Abstract
Acute kidney injury (AKI) is associated with poor prognosis among patients with acute heart failure (AHF). Early documentation of impaired kidney function through simple examination may provide risk reduction in such patients. The present study aims to reveal an association between cellular casts and hospital-acquired AKI in AHF. This study included patients with AHF who underwent urinalysis, including urinary sediment analysis within 24 hours post admission. AKI was defined as an increase of ≥0.3 mg/dL within 48 hours or ≥1.5 times in serum creatinine level in contrast to baseline creatinine level. In this study, 114 patients with AHF (age, 75 ± 14 years; male, 59.7%) were included. Of them, 40 (35%) developed hospital-acquired AKI. Cellular casts were detected in 30 patients (26%) prior to AKI development and related to hospital-acquired AKI in the multivariate logistic regression analysis (odds ratio, 2.80; 95% confidence interval, 1.04–7.49; P = 0.041). In conclusion, cellular casts are observed occasionally in patients with AHF and potentially useful markers for development of AKI during hospitalization.
Similar content being viewed by others
Introduction
The growth of elderly population in Japan and improved outcome of patients with cardiac disease due to modern therapeutic progress have led to an increasing incidence of acute heart failure (AHF). Despite improvements in therapy for AHF, its mortality rate remains unacceptable1,2. There is a close interrelationship between heart and kidney diseases, known as cardiorenal syndrome (CRS). Impaired kidney function including acute kidney injury (AKI) is common among such patients3,4 and is reported to be associated with short– and long–term prognosis in patients with AHF5,6. Early documentation of impaired kidney function may provide risk reduction in such patients. However, creatinine is an unreliable marker for detection of early phase of renal impairment in AHF7. Furthermore, plasma neutrophil gelatinase–associated lipocalin (NGAL) failed to indicate its usefulness8. Until now, various biomarkers for detection of renal impairment were proposed (e.g., kidney injury molecular–1 [KIM–1], cystatin C, interleukin–6, interleukin–8, interleukin–18, N–acetyl–β–D–glucosaminidase [NAG], and liver fatty acid–binding protein [LFABP]), but their usefulness has not been confirmed9. Although a combination of such biomarkers may be useful, high cost and complexity are nonnegligible problems. Therefore, early detection of AKI through a simple examination is necessary.
Microscopic examination of the urine sediment is commonly performed, and its clinical value as a noninvasive detector of renal damage has been confirmed10,11. For instance, cellular casts can predict subsequent renal relapse in patients with systemic lupus erythematosus12. Bagshaw et al. reported the potential usefulness of urine sediment examination for the prediction of AKI in sepsis13. However, the clinical impact of urinary cast on renal function has not been sufficiently explored in patients with AHF. The present study aimed to clarify the clinical usefulness of urinary casts and whether they detect an early phase of AKI in AHF.
Results
Impact of cellular casts in the short term
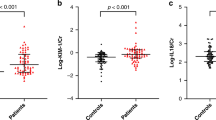
The present study included 114 patients with AHF (age, 75 ± 14 years; male, 59.7%). The patient characteristics are shown in Table 1. Of all patients, 40 (35%) developed hospital-acquired AKI, which developed 5 days (3–10) after admission. Cellular casts were detected in 30 patients (26%). Hyaline cast with and without other casts were found in 30 (26%) and 23 (20%) patients, respectively. Cellular casts were related to a higher serum creatinine level on admission and at discharge, as well as their absolute difference during hospitalization (Fig. 1A–C). Uni– and multivariate logistic regression analyses are presented in Table 2. Cellular casts were significantly associated with hospital-acquired AKI (odds ratio [OR], 2.86; 95% confidence interval [CI], 1.21–6.75; P = 0.017), whereas hyaline cast was not (OR, 1.96; 95% CI, 0.77–4.96; P = 0.156). The result regarding hyaline cast did not depend on its number. These results persisted after adjustment for age, estimated glomerular filtration rate (eGFR), hemoglobin, and diabetes mellitus (DM) on admission (OR in cellular cast, 2.80; 95% CI, 1.04–7.49; P = 0.041; OR in hyaline cast, 1.39; 95% CI, 0.48–4.00; P = 0.543). The prevalence of hospital-acquired AKI did not depend on serum creatinine level on admission among patients with serum creatinine level of ≥2.0 mg/dL (Fig. 2).
