5. However, patients with hypomorphic mutations in RAG1 or RAG2 that display partial V(D)J recombination activity can give rise to a wide spectrum of clinical and immunological phenotypes, ranging from Omenn syndrome (OS) to atypical SCID6. In typical OS patients, circulating B cells are mostly absent, whereas the levels of T cells are normal or elevated with a restricted T-cell receptor (TCR) repertoire7. Patients are defined to present atypical SCID when correlated conditions do not fully satisfy the criteria for OS8. While approximately 15% of the SCID-suffering infants in the United States have RAG1 mutations9, the underlying molecular mechanisms of such phenotypic heterogeneity remain unclear.
Recently, crystal structure analysis of RAG protein complex revealed the role of conserved residues and frequently occurring mutations in patients10,11. Based on the structure of the RAG1-RAG2 protein complex, missense mutations leading to SCID or OS can be categorized into four groups: (1) mutations destabilizing the tertiary structure of RAG1-RAG2; (2) mutations affecting polar residues involved in DNA binding; (3) mutations surrounding the active sites; and (4) mutations located at the interface of RAG1 and RAG210. Among these, R229 of RAG2 that forms salt bridges with D546 of RAG110 is regarded to be critical for the development of SCID or OS in patients5,8,12. In addition, homozygous Rag2-R229Q/Enhanced green fluorescent protein (EGFP) mutant mice (hereafter Rag2 KI/EGFP), developed by Marrella and colleagues13, presented clinical and immunological phenotypes remarkably similar to human OS with severe alopecia, erythroderma, infiltration by T lymphocytes and eosinophils into the skin and gut, and complete absence of B cells. However, this mouse model expressed the mutant protein, in which the endogenous Rag2 gene was targeted by a construct containing Rag2-R229Q with an EGFP tag at the N terminus. Furthermore, they created the mutant mice by gene targeting method using mouse 129/Sv embryo-derived stem (ES) cells and performed analysis on a mixed C57BL/6 X129/Sv genetic background13. Although the Rag2 KI/EGFP mice used as murine OS model helped us to understand the detailed pathogenesis of OS and autoimmunity, whether the R229Q mutation itself is sufficient to cause full development of OS and severely affect immunological disorder is still not clear.
Animal models have been providing valuable clues to the aetiology and the molecular pathogenesis of human genetic diseases caused by several types of mutations. Recently, a clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9, identified in bacteria and archaea as the heritable and adaptive immune system, has become a powerful tool for genome editing in eukaryotic cells due to its accuracy, simplicity, and high efficiency14. The CRISPR/Cas9 system is composed of Cas9 endonuclease and a synthetic single guide RNA (sgRNA) that contains a targeting sequence (crRNA) and a Cas9 nuclease-recruiting sequence [transactivating crRNA (tracrRNA)]14. The sgRNA includes 20 nucleotides that are complementary to a target sequence upstream of a PAM sequence (NGG) and directs the Cas9 endonuclease to a specific location in the genome. The Cas9 endonuclease, along with an sgRNA, introduces sequence specific DNA double-strand breaks which results in non-homologous end joining (NHEJ)-mediated insertions and deletions or homology-directed repair (HDR)-mediated repair in the presence of donor templates. In mice, CRISPR/Cas9 has been successfully used for generating knockout or knock-in mice that mimic human diseases15.
In this study, we generated Rag2-R229Q knock-in mice by CRISPR/Cas9-mediated gene editing in C57BL/6 zygotes. Since Rag2 KI/EGFP mouse model has been used to study pathogenesis of OS13 and the R229Q residue of RAG2 has been considered important for lymphocyte development in patients5,8,12, our first intention was to set them up as a potential model for gene therapy of SCID and OS. However, our Rag2-R229Q mutant mouse model exhibited a milder immunological phenotype in lymphocyte development than in the previous Rag2 KI/EGFP mouse model13. Furthermore, we could not observe clinical evidence for OS, possibly due to the absence of a reporter gene and/or the influence of genetic background. Accordingly, our results clearly demonstrated that identical mutation in RAG2 give rise to distinct in vivo phenotypes depending on external factors, while the R229Q mutation of Rag2 by itself is not sufficient to cause the full development of OS and SCID in mice.
