Abstract
The chemical composition of the Gaoping River in Taiwan reflects the weathering of both silicate and carbonate rocks found in its metasedimentary catchment. Major dissolved ion chemistry and radiocarbon signatures of dissolved inorganic carbon (DIC) reveal the importance of pyrite-derived sulphuric acid weathering on silicates and carbonates. Two-thirds of the dissolved load of the Gaoping River derives from sulphuric acid-mediated weathering of rocks within its catchment. This is reflected in the lowest reported signatures DI14C for a small mountainous river (43 to 71 percent modern carbon), with rock-derived carbonate constituting a 14C-free DIC source. Using an inverse modelling approach integrating riverine major dissolved ion chemistry and DI14C, we provide quantitative constraints of mineral weathering pathways and calculate atmospheric CO2 fluxes resulting from the erosion of the Taiwan orogeny over geological timescales. The results reveal that weathering on Taiwan releases 0.31 ± 0.12 MtC/yr, which is offset by burial of terrestrial biospheric organic carbon in offshore sediments. The latter tips the balance with respect to the total CO2 budget of Taiwan such that the overall system acts as a net sink, with 0.24 ± 0.13 MtC/yr of atmospheric CO2 consumed over geological timescales.
Similar content being viewed by others
Introduction
Taiwan is one of the most rapidly uplifting orogens, with erosion rates in the order of 3–6 mm/yr continuously exposing fresh minerals for chemical weathering1,2. Together with volcanic activity, metamorphic degassing, and the organic carbon cycle, chemical weathering of minerals exerts a key control on atmospheric chemistry over geologic timescales3. Orogenies sustain high rates of physical erosion and are classically invoked as major CO2 sinks due to the weathering of silicates by carbonic acid4,5. While carbonic acid as a weathering agent is widely considered the most important, recent work has highlighted that sulphuric acid weathering of carbonates plays an important role in catchments that contain abundant pyrite2,6,7,8,9 and acts as a major source of CO2 to the atmosphere over geological timescales10,11,12. Pyrite oxidises to sulphuric acid giving rise to river dissolved sulphate6,8 following the weathering pathway10:
Together with carbonic acid and sulphuric acid stemming from weathering reaction (1), silicate and carbonate mineral weathering proceeds as follows
The trails of evidence of these weathering reaction pathways (2–5) lead to unique signatures in the dissolved ion load (see Table 1). The theoretical radiocarbon isotopic composition of DIC (hereafter DI14C) arising from these pathways are characteristic for different weathering mechanisms. In reaction (2), carbon is sourced primarily from the atmosphere, which exhibits a modern signature, expressed as 100% modern carbon (pMC). Reaction (3) does not involve carbon and only adds sulphate to the dissolved river load. In reaction pathway (4), 50% of bicarbonate carbon is sourced from the atmosphere and the other half from the radiocarbon-dead lithosphere, hence characterised by a pMC = 50 “fingerprint”, while in the case of (5), bicarbonate is entirely derived from the radiocarbon-dead lithospheric source (i.e., pMC = 0). Radiocarbon has considerable advantages over stable carbon isotopic compositions of DIC by incorporating a correction for fractionation and exhibiting lower end-member uncertainty13,14. Additionally, the weathering of silicates and carbonates release characteristic assemblages of major cations (calcium, magnesium, and sodium) to the river dissolved load that are further indicative of the mineral species undergoing chemical weathering5.
Globally, the reaction pathway of silicates and carbonates dictates the net effect of weathering on atmospheric CO23,4, with the dissolution of carbonates by sulphuric acid acting as a CO2 source over geological time scales10. Using a novel approach, we quantitatively disentangle the inputs of silicate and carbonate weathering via carbonic and sulphuric acid dissolution by determining DI14C and dissolved ion compositions within the Gaoping River catchment of Taiwan, leading to new quantitative estimates on the effect of the Taiwan orogeny on atmospheric chemistry.
Study Area and Methods
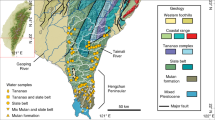
The Gaoping River covers a length of 170 km and drains 3257 km2 in southwest Taiwan and is the island’s second largest river as measured by sediment discharge6,15. Nearly half of its catchment is situated above 1000 m elevation, reaching a maximum of 3997 masl15. Within the catchment, (meta) sedimentary rocks ranging from shales to conglomerates of ages spanning from the Mesozoic to Pleistocene are exposed (see appendix for details and references). Due to the monsoonal climate, >90% of river discharge takes place in the flood season (focused in June to October)15.