Serum creatinine level between patients with and without cellular casts. (A) Serum creatinine level on admission was significantly higher in those who presented with cellular casts. (B) Serum creatinine level at discharge was significantly higher in those who presented with cellular casts similar to that on admission. (C) Patients with cellular casts indicated an increase in serum creatinine level at the time of discharge, whereas there were few changes in those who presented without them.
Relationship between the prevalence of hospital-acquired AKI and serum creatinine level. Caption: A cubic spline curve demonstrated that the prevalence of hospital-acquired AKI did not depend on serum creatinine level on admission among patients with serum creatinine level of ≥2.0 mg/dL. AKI: acute kidney injury; CI: confidence interval.
Sensitivity and specificity
At cutoff of 4 kinds of urinary casts, specificity was 98.65%, sensitivity was 27.50%, and likelihood ratio was 20.35 for prediction of hospital-acquired AKI. Specificity was 81.08%, sensitivity was 40.00%, and likelihood ratio was 2.11 if cutoff was set as presence or absence of urinary casts.
Invasive procedure
In the present cohort, 45 patients (39%) underwent any invasive procedure for diagnosis or treatment of underlying cardiovascular diseases; coronary angiography (CAG) alone was performed for 31 patients, elective percutaneous coronary intervention (PCI) on the day different from the date of CAG for 4 patients, ad hoc PCI for 6 patients, pacemaker implantation for 2 patients, cardiac resynchronization therapy for 1 patient, and thoracic surgery for 1 patient. In the procedures of CAG and PCI, the amount of contrast medium was 40 (25–100) ml in CAG alone, 59 (86–102) ml for CAG and 70 (60–100) ml for PCI in elective PCI, and 90 (40–140) in ad hoc PCI. The AKI was detected in 14 of 41 (34%) patients who received CAG or PCI. Although univariate logistic regression analysis demonstrated that no significant association between the amount of contrast medium used in such procedures and AKI, a patient with granular casts, in whom eGFR was 50 ml/min/1.73 m2 and injected contrast medium for CAG was 45 ml, developed contrast-associated AKI. The remaining four patients, who underwent invasive procedures such as thoracic surgery, pacemaker implantation, or cardiac resynchronization therapy, did not develop AKI.
Long–term impact of cellular casts
Of 114 patients, 4 died during hospitalization, and 110 were followed up in the outpatient clinic. The median follow–up duration was 453 days (115–770). Thirty–five patients (32%) had worsening renal function (WRF) at least one time within a year after discharge. The results of the univariate Cox regression analysis are shown in Table 3. Cellular casts were not associated with 1–year WRF (hazard ratio (HR), 1.13; 95% CI, 0.53–2.37; P = 0.756).
Discussion
The present study demonstrated potential usefulness of urinary casts for the evaluation of renal function in AHF. First, they might be an early predictor of AKI during hospitalization. Whereas NGAL was reported to increase one day prior to the increase in serum creatinine level14, cellular casts were observed 5 days (median) prior to AKI. The characteristics may be useful for risk stratification and early intervention. Second, urinalysis demonstrated high specificity despite it is a daily, easy, and low–cost examination that differed from other time–consuming and expensive biomarkers.
In the present study, approximately a quarter of all patients presented with any cellular casts. Impaired kidney function includes various lesions such as a tubular, glomerular and vascular, and interstitial. In general, most of cellular casts often appear in patients with acute tubular necrosis (ATN)11 rather than those who present with interstitial lesions. Some cellular casts, for instance, red blood cell casts, appear in glomerular disease15. Our results suggested that some patients with AHF may present with renal parenchymal lesions, including tubular, glomerular and vascular, potentially or apparently. Renal injury lesions have been seldom mentioned in the topic on AKI in those with AHF. Our study showed that urinary casts would be useful to predict AKI development with high specificity, whereas they might not contribute to rule-out AKI because of its low sensitivity. This weak point is reasonable because there are various etiologies of AKI such as interstitial damage which is not associated with urinary casts.