Results
Generation of Rag2-R229Q mice by CRISPR/Cas9-mediated gene editing
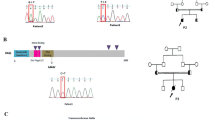
To establish a murine model of severe immunodeficiency with SCID or OS, we generated a Rag2 knock-in mouse carrying the R229Q mutation by CRISPR/Cas9-mediated gene editing. The R229 residue of RAG2, frequently identified in patients with SCID or OS, is considered to be critical for the development of SCID or OS5,8,12. In accordance with this, a previous work13 has shown that Rag2 KI/EGFP mice displayed immunological and clinical phenotypes similar to those of human OS; however, they generated mutant mice in which an EGFP tag is fused to endogenous Rag2 and were bred on a mixed C57BL6 X 129/Sv genetic background. To remove the effects of genetic background and an artificial tag, multiple sgRNAs and a single-stranded oligodeoxyribonucleotide (ssODN) were designed for targeting the exon3 of Rag2 to introduce the R229Q mutation on the pure C57BL/6 genetic background (Fig. 1A). We constructed the ssODN with silent mutations to prevent re-cleaving by CRISPR/Cas9 and to aid Polymerase chain reaction (PCR)-based genotype methods. After co-injection of Cas9 mRNA, sgRNAs, and ssODN into pure C57BL/6 zygotes, we screened the founder mice by PCR amplification on the Rag2 locus. Among 27 newborns, we obtained one targeted knock-in mouse (Supplementary Table S1). To test germline transmission and reduce potential risk of off-target effects, the mutant mouse was continually bred with C57BL/6 wild-type mice and confirmed the presence of the Rag2-R229Q mutation in their progeny through genomic sequencing (Fig. 1B). The heterozygous mutant mice were subsequently intercrossed to generate homozygous mutant mice (Fig. 1C). The homozygous Rag2-R229Q knock-in (hereafter Rag2 KI) mice were fertile and survived into adulthood with no obvious development defects, compared to wild-type strains (hereafter Rag2 WT; data not shown). It is worthwhile to note that unlike Rag2 KI/EGFP mice, our Rag2 KI mice did not show any pathological phenotypes of human OS such as severe alopecia and skin erythroderma (Supplementary Fig. S1).
Introduction of the R229Q mutation in Rag2 locus by CRISPR/Cas9-mediated gene editing. (A) A schematic representation of sgRNA targeting sites in Rag2 and sequence of ssODN. The sgRNA targets and PAM sequences are shown in blue and red, respectively. The 173-bp ssODN donor template is presented under the genomic DNA with a R229Q mutant sequence in green. The silent mutations used to prevent re-cutting by Cas9 are underlined, whereas the bold and underlined sequences are the ssODN donor template sequences that were successfully introduced into Rag2 locus. (B) PCR analysis of genomic DNA from wild-type and Rag2-R229Q knock-in mice. Specific bands were observed after genotyping at the expected DNA size: Rag2 wild-type (WT) = 300 bp; Rag2 knock-in (KI) = 188 bp. (C) Comparison of genomic DNA sequences between Rag2 wild-type and Rag2 R229Q mutation.
A mild impairment of early T cell development in Rag2-R229Q knock-in mice
To investigate the effects of the Rag2-R229Q mutation on T cell development, we performed fluorescence-activated cell sorting (FACS) analysis for thymocytes in Rag2 KI mice. During T cell development, immature T cell progenitors in the thymus progress from the CD4−CD8− double-negative (DN) to the CD4+CD8+ double-positive (DP) stage. According to previous Rag2 KI/EGFP mouse model, most thymocytes were at DN stages (3% in Rag2 WT versus 89% in Rag2 KI/EGFP)13. In contrast, only minor population of thymocytes from Rag2 KI mice was observed at DN stage (3.8% in Rag2 WT versus 15.5% in Rag2 KI, Fig. 2A). Nevertheless, we found that Rag2 KI mice showed a marked increase in the number and percentage of DN population, suggesting that thymocyte development was partially arrested at the DN stage (Fig. 2A and Supplementary Fig. S2). The DN population is divided according to the expression of CD44 and CD25, into four subsets: CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3), and CD44−CD25− (DN4)16. TCRβ rearrangement is initiated by Dβ-Jβ recombination during transition from the DN2 to DN3 stage and completed by Vβ-DJβ joining at the DN3 stage17. Further analysis revealed that the DN3 cell numbers from Rag2 KI mice were highly accumulated, whereas the DN4 cell numbers were reduced, indicating the arrest of thymocyte development at the DN3 stage (Fig. 2B and Supplementary Fig. S2). While mice lacking either RAG1 or RAG2 displayed a complete block in thymocyte development at the DN3 stage18,19, our data support that the R229 residue of Rag2 affects rearrangement of TCRβ locus.
Impaired T cell development in Rag2-R229Q KI mice. Flow cytometric analyses were performed on wild-type (hereafter Rag2 WT) and homozygous Rag2-R229Q knock-in (hereafter Rag2 KI) mice. (A) Thymocytes were stained with CD8 and CD4 antibodies. Percentages of each quadrant are summarized in the bar graph (n = 8). DN, Double negative; DP, Double-positive. (B) CD4−CD8− (DN) cells were gated and stained with CD25 and CD44 antibodies. Percentages of each quadrant were summarized in the bar graph. DN1, CD44+CD25−; DN2, CD44+CD25+; DN3, CD44−CD25+; DN4, CD44−CD25−. (C) CD4+CD8+ (DP) cells were gated and stained with TCRβ and CD69 antibodies. Numbers in the plots indicate the percentages of cells in each quadrant. Percentages of each quadrant are summarized in the bar graph. (D) TCRβ expressions on the indicated thymocyte subsets were analysed by flow cytometry. Gray-tinted histograms, Rag2 WT; black histograms, Rag2 KI. Bar graphs show mean ± standard error of the mean (SEM). n = 8 per group. ***p < 0.001.