Surface water samples were collected from the Gaoping River catchment during the dry season in February 2017 and 2018 as well as during the wet season in June and October 2017 (Fig. 1). Filtered water samples were enclosed in 12 ml glass exetainer vials without head-space and pre-poisoned with 12 µl of dried HgCl2 saturated solution. One and six millilitre aliquots were purged with He, acidified with 150 µl 85% H3PO4, and measured for their DI13C and DI14C isotope compositions, respectively, using online CO2 sparging-mass spectrometry setups described elsewhere16,17. Radiocarbon values are reported in units pMC18. Concentrations of major cations and anions were measured by ion chromatography with details reported in the appendix. Bicarbonate concentrations where calculated by charge balance, following previous Taiwanese river studies2,8,12,19. Inputs from rainwater were removed by assuming all chloride is sourced from the atmosphere and by subtracting ions using ratios typical for rainwater (Ca/Na = 0.023; Mg/Na = 0.11; Cl/Na = 1.15; S/Na = 0.06; HCO3/Na = 0.004)5,7.
Gaoping River catchment. Geological and hydrological overview with sampling locations. Redrawn after geologic map of Taiwan44, which is openly accessible (https://data.gov.tw/dataset/11004). Topographic features were added from open access digital elevation data (https://data.gov.tw/dataset/35430) and shaded with QGIS (QGIS Development Team (2018). QGIS Geographic Information System. Open Source Geospatial Foundation Project. www.qgis.org). The Gaoping River catchment was outlined from open access data from the Taiwanese government (https://www.wra07.gov.tw/12594/12595/12602/12605/70918/) and the final figure was generated with CorelDRAW (www.coreldraw.com).
Using a constrained linear least-squares approach with the Matlab solver lsqlin, contributions from weathering reactions (2–5) are assessed from the mineral unit-normalised stoichiometries (see definition below), DI14C fingerprints, and ionic ratios characteristic for different weathering reactions listed in Table 1. Here we use α to denote the relative contributions stemming from the carbonic acid weathering of silicates (αSilicate,H2CO3) and carbonates (αCarbonate,H2CO3) and sulphuric acid weathering of silicates (αSilicate,H2SO4) and carbonates (αCarbonate,H2SO4). The model output is bound by three equality constraints: (1) the sum of the weathering contributions αi for the mineral-normalised ion concentrations for the four reactions equals 100%, (2) measured and modelled mineral unit-normalised SO4 must be equal, and (3) the measured and modelled DI14C must be equal. Additional constraints are included to find the best least-squares fit between modelled and measured dissolved major ion composition based on ratios between calcium, magnesium, and sodium and the sum of sulphate and bicarbonate, which are indicative of different weathering pathways from silicates and carbonates (see Table 1 and appendix for equations). Here, we define a “mineral unit” as (Ca, Mg, 2Na, 2 K)SiO3 for silicates and (Ca,Mg,2Na,2 K)CO3 for carbonates. Within each silicate and carbonate mineral unit, calcium is interchangeable with charge balance equivalent amounts of magnesium, sodium, and potassium. For silicates, the relative molar abundances of these cations are typically 0.35 ± 0.25 for Ca/Na and 0.25 ± 0.2 for Mg/Na. For carbonates, Ca/Na and Mg/Na are 60 ± 30 and 30 ± 15, respectively5,7, with the uncertainties taken to represent 2-σ uncertainty in the model. Potassium is also present in carbonate mineral lattices in minor quantities (Ca/K ≈ 250–350)20, besides its presence in micas and potassic feldspars. Based on these ratios and the ideal mineral unit definition introduced here, the average formula for carbonates and for silicates is Ca0.66Mg0.33Na0.011K0.0055CO3 and Ca0.30Mg0.21Na0.85K0.14SiO3, respectively. Model output uncertainty was quantified by applying a Monte Carlo approach (10,000 iterations) propagating analytical uncertainties from measured ionic ratios (5% relative 1-σ uncertainty assigned), measured DI14C uncertainty (≈1% 1-σ uncertainty based on analytical uncertainty), uncertainty in the cation composition of the weathered minerals, and uncertainty in the radiocarbon signature of the carbonic acid weathering agent. In the case of the latter, an end member signature of 100 ± 5 (1-σ uncertainty) pMC was assigned, with the uncertainty stemming from possible contributions of respired soil organic carbon21,22 or degraded kerogen, which attenuate the atmospheric carbon signature with the addition of bomb and aged carbon isotopic signatures. Used equations are reported in the appendix.