The pathophysiology of renal insufficiency in patients with AHF has not been sufficiently understood16 because the causal relationship in CRS would be different in each case. Appearance of urinary cellular casts might be the result of CRS and reflect renal parenchymal damage due to various mechanisms. A previous study which evaluated urinary NGAL as a marker for tubular damage indicated that patients with heart failure would suffer from a combination of reduced GFR and tubular damage17. One of possible mechanisms may be the excessive activation of renin–angiotensin–aldosterone–system (RAAS) which can impair the kidneys via renal vasoconstriction yielding impairment of tubular epithelial cells18,19,20. Furthermore, renal hypoperfusion caused by cardiac dysfunction, venous congestion21,22, activation of sympathetic nerve system, and oxidative injury23 may also contribute to the renal parenchymal lesions. Renal tubular injury can substantially decrease glomerular filtration by a tubulo–glomerular feedback mechanism24,25; the influence might persist for a few weeks, but not for a long–term period as urinary cellular casts were not related to 1–year WRF in the present study. Considering such results, an appearance of urinary cellular casts or hospital-acquired AKI in some AHF patients may be due to reversible causes such as temporal venous congestion and activation of RAAS.
As compared with cellular casts, hyaline cast demonstrated nonsignificant and weaker correlation with hospital-acquired AKI. The result might be due to beta–error because of the small number of patients. Although hyaline cast is generally regarded as nonspecific26, the clinical impact may differ in various comorbidities. The clinical implication of hyaline cast in AHF might not be determined in the present study.
The present study included some limitations. First, due to the small number of patients, it is difficult to assess the clinical impact of each cellular cast on hospital-acquired AKI and long-term WRF. Second, patients without urinalysis on admission were not included, which might have yielded selection bias. Finally, urinalysis was performed by a single technician, and the photographs of casts were not routinely taken in our laboratory, which means future verification is difficult using the present data. However, the findings of the present study provided an important clue in the evaluation and treatment of AKI in AHF. Future studies with sufficient number of patients are needed to confirm the results of the present study.
Conclusion
Cellular casts are observed occasionally in patients with AHF and are potentially useful markers for early documentation of hospital-acquired AKI.
Methods
Study population
This study included patients with AHF who were hospitalized in Kyorin University Hospital from January 2015 to April 2018. A diagnosis of AHF is defined as rapid–onset heart failure, new or worsening signs and symptoms of heart failure requiring urgent therapy and hospitalization, based on the Framingham criteria27.
Inclusion criteria
The patients underwent urinalysis including urinary sediment analysis and urine biochemistry within 24 hours post admission for AHF treatment.
Exclusion criteria
The patients who developed acute coronary syndrome, hemodialysis, or peritoneal dialysis were excluded from the study.
Data collection
The patients’ background and outcomes were collected through an interview and medical record review. Blood examination, including creatinine, hemoglobin, uric acid, and B type natriuretic peptide was performed on admission in all patients. Serum creatinine was measured by technicians without the knowledge of each clinical course and findings of urinalysis. Follow–up data were acquired by a medical record review or interview at the outpatient clinic.
Urinalysis
Urinalysis was conducted by a highly experienced clinical technologist without the knowledge of each patient’s clinical course and serum creatinine level within 24 hours after admission. Urinalysis consists of urinary sediment analysis and urine biochemistry, including β2 microglobulin and NAG. In preparing the urine sediment examination, 10 mL of urine was centrifuged at 500 gravity (1500 rpm; radius, 20 cm) for 5 min. Urine sediment was assessed in undyed bright–field observation. Urinary casts were counted in 20–30 fields with high power field (400×). Urinary casts were judged as present if they were observed in at least 1 field. Urinary casts were classified as hyaline, epithelial, granular, waxy, red blood cell, and fatty casts. Any casts except for hyaline cast are described as “cellular casts” in the present study. “All casts” mean both cellular and hyaline casts.