To further characterize the population of DP cells, we analysed the cells using antibodies to TCRβ and CD69. Despite the arrest of thymocyte development at the DN3 stage, the percentage of DP cells undergoing positive selection was comparable in both Rag2 WT and KI mice (5.5% in WT versus 5.3% in KI, Fig. 2C). While positively selected thymocytes are developed into mature CD4+ and CD8+ single-positive (SP) T cells that express TCRαβ, CD4+ and CD8+ SP T cells from the thymus of Rag2 KI mice exhibited high TCRβ expression in both Rag2 WT and KI mice, suggesting that surviving T cells were accumulated as SP T cells (Fig. 2D). Taken together, our results indicate that Rag2 KI mice exhibited enhanced accumulation of thymocytes at the DN stage and led to the impairment of thymocyte development at the DN-to-DP transition. However, the majority of surviving T cells were typically differentiated to CD4+ and CD8+ SP T cells in the thymus of Rag2 KI mice.
An alteration of T cell differentiation in the periphery of Rag2-R229Q knock-in mice
We then examined the T cell compartment in peripheral lymphoid organs (spleen and inguinal lymph nodes) and non-lymphoid organs (lung and liver). Consistent with the reduction in the number of T lymphocytes in the thymus, the absolute numbers of lymphocytes in peripheral lymphoid and non-lymphoid organs were mostly reduced in Rag2 KI (Supplementary Fig. S3). However, the percentages of CD8+ as well as CD4+ T lymphocytes were varied between tissues, suggesting the abnormal distribution of peripheral T cells (Fig. 3A). SP T cells in the thymus can be divided into naïve and memory cells by the expression of CD44 and CD62L20. In patients with OS, it has been reported that T cells are highly activated in the peripheral blood21. Moreover, Rag2 KI/EGFP mice showed that activated T cells infiltrate gut and skin and the majority (~80%) of T cell subsets from lymph nodes were CD44+CD62L−, implying that SP T lymphocytes were predominantly present as effector/memory-like cells13. However, the percentage of CD44+CD62L− T cells from lymph nodes of Rag2 KI mice was slightly increased (Fig. 3B,C). Therefore, these results suggested that although the Rag2-R229Q mutation affects T cell differentiation in periphery, the extent to which the mutation contributes to T cell activation was not as severe as previously reported from Rag2 KI/EGFP mice13.
T cells and their phenotype in lymphoid and non-lymphoid organs. Cells were isolated from the spleen (SP), lymph node (LN), lungs (LG), and liver (LV) of Rag2 WT and KI mice. (A) Expressions of CD8 and CD4 on lymphocytes were analysed by flow cytometry. Numbers in the plots indicate the frequency of CD8+ and CD4+ cells in the indicated tissues, and the frequency of CD8+ and CD4+ cells were summarized in the bar graph. (B,C) CD8+ (B) and CD4+ (C) T cells were analysed for CD44 and CD62L expression. Numbers in the plots indicate the percentages of cells in each quadrant. Frequency of CD44+CD62L− are summarized in the bar graph. Bar graphs show mean ± SEM. n = 8 per group. *p < 0.05, **p < 0.01, ***p < 0.001.
Enhanced effector/memory-like phenotype of FoxP3+CD4+ regulatory T cells in Rag2-R229Q knock-in mice
Next, we determined the presence of naturally occurring FoxP3+CD4+ regulatory T (Treg) cells, which play a crucial regulatory role in the pathogenesis of autoimmune diseases22. Interestingly, OS patients generally develop autoimmune phenomena with immunodeficiency23. However, a possible explanation for this paradoxical observation is the severe reduction in the number of Treg cells in Rag2 KI/EGFP mice13. To test whether Treg cell population was changed or not in Rag2 KI mice, we analysed the expression of FoxP3 that acts as the transcription factor of regulatory T cell lineage specification on CD4+ T cells. Surprisingly, FACS analysis revealed that the percentage of CD4+ T cells expressing FoxP3 was increased in Rag2 KI mice, while the absolute numbers of FoxP3+ Treg cells were comparable between Rag2 WT and KI mice (Fig. 4A). Moreover, FoxP3+CD4+ Treg cells obtained from the spleen, inguinal lymph nodes, lungs, and livers displayed enhanced effector/memory-like (CD44+CD62L−) phenotype in Rag2 KI mice (Fig. 4B). Therefore, these findings indicate that the R229Q mutation of Rag2 per se does not contribute to the reduction of Treg cells and the development of autoimmunity.