Results and Discussion
Over the sampled time interval, pH averaged 7.9 ± 0.2 (n = 13) and bicarbonate represented the major anion (2.3 ± 0.6 mmol/l) followed by sulphate (0.9 ± 0.4 mmol/l) and chloride (0.2 ± 0.3 mmol/l) (supplemental Table 1). For the cations, calcium was the most abundant (1.4 ± 0.4 mmol/l n = 13) followed by magnesium and sodium (both 0.5 ± 0.2 mmol/l), and potassium (0.2 ± 0.2 mmol/l). Carbon isotopic compositions were centred at −5.3 ± 1.3‰ for DI13C (n = 13), while DI14C values ranged from 43 to 68 pMC (average 54 ± 8 pMC n = 20) (supplemental Table 1). One sample contained 0.02 mmol/l of ammonium that was presumably sourced from anthropogenic inputs and was also characterized by the highest nitrate concentrations suggesting that nitrification induced mineral weathering was ongoing23,24. As this additional weathering pathway could not be deconvolved using the approaches devised here, this sample (2018FebAR) was not considered further in this study.
Taiwanese bedrock primarily consists of (metamorphosed) sandstones, siltstones, and claystones, up to a metamorphic grade of amphibolite facies with no reports of evaporitic deposits occurring on the island25 (see also Supplemental Table 2). In agreement with these geological observations, sulphur isotopes in sulphate24,26 demonstrate that pyrite oxidation is the predominant source of dissolved sulphate in Taiwanese rivers6,24,26. Following the theoretical outline provided in the introduction, rainwater-corrected river data from the Gaoping River are plotted in Fig. 2a showing the mixing of the four end members, which adhere to theoretical expectations in the case of quaternary mixing of solute derived from silicate and carbonate weathering via sulphuric and carbonic acid in the absence of evaporite contributions (Fig. 2a). The relatively high concentrations of chloride ions are similar to those of New Zealand rivers within catchments that receive high amounts of precipitation, which is also attributed to cyclic salt input27. Based on the measured DI13C values, soil organic matter and kerogen degradation are considered to contribute subordinately to the DIC pool. Similarly low contributions are also inferred for other Taiwanese rivers where most DI13C values suggest a predominantly carbonate source for riverine DIC12, supporting the assumption in our model that DIC is exclusively carbonate mineral-derived. Figure 2b shows the effect of ternary mixing between reactions (2), (4), and (5). Excess sulphate is introduced into the system via reaction (3), which has no effect on the DI14C composition, yet causes the data points to lie outside the boundaries for ternary mixing. By applying the equations outlined in the methods and using the stoichiometric constraints summarised in Table 1 on the data reported in Table S1, the relative contributions stemming from mineral weathering pathways are calculated. These calculations deconvolve the excess sulphate contributions in order to allow quantification of the weathering contributions controlling DI14C under the constraint of ionic composition adhering to the quaternary mixing line. The weathering apportionment did not reveal any clear patterns as a function of sampling season or location. Averaged over the catchment and the seasons, and based on molar mineral unit-normalised weathering, αSilicate,H2CO3 and αCarbonate,H2CO3 averaged 34 ± 5% and 0.5% (−0.5/+2%) respectively, while αSilicate,H2SO4 and αCarbonate,H2SO4 averaged 14 ± 9% and 53 ± 8%, respectively (see also Supplemental Fig. S1–S13). Total weathered silicates (αSilicate,H2CO3 + αSilicate,H2SO4) contribute 46 ± 8% and carbonates (αCarbonate,H2CO3 + αCarbonate,H2SO4) contribute 54 ± 8% to the total dissolved ion load, which is broadly consistent with previous observations of major cation chemistry from the Gaoping River2,19,28 (see Appendix Fig. S16). Integrated over the Gaoping River catchment, the weathering effect of carbonic acid (αSilicate,H2CO3 + αCarbonate,H2CO3 = 33 ± 6%) and sulphuric acid (αSilicate,H2SO4 + αCarbonate,H2SO4 = 67 ± 6%) account for one-third and two-thirds of the total weathering, respectively. The results reveal that carbonates are almost exclusively weathered by sulphuric acid. This is most likely due to the limited abundance of rock carbonate (as siliciclastic metasedimentary units dominate in the catchment of the Gaoping River), a high supply of sulphuric acid, and the rapid reaction kinetics of sulphuric acid with carbonates. The excess sulphuric acid continues to weather silicates. The weathering patterns observed for the Gaoping catchment operate comparably for other Taiwanese catchments including the Liwu8, Taimaili12, and Chenyoulan12 rivers. This is evidenced by their adherence to the quaternary mixing trend, encouraging the extrapolation of the results generated here to the entire Taiwan orogenic belt (see appendix).