Definition of technical terms
Hospital-acquired AKI was defined as an increase in serum creatinine level of ≥0.3 mg/dL within 48 hours or ≥1.5 times from the baseline value28. One-year WRF was defined as an increase of 0.3 mg/dL or more in the serum creatinine level compared to baseline creatinine level at discharge within a year after discharge29,30,31. Ischemic heart disease was diagnosed if patients presented with any of the following: left anterior descending artery, left circumflex artery, or right coronary artery stenosis ≥75% or left main trunk stenosis ≥50% if they underwent coronary angiography or computed tomography. Dyslipidemia was diagnosed if one of the following criteria was fulfilled: low–density lipoprotein cholesterol level (calculated as total cholesterol – high–density lipoprotein (HDL) – triglyceride/5) of ≥140 mg/dL, HDL cholesterol level of <40 mg/dL, or triglyceride level of ≥150 mg/dL. Hypertension was defined as systolic blood pressure (SBP) of ≥140 mmHg and diastolic blood pressure of ≥90 mmHg32. Diagnosis of DM was defined with the following criteria: self–reported diabetes or use of antidiabetic medications based on the questionnaire, fasting plasma glucose level ≥126 mg/dL, or hemoglobin A1c (HbA1c) level ≥6.5%33. Hyperuricemia was defined as serum uric acid value of ≥7.0 mg/dL.
Endpoints
The primary endpoint was hospital-acquired AKI. The secondary endpoint was 1–year WRF.
Ethical Principles
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki34 in line with the Ethical Guidelines for Epidemiological Research by the Japanese government. The study was approved by the ethics committee at Kyorin University. According to the guidelines, the study satisfied the conditions to waive the requirement for informed consent from individual participants. Therefore, informed consent was waived, which was approved by the ethics committee.
Statistical analysis
No preceding study evaluated a clinical impact of urinary casts on AKI in patients with AHF. We assumed the number of patients required for the analysis based on the previous study evaluating the clinical value of urinalysis in sepsis although the underlying condition was different. According to the study, the incidence of AKI was 33.3% and 7.4% in patients with and without cellular casts13. We assumed the prevalence of cellular casts in reference to the first 40 patients in the present study, which indicated a 30% prevalence rate. Based on 80% power and a significance level of 0.05, 93 patients were required. Numerical data are presented as mean ± standard deviation if the data followed a normal distribution. Otherwise, data are displayed as median and interquartile range (Q1–Q3) values. Categorical variables are expressed as absolute numbers or percentages. Continuous variables were analyzed using the unpaired Student t–test or Mann–Whitney U test, while categorical variables were analyzed using Fisher exact test or χ2 tests, as appropriate. The association between urinary casts and hospital-acquired AKI was assessed using uni– and multivariate logistic regression analyses and expressed as OR, 95% CI, and P value. Variables with a P value < 0.10 were retained for the multivariate logistic regression analysis with least absolute shrinkage and selection operator. Sensitivity, specificity, and likelihood ratio of urinary casts for hospital-acquired AKI were calculated. In sensitivity analysis, different assessment was performed. Presence of cellular casts and total numbers of each cast were evaluated. Follow–up began on the date of discharge. The data in the present study were collected on June 2018. The follow–up was completed at the date at which patients died or were censored. The risk of 1–year WRF was assessed using Cox regression analysis expressed as HR, 95% CI, and P value. Statistical significance was set at P value < 0.05. All statistical analyses were carried out using Stata software version 14 (StataCorp, College Station, TX).
Data Availability
We agree with the statement.
References
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 33, 1750–1757 (2012).
Lam, C. S., Donal, E., Kraigher-Krainer, E. & Vasan, R. S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 13, 18–28 (2011).
Metra, M. et al. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circ Heart Fail 11, e004644 (2018).
Belziti, C. A., Bagnati, R., Ledesma, P., Vulcano, N. & Fernandez, S. Worsening renal function in patients admitted with acute decompensated heart failure: incidence, risk factors and prognostic implications. Rev Esp Cardiol 63, 294–302 (2010).
Damman, K. et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 13, 599–608 (2007).
Smith, G. L. et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 47, 1987–1996 (2006).
Mishra, J. et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365, 1231–1238 (2005).
Maisel, A. S. et al. Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury During Acute Heart Failure Hospitalizations: The AKINESIS Study. J Am Coll Cardiol 68, 1420–1431 (2016).