FoxP3+ Treg cells in Rag2 KI mice. Cells were isolated from the spleen (SP), lymph node (LN), lungs (LG), and liver (LV) of Rag2 WT and KI mice. (A) Expressions of FoxP3 and CD25 on CD4+ T cells were analysed by flow cytometry. Numbers in the plots indicate the frequency of FoxP3+ cells in the indicated tissues, and the frequency and absolute number of FoxP3+ cells are summarized in the bar graph. (B) FoxP3+ Treg cells were analysed for CD44 and CD62L expression. Numbers in the plots indicate the percentages of cells in each quadrant. Frequency of CD44+CD62L− cells is summarized in the bar graph. Bar graphs show mean ± SEM. n = 8 per group. *p < 0.05, **p < 0.01, ***p < 0.001.
Little disparity of TCRVβ diversity in Rag2-R229Q knock-in mice
Since T cell development of Rag2 KI mice was arrested at the DN3 stage, we further investigated the repertoire of T cell receptor beta chain variable genes (TCRVβ) from the spleens of Rag2 WT and KI mice. As shown in Fig. 5, there was no significant difference in the frequencies of TCRVβ family members between Rag2 WT and KI mice. We also analysed the TCRVβ diversity by diverse indices such as Shannon-Weaver index H, Inverse Simpson’s index 1/λ, Pielou’s evenness, and Diversity Evenness score (DE50). Although the diverse indices proposed that there was a decrease in the scores representing the TCRVβ diversity in Rag2 KI mice, the difference between Rag2 WT and KI mice was not statistically significant (Table 1).
A mild impairment of B cell development in Rag2-R229Q knock-in mice
In patients with OS or SCID, circulating B lymphocytes are generally absent23. We therefore accessed the role of Rag2-R229Q mutation in B cell development. Although the frequency and absolute number of B220+ cells from Rag2 KI mice were reduced in the bone marrow, spleen, and inguinal lymph nodes compared with Rag2 WT mice, B cells were not absent (Supplementary Fig. S4). To delineate the transition of B cells, we stained bone marrow cells with B220 and CD2 antibodies. Rag2 KI mice showed a significant reduction in B22010CD2+ pre-B cells and B220hiCD2+ cells including immature and mature recirculating B cells (Fig. 6A and Supplementary Fig. S4). The immature transitional B cells migrate to the peripheral blood and complete their maturation in the spleen24. To discriminate between transitional B cells and mature recirculating B cells, we further discriminated them by the expression of IgM and IgD. FACS analyses from bone marrow cells revealed that the percentage of IgD+IgM− transitional B cells was reduced in Rag2 KI mice, while the absolute numbers of IgD+IgM− transitional B cells were comparable between Rag2 WT and KI mice (Fig. 6B and Supplementary Fig. S4). In contrast to transitional B cells, the percentage of mature recirculating B cells was increased in Rag2 KI mice (Fig. 6B). However, the number of mature recirculating B cells was decreased, possibly due to a reduction in total B cells from the bone marrow of Rag2 KI mice (Supplementary Fig. S4). We next examined the percentages of IgD+IgM− transitional B and IgD+IgM+ mature follicular B cells in the spleen and lymph nodes. While the percentage of IgD+IgM− transitional B cells was only reduced in the spleen of Rag2 KI mice, that of IgD+IgM+ mature follicular B cells was increased in both the spleen and lymph nodes of Rag2 KI mice (Fig. 6C). Further analysis was performed with CD21 and CD23 antibodies to discriminate between mature follicular B and marginal zone B cells. The frequency of CD21+CD23+ mature follicular B cells was increased whereas that of CD21+CD23− marginal zone B cells was decreased (Fig. 6D). However, absolute numbers of transitional, mature follicular, and marginal zone B cells were reduced in peripheral lymphoid organs of Rag2 KI mice compared with Rag2 WT mice except those of marginal zone B cells in the spleen (Supplementary Fig. S4). Accordingly, these results indicate that Rag2-R229Q mutation impairs B as well as T cell development; however, the effect is not as severe as previously reported from Rag2 KI/EGFP mutant mice13.
Impaired B cell development in Rag2 KI mice. Cells were isolated from the bone marrow (BM) (A and B), spleen (SP), and lymph node (LN) (C and D) of Rag2 WT and KI mice. (A) Expressions of B220 and CD2 on lymphocytes were analysed by flow cytometry. Numbers in the plots indicate the percentage of B22010CD2+ and B220hiCD2− cells in BM. The frequency of B220loCD2+ or B220hiCD2− cells is summarized in the bar graph. (B) Expressions of IgM and IgD on B220+ cells were analysed by flow cytometry. Numbers in the plots indicate the frequency of IgD+IgM− and IgD+IgM+ cells in BM. The frequency of IgD+IgM− and IgD+IgM+ cells is summarized in the bar graph. (C) Expressions of IgM and IgD on B220+ cells were analysed by flow cytometry. Numbers in the plots indicate the frequency of IgD+IgM− and IgD+IgM+ cells in SP and LN. The frequency of IgD+IgM− or IgD+IgM+ cells is summarized in the bar graph. (D) B220+ cells were analysed for CD21 and CD23 expression. Numbers in the plots indicate the percentages of CD21+CD23− and CD21+CD23+ cells in SP and LN. Frequency of CD21+CD23− or CD21+CD23+ cells is summarized in the bar graph. Bar graphs show mean ± SEM. n = 8 per group. *p < 0.05, ***p < 0.001.