Mineral weathering end-member mixing. (a) Mineral unit-normalised sulphate versus bicarbonate abundance. The Gaoping River data collected over multiple years in relation to the quaternary end-member mixing line. Literature data points where ionic compositions reflect incursion of seawater plot away from the quaternary mixing line. One data point from this study (datapoint with highest mineral unit normalised sulphate) deviated from the mixing line due to high ammonium amounts that are attributed to anthropogenic inputs and therefore wasn’t considered further in this study. Removing these anomalies, the data from this study correlates with R2 = 0.99, and for all studies with R2 = 0.99. (b) Mineral unit-normalised sulphate versus pMC of DIC. The three mineral weathering reactions producing bicarbonate span a ternary mixing triangle. All of the data lie outside of this mixing ternary due to the addition of sulphate from sulphuric acid weathering of silicates. The latter process, which leaves DI14C on the x-axis unaffected, adds sulphate until a theoretical maximum of one mole per weathered mineral unit, representing 100% silicate weathering by sulphuric acid.
In the case of Taiwan and major orogenic phases (e.g. Himalayan orogeny), meta-sediments are uplifted bearing the mineral ingredients to both sequester CO2 and also release it to the atmosphere4,10,29. Based on Taiwanese long-term discharge measurements (4.98 × 1013 l/yr1), the average mineral-unit equivalent normalised dissolved ion load (2.5 ± 0.9 mmol/l n = 63) observed for the Gaoping River catchment (this study2,19,28), and the calculated mineral weathering apportionment with propagated uncertainty, 0.50 ± 0.19 MtC/yr are removed from the atmosphere as a result of carbonic acid weathering of silicates following carbonate precipitation in the oceans. In contrast, 0.40 ± 0.16 MtC/yr are released long term as a result of sulphuric acid weathering of carbonates. While sulphuric acid weathering of silicates does not directly involve carbon, for each silicate mineral unit weathered, ≈0.45 mineral units of carbonate can be precipitated by combining the dissolved cations generated with bicarbonate, which then releases CO2 to the atmosphere. Based on the ideal mineral unit formula, 0.30 moles of calcium are released upon weathering of one silicate unit and 0.66 moles of calcium are needed for one carbonate unit. Over geological timescales, sulphuric acid weathering of silicates on Taiwan may thus result in the release of 0.09 ± 0.07 MtC/yr (see second panel in Fig. 2). The net release of CO2 to the atmosphere from chemical weathering of minerals as interpreted in this study is consistent with previous work incorporating considerations of alkalinity and DIC delivered to the ocean30. CO2 release is expected to occur on timescales longer than that of carbonate burial (105-106 years), yet shorter than pyrite burial (107 years)30 (see Fig. S14 in appendix). In addition to chemical weathering of minerals, the weathering of kerogen (CO2 source), quantified by river dissolved rhenium, results in the release of 0.27–0.47 MtC/yr29. To counterbalance this, the burial of terrestrial biospheric carbon in adjacent ocean sediments31,32, quantified previously using radiocarbon as a tracer for modern sedimentary organic matter, removes 0.5–0.6 MtC/yr32,33. In terms of overall carbon balance, the long-term weathering effect of silicates, carbonates, and kerogen on land, together with offshore burial of terrestrial biospheric organic carbon, results in the removal of 0.24 ± 0.13 MtC/yr from the atmosphere. However, the total weathering effect on land acts as a net source of CO2 to the atmosphere, releasing 0.31 ± 0.12 MtC/yr (see Fig. 3). These quantities illustrate the fine balance between weathering mechanisms, and of the need to disentangle underlying processes for accurate assessment of the net effects of weathering budgets on atmospheric chemistry. The Taiwan orogeny shows that the weathering of metasedimentary catchments rich in pyrite and kerogen significantly influence the carbon budget of an orogenic entity.