Vanmassenhove, J., Vanholder, R., Nagler, E. & Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 28, 254–273 (2013).
Fogazzi, G. B., Ferrari, B., Garigali, G., Simonini, P. & Consonni, D. Urinary sediment findings in acute interstitial nephritis. Am J Kidney Dis 60, 330–332 (2012).
Perazella, M. A., Coca, S. G., Kanbay, M., Brewster, U. C. & Parikh, C. R. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 3, 1615–1619 (2008).
Hebert, L. A., Dillon, J. J., Middendorf, D. F., Lewis, E. J. & Peter, J. B. Relationship between appearance of urinary red blood cell/white blood cell casts and the onset of renal relapse in systemic lupus erythematosus. Am J Kidney Dis 26, 432–438 (1995).
Bagshaw, S. M. et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 27, 582–588 (2012).
Cruz, D. N. et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 36, 444–451 (2010).
Hebert, L. A., Parikh, S., Prosek, J., Nadasdy, T. & Rovin, B. H. Differential diagnosis of glomerular disease: a systematic and inclusive approach. Am J Nephrol 38, 253–266 (2013).
Dupont, M. et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail 14, 597–604 (2012).
Damman, K., van Veldhuisen, D. J., Navis, G., Voors, A. A. & Hillege, H. L. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail 10, 997–1000 (2008).
Pokhrel, N., Maharjan, N., Dhakal, B. & Arora, R. R. Cardiorenal syndrome: A literature review. Exp Clin Cardiol 13, 165–170 (2008).
Bongartz, L. G., Cramer, M. J., Doevendans, P. A., Joles, J. A. & Braam, B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 26, 11–17 (2005).
Struthers, A. D. & MacDonald, T. M. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res 61, 663–670 (2004).
Damman, K. et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9, 872–878 (2007).
Damman, K. et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53, 582–588 (2009).
Bock, J. S. & Gottlieb, S. S. Cardiorenal syndrome: new perspectives. Circulation 121, 2592–2600 (2010).
Thurau, K. & Boylan, J. W. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 61, 308–315 (1976).
Rosen, S. & Stillman, I. E. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol 19, 871–875 (2008).
Lindner, L. E. & Haber, M. H. Hyaline casts in the urine: mechanism of formation and morphologic transformations. Am J Clin Pathol 80, 347–352 (1983).
Ho, K. K., Anderson, K. M., Kannel, W. B., Grossman, W. & Levy, D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88, 107–115 (1993).
Mehta, R. L. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11, R31 (2007).
Metra, M., Cotter, G., Gheorghiade, M., Dei Cas, L. & Voors, A. A. The role of the kidney in heart failure. Eur Heart J 33, 2135–2142 (2012).
Gottlieb, S. S. et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail 8, 136–141 (2002).
Lassus, J. et al. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 28, 1841–1847 (2007).
James, P. A. et al. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014).
American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1, S14–80 (2014).
Shephard, D. A. The 1975 Declaration of Helsinki and consent. Canadian Medical Association journal 115, 1191–1192 (1976).
Acknowledgements
We greatly appreciate Prof. Yasuhiro Ando for the valuable advice, especially regarding urinary casts. This work was supported by JSPS KAKENHI Grant Number 17K18085.
Author information
Authors and Affiliations
Contributions
Satoshi Higuchi, MD, PhD designed the study, analyzed and interpreted data, and drafted manuscript. Yusuke Kabeya, MD, MPH, PhD, Kenichi Matsushita, MD, PhD analyzed and interpreted data, and revised the manuscript critically for important intellectual content. Satoko Yamasaki, MD, Hiroaki Ohnishi, MD, PhD, and Hideaki Yoshino, MD, PhD revised the manuscript critically for important intellectual content.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Higuchi, S., Kabeya, Y., Matsushita, K. et al. Urinary cast is a useful predictor of acute kidney injury in acute heart failure. Sci Rep 9, 4352 (2019). https://doi.org/10.1038/s41598-019-39470-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39470-1
This article is cited by
-
Rational selection of a biomarker panel targeting unmet clinical needs in kidney injury
Clinical Proteomics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.