OS patients are generally characterized by alopecia, skin erythroderma, and severe immunodeficiency. As mentioned above, we could not observe any symptoms related to alopecia and erythroderma with age in Rag2 KI mice (Supplementary Fig. S1). Besides, Rag2 KI mice did not lead to a severe immunodeficiency with T−B− SCID or T+B− OS. Additional OS phenotypes present low serum immunoglobulins but paradoxically, elevated serum IgE. However, there were no significant changes in serum immunoglobulins except IgG1 (Fig. 7). Taken together, the results presented here suggest that R229Q mutation per se does not lead to severe immunodeficiency in mice.
Discussion
The relationship between genotype and phenotype in monogenic diseases is important to predict the onset, penetrance, and severity of diseases. However, accumulating evidence suggests that identical or similar RAG mutations in relatives can lead to different clinical phenotypes12,25,26,27, indicating that mutations involved in V(D)J recombinase activity are not solely responsible for the clinical outcome. Therefore, investigating the pathogenesis of the RAG gene variant is a challenging task. Although, several algorithms have been developed to predict the pathogenicity of the RAG gene variants, these approaches have failed to elucidate the cause of the phenotypic variation23. While several studies proposed that additional factors may be required for determining distinct phenotypes from identical or similar RAG mutations8, there was no direct evidence that such factors are important contributors to the development of different phenotypes in mice. In the present study, we generated the Rag2 KI mice by the CRISPR/Cas9 system and compared the immunological phenotypes with Rag2 KI/EGFP mice as previously reported13. Intriguingly, Rag2 KI mice displayed milder immunological phenotypes than Rag2 KI/EGFP mice and there were no clues to the determinants of OS phenotypes including severe alopecia, B cell numbers, and elevated IgE levels in Rag2 KI mice. Therefore, R229Q mutation per se is not sufficient for the development of OS and SCID in mice.
The R229Q mutation of Rag2 is well described in patients leading to severe immunodeficiency with T−B−SCID or OS5,8,12. Biochemical analysis of recombinant RAG2 proteins suggests that the R229 residue is critical for DNA binding and catalysis28. While Rag2 KI/EGFP mice recapitulate human OS13, we initially expected that our Rag2 KI mice will give rise to an immunodeficiency characterized by SCID or OS. How can identical R229Q mutations give rise to different immunological phenotypes in mice? The discrepancy may be explained by the existence of additional factors required for the development of OS. One possibility is the potential effect of EGFP on Rag2-R229Q mutation. Although the EGFP-tagged gene served as a useful reporter system to monitor its tissue specific gene expression in living animals, growing evidences suggested that EGFP itself can alter important cellular functions such as protein stability29. In RAG2-transgenic mice, it was revealed that the half-life of RAG2-GFP was 2–3 days, probably much longer than that of the endogenous RAG2 protein30. Since the expression of RAG proteins is restricted to immature lymphocytes, the long-term effect of R229Q mutation on lymphocytes of Rag2 KI/EGFP mice can lead to severe immunodeficiency. Another plausible explanation is the influence of genetic background. We established the Rag2 KI mice on a C57BL/6 background, while all experiments from Rag2 KI/EGFP were performed on a mixed 129/Sv and C57BL/6 genetic background. However, we previously reported that the Rag2 knockout mice, generated by CRISPR/Cas9-mediated gene editing on C57BL/6 and FVB/N genetic backgrounds, showed similar immunological phenotypes observed on the mixed 129/Sv and C57BL/6 genetic background31. Based on such observations, we thought that the EGFP tag might be a major cause of the discrepant phenotypes of Rag2-R229Q knock-in mice. Nevertheless, we may not exclude the potential effects of the genetic background in the mice. Therefore, our results suggest that phenotype variability in a given genotype may stem from the difference in the gene constructs and mouse strains.
Since the RAG proteins are not expressed in mature lymphocytes, the steps required for isolation of immature lymphocytes from patients should be preceded by the appropriate method. For this reason, it was difficult to study the pathogenesis of the RAG variants in vivo23. Therefore, the development of mouse models mimicking human immunodeficiency (SCID and OS) allowed us to investigate the pathogenesis of immunological disorders. Although our data suggested that an identical mutation in RAG2 can give rise to distinct phenotypic patterns with heterogeneous clinical manifestations, there is little debate about the importance of mouse models. In parallel, hypomorphic RAG mutations were recently identified in patients with combined immunodeficiency associated with granulomas and/or autoimmunity (CID-G/AI)32. However, the lack of appropriate mouse models recapitulating the CID–G/AI is a stumbling block to characterize the pathophysiology of this phenotype23. Overall, our data propose that generating mouse models for heterogeneous conditions should consider the pathogenic mutations and mouse strains with the complexity of factors affecting the in vivo phenotypes.
Materials and Methods
Preparation of CRISPR/Cas9 mRNA and sgRNAs
Cas9 mRNA was synthesized as described previously33 with slight modifications. In brief, we used the mMESSAGE mMACHINE T7 Ultra kit (ThermoFisher Scientific, #AM1345) to synthesize Cas9 mRNA. The resulting products were diluted into diethyl pyrocarbonate (Sigma Aldrich, #D5758)-treated injection buffer (0.25 mM EDTA pH 8.0 and 10 mM Tris pH 7.4). The sgRNAs targeting exon3 of Rag2 were obtained from ToolGen, Inc. (Seoul, Republic of Korea). To avoid potential risk of off-target effects, each sgRNA was designed to recognize a specific target sequence that does not share homology with any sequence of mouse genome. After screening the sgRNA cleavage efficiency in vitro, two sgRNAs were selected for zygote injection. The sequences of Rag2 sgRNAs used in injection are listed as follows: sgRNA #1 (5′- GTTAGCAGGGCGTATATTACTGG-3′) and sgRNA #2 (5′-CTTATTCTATACAAGTTAGCAGG-3′).
Preparation of the donor template
The ssODN donor template was designed to contain 173 nucleotides with homology arms, a R229Q mutation at Rag2 gene, and blocking silent mutations to prevent re-cutting by Cas9 to aid PCR-based genotype analysis. Polyacrylamide gel electrophoresis (PAGE)-purified ssODN, oriented in the antisense direction, was synthesized from Integrated DNA Technologies (IDT, USA) and dissolved in the injection buffer (0.25 mM EDTA pH 8.0 and 10 mM Tris pH 7.4). The sequence of the ssODN was as follows: 5′-CACTGGAGACAGAGATTCCTCCTGGCAAGACTGTGCAATTCACTGCTGGGGTACCCAGGGGAAGGTCCACGCGAATGCGGTAAAGATTTGCTGGCTGGATGTTACTGGCAAGTGAGTGTCCTCCCAAAATATAAACGGTATCGTTTCTGGCAATAGAAACATGAAAAGACAGC-3′
Animals and microinjection
C57BL/6JBomTac mice were purchased from Taconic Biosciences (Dae Han Biolink Co., Ltd., Chungbuk, Republic of Korea). To obtain mouse zygotes, female C57BL/6J mice (6‒8 weeks) were superovulated by intra-peritoneal injections of 5 IU pregnant mare serum gonadoptropin (Daesung Inc., Republic of Korea) and 5 IU human chorionic gonadotropin (Daesung Inc., Republic of Korea) at a 48-h interval. Mouse zygotes were collected by mating the superovulated females and wild-type C57BL/6J males. Prior to microinjection, Cas9 mRNA (20 ng/μl), two Rag2-sgRNAs (100 ng/μl in each), ssODN (200 ng/μl), and Scr7 (1 mM) were mixed in injection buffer (0.25 mM EDTA, pH 8.0 and 10 mM Tris, pH 7.4) and injected into the cytoplasm using a piezo-driven manipulator (Prime Tech). The manipulated embryos were transferred into the oviducts of foster mothers to produce mutant mice. All animal care and experiments were performed in accordance with the guidelines for Korean Food and Drug Administration (KFDA) and were approved by the Institutional Animal Care and Use Committees (IACUC) of the Laboratory Animal Research Center at Yonsei University (Permit Number: 201507-390-01). All mice were maintained in the specific pathogen–free (SPF) facility of the Yonsei Laboratory Animal Research Center.
Founder screening and genotyping PCR
Founder mice were identified by PAGE-based PCR genotyping from tail biopsies34. PCR amplification was performed with the primers encompassing the sgRNA target sites. Following PAGE-based PCR genotyping, the resulting PCR products were isolated using the DNA purification kit (iNtRON Biotechnology, #17290) and then cloned into the TA cloning vector (SolGent Co., Ltd., Republic of Korea). The mutant sequence of cloned PCR products was confirmed by DNA sequencing. For routine genotyping, the following primers were used for detecting both wild-type and mutant alleles: WT-F (5′-TGT CCCTGAACCCAGATACG-3′), MT-F (5′-AACATCCAGCCAGCT-3′), WT-R (5′-TTAGCAGGGCGTATATTACT-3′), and MT-R (5′-AGGCTGCAGACCATCCTTTT-3′).
Sample processing for the flow cytometry analysis
To obtain single-cell suspensions, lymphocytes were isolated from the thymus, spleen, inguinal lymph nodes, lungs, and livers as described before35. The prepared cells were stained with the following antibodies: anti-CD4-BV605 (RM4–5, BioLegend), anti-PD-1-BV421 (RMP1–13, BioLegend), anti-CD2-PE-Cy7 (RM2–5, BioLegend), anti-CD23-PerCP-Cy5.5 (B3B4, BioLegend), anti-CD21/35-BV421 (7E9, BioLegend), anti-CD43-PE (1B11, BioLegend), anti-IgM-APC (RMM-1, BioLegend), anti-IgD-BV605 (11-26c.2a, BioLegend), anti-CD8-PerCP-Cy5.5 (53-6.7, eBioscience), anti-CD25-FITC (PC61.5, eBioscience), anti-CD3-PE-Cy7 (145-2C11, BD Biosciences), anti-CD44-V450 (IM7, BD Biosciences), anti-CD45R/B220-FITC (RA3-6B2, BD Biosciences), anti-CD62L-FITC (MEL-14, BD Biosciences), anti-CD69-PE (H1.2F3, BD Biosciences), and anti-TCR-β-FITC (H57-597, BD Biosciences). For the staining of transcription factor FoxP3, the cells were stained using anti-FoxP3-APC (FJK-16s, eBioscience) after permeabilization/fixation step. In all experiments, cells were labelled with the Live/Dead fixable Stain Kit (eBioscience) to discriminate the live and dead cells. The data were acquired by FACS CANTO II (BD Biosciences) and analysed by FlowJo software (Tree Star).
Analysis of serum immunoglobulins
Serum immunoglobulin levels (IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM) of mice were analysed by a multiplex bead-based assay panel (BioLegend, cat #740495) using fluorescence-encoded beads. The samples were prepared according to manufacturer’s instructions and analysed by FACS CANTO II (BD Biosciences). The serum immunoglobulin level of IgE was measured using a Mouse IgE ELISA MAX kit (BioLegend).
TCRVβ repertoire analysis
CD90.2+ cells were isolated from the spleen using positive selection (Miltenyi Biotec). The isolated cells were stored in RNAprotect® cell reagent (QIAGEN), and quantitative TCRVβ repertoire was determined by Repertoire Genesis, Inc. based on next generation sequencing (NGS) analysis. The diversity of TCRVβ repertoire analysis is described by Shannon-Weaver index H’, Inverse Simpson’s index 1/λ, Pielou’s evenness, and Diversity Evenness score (DE50), which are provided by Repertoire Genesis, Inc.
Statistical analysis
Data were analysed by two-tailed unpaired Student’s t-test using GraphPad Prism software. A p value less than 0.05 was considered statistically significant.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cooper, M. D. & Alder, M. N. The evolution of adaptive immune systems. Cell 124, 815–822, https://doi.org/10.1016/j.cell.2006.02.001 (2006).
Bassing, C. H. et al. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature 405, 583–586, https://doi.org/10.1038/35014635 (2000).
Sadofsky, M. J. The RAG proteins in V(D)J recombination: more than just a nuclease. Nucleic acids research 29, 1399–1409 (2001).
Bassing, C. H., Swat, W. & Alt, F. W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109(Suppl), S45–55 (2002).
Schwarz, K. et al. RAG mutations in human B cell-negative SCID. Science 274, 97–99 (1996).
Giblin, W. et al. Leaky severe combined immunodeficiency and aberrant DNA rearrangements due to a hypomorphic RAG1 mutation. Blood 113, 2965–2975, https://doi.org/10.1182/blood-2008-07-165167 (2009).
Kato, M. et al. Omenn syndrome–review of several phenotypes of Omenn syndrome and RAG1/RAG2 mutations in Japan. Allergology international: official journal of the Japanese Society of Allergology 55, 115–119, https://doi.org/10.2332/allergolint.55.115 (2006).
Villa, A. et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 97, 81–88 (2001).
Kobrynski, L. Newborn screening for severe combined immune deficiency (technical and political aspects). Current opinion in allergy and clinical immunology 15, 539–546, https://doi.org/10.1097/ACI.0000000000000221 (2015).
Kim, M. S., Lapkouski, M., Yang, W. & Gellert, M. Crystal structure of the V(D)J recombinase RAG1-RAG2. Nature 518, 507–511, https://doi.org/10.1038/nature14174 (2015).
Ru, H. et al. Molecular Mechanism of V(D)J Recombination from Synaptic RAG1-RAG2 Complex Structures. Cell 163, 1138–1152, https://doi.org/10.1016/j.cell.2015.10.055 (2015).
Corneo, B. et al. Identical mutations in RAG1 or RAG2 genes leading to defective V(D)J recombinase activity can cause either T-B-severe combined immune deficiency or Omenn syndrome. Blood 97, 2772–2776 (2001).
Marrella, V. et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. The Journal of clinical investigation 117, 1260–1269, https://doi.org/10.1172/JCI30928 (2007).
Komor, A. C., Badran, A. H. & Liu, D. R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 169, 559, https://doi.org/10.1016/j.cell.2017.04.005 (2017).
Martinez-Lage, M., Torres-Ruiz, R. & Rodriguez-Perales, S. CRISPR/Cas9Technology: Applications and Human Disease Modeling. Progress in molecular biology and translational science 152, 23–48, https://doi.org/10.1016/bs.pmbts.2017.09.002 (2017).
Liu, G. et al. Direct detection of FoxP3 expression in thymic double-negative CD4-CD8- cells by flow cytometry. Scientific reports 4, 5781, https://doi.org/10.1038/srep05781 (2014).
Farcot, E. et al. TCR beta allelic exclusion in dynamical models of V(D)J recombination based on allele independence. Journal of immunology 185, 1622–1632, https://doi.org/10.4049/jimmunol.0904182 (2010).
Ott de Bruin, L. et al. Rapid generation of novel models of RAG1 deficiency by CRISPR/Cas9-induced mutagenesis in murine zygotes. Oncotarget 7, 12962–12974, https://doi.org/10.18632/oncotarget.7341 (2016).
Laurent, J., Bosco, N., Marche, P. N. & Ceredig, R. New insights into the proliferation and differentiation of early mouse thymocytes. International immunology 16, 1069–1080, https://doi.org/10.1093/intimm/dxh108 (2004).
Hu, J. & August, A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. Journal of immunology 180, 6544–6552 (2008).
Elnour, I. B., Ahmed, S., Halim, K. & Nirmala, V. Omenn’s Syndrome: A rare primary immunodeficiency disorder. Sultan Qaboos University medical journal 7, 133–138 (2007).
Long, S. A. & Buckner, J. H. CD4+FOXP3+T regulatory cells in human autoimmunity: more than a numbers game. Journal of immunology 187, 2061–2066, https://doi.org/10.4049/jimmunol.1003224 (2011).
Notarangelo, L. D., Kim, M. S., Walter, J. E. & Lee, Y. N. Human RAG mutations: biochemistry and clinical implications. Nature reviews. Immunology 16, 234–246, https://doi.org/10.1038/nri.2016.28 (2016).
Sims, G. P. et al. Identification and characterization of circulating human transitional B cells. Blood 105, 4390–4398, https://doi.org/10.1182/blood-2004-11-4284 (2005).
H, I. J. et al. Similar recombination-activating gene (RAG) mutations result in similar immunobiological effects but in different clinical phenotypes. The Journal of allergy and clinical immunology 133, 1124–1133, https://doi.org/10.1016/j.jaci.2013.11.028 (2014).
Chou, J. et al. A novel homozygous mutation in recombination activating gene 2 in 2 relatives with different clinical phenotypes: Omenn syndrome and hyper-IgM syndrome. The Journal of allergy and clinical immunology 130, 1414–1416, https://doi.org/10.1016/j.jaci.2012.06.012 (2012).
Pasic, S., Djuricic, S., Ristic, G. & Slavkovic, B. Recombinase-activating gene 1 immunodeficiency: different immunological phenotypes in three siblings. Acta paediatrica 98, 1062–1064, https://doi.org/10.1111/j.1651-2227.2009.01250.x (2009).
Fugmann, S. D. & Schatz, D. G. Identification of basic residues in RAG2 critical for DNA binding by the RAG1-RAG2 complex. Molecular cell 8, 899–910 (2001).
Baens, M. et al. The dark side of EGFP: defective polyubiquitination. PloS one 1, e54, https://doi.org/10.1371/journal.pone.0000054 (2006).
Nagaoka, H., Gonzalez-Aseguinolaza, G., Tsuji, M. & Nussenzweig, M. C. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. The Journal of experimental medicine 191, 2113–2120 (2000).
Lee, J. H. et al. Differences between immunodeficient mice generated by classical gene targeting and CRISPR/Cas9-mediated gene knockout. Transgenic research 27, 241–251, https://doi.org/10.1007/s11248-018-0069-y (2018).
Schuetz, C. et al. An immunodeficiency disease with RAG mutations and granulomas. The New England journal of medicine 358, 2030–2038, https://doi.org/10.1056/NEJMoa073966 (2008).
Sung, Y. H. et al. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome research 24, 125–131, https://doi.org/10.1101/gr.163394.113 (2014).
Zhu, X. et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Scientific reports 4, 6420, https://doi.org/10.1038/srep06420 (2014).
Lee, A. et al. IL-4 Induced Innate CD8+T Cells Control Persistent Viral Infection. PLoS pathogens 11, e1005193, https://doi.org/10.1371/journal.ppat.1005193 (2015).
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST; 2018R1A2A1A05022746, 2017R1A4A1015328, 2018M3A9H3024850, 2018R1A2A1A05076997). and the Korea Food and Drug Administration (14182MFDS978).
Author information
Authors and Affiliations
Contributions
Y.J. wrote the manuscript with support from A.L., H.-W.L., and S.-J.H. Y.J. and A.L. designed and performed the experiments. J.-H.O. contributed reagents/materials/analysis tools. H.-W.L. and S.-J.H. supervised the project.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, Y., Lee, A., Oh, J.H. et al. The R229Q mutation of Rag2 does not characterize severe immunodeficiency in mice. Sci Rep 9, 4415 (2019). https://doi.org/10.1038/s41598-019-39496-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39496-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.