The carbon budget presented here should be viewed conservatively, as two additional sources of CO2 to the atmosphere, authigenic silicate formation34 and metamorphic degassing35, are not considered. Considerable uncertainty lies in the former, as this process is limited by reactive silica availability in the oceans34. In the case of the latter process, the emission of CO2 and volatile hydrocarbons (e.g. CH4) further offset net carbon sequestration as a result of earth surface geochemical pathways and may tip the integrated surface and deep Earth geochemical cycles of an orogeny towards acting as a carbon source to the atmosphere35,36. Changes through time can be expected with erosional events2 as a result of major typhoons37 and mineral weathering pathways modified by fertiliser usage23. From the available data, the key variable compensating the release of CO2 to the atmosphere due to chemical weathering is the export and preservation efficiency of terrestrial biospheric organic carbon in offshore sediments – a particularly dynamic component that is responsive to tectonic38, climatic31,39 and anthropogenic40 influences.
The relative importance of sulphuric acid-mediated weathering depends on the presence and weathering of pyrite in exposed lithologies. The combined contributions of the Gaoping, Mackenzie (northern Canada), and Jialing (southern China) rivers deliver around 12% of sulphide-derived sulphate to the oceans, while their catchments encompass less than 2% of the Earth’s land surface7, highlighting the uneven distribution and localized occurrence of sulphuric acid-mediated weathering. In metasedimentary catchments such as those of the Gaoping River on Taiwan, weathering by sulphuric acid may contribute significantly to mineral dissolution. Here, it accounts for approximately two-thirds of total mineral dissolution, with carbonates almost entirely dissolved by sulphuric acid. The chemical weathering carbon budget of Taiwan supports the hypothesis that sulphuric acid mediated weathering in tandem with orogenic activity may well have affected atmospheric chemistry over geologic time10. Since the rise in atmospheric oxygen in the Precambrian, pyrite oxidation and sulphuric acid evolved therefrom has affected ocean chemistry, biologic activity, and atmospheric oxygen concentrations41,42,43. Mineral weathering by sulphuric acid has important implications for atmospheric CO2 inventories over geologic time10,30, with CO2 uptake and release governed by the fine balance between organic carbon burial in sediments and silicate, carbonate, and kerogen weathering controlled by the activity of oxygen, carbonic acid, and sulphuric acid. The total effect of orogenic activity on atmospheric chemistry remains a matter for discussion36, yet it is clear that the presence of sulphuric acid and its involvement in carbonate and silicate mineral weathering acts as a strong buffer on atmospheric CO2 uptake.
References
Dadson, S. J. et al. Links between erosion, runoff variability and seismicity in the Taiwan orogen. Nature 426, 648–651, https://doi.org/10.1038/nature02150 (2003).
Emberson, R., Hovius, N., Galy, A. & Odin, M. Oxidation of sulfides and rapid weathering in recent landslides. Earth Surface Dynamics 4, 727–742, https://doi.org/10.5194/esurf-4-727-2016 (2016).
Berner, R. A. Atmospheric carbon dioxide levels over Phanerozoic time. Science 249, 1382–1386, https://doi.org/10.1126/science.249.4975.1382 (1990).
Raymo, M. E. & Ruddiman, W. F. Tectonic forcing of late Cenozoic climate. Nature 359, 117, https://doi.org/10.1038/359117a0 (1992).
Gaillardet, J., Dupré, B., Louvat, P. & Allègre, C. J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical Geology 159, 3–30, https://doi.org/10.1016/S0009-2541(99)00031-5 (1999).
Das, A., Chung, C. H. & You, C. F. Disproportionately high rates of sulfide oxidation from mountainous river basins of Taiwan orogeny: Sulfur isotope evidence. Geophysical Research Letters 39, https://doi.org/10.1029/2012GL051549 (2012).
Burke, A. et al. Sulfur isotopes in rivers: Insights into global weathering budgets, pyrite oxidation, and the modern sulfur cycle. Earth and Planetary Science Letters 496, 168–177, https://doi.org/10.1016/j.epsl.2018.05.022 (2018).
Calmels, D. et al. Contribution of deep groundwater to the weathering budget in a rapidly eroding mountain belt, Taiwan. Earth and Planetary Science Letters 303, 48–58, https://doi.org/10.1016/j.epsl.2010.12.032 (2011).
Yoshimura, K. et al. Geochemical and stable isotope studies on natural water in the Taroko Gorge karst area, Taiwan—chemical weathering of carbonate rocks by deep source CO2 and sulfuric acid. Chemical Geology 177, 415–430, https://doi.org/10.1016/S0009-2541(00)00423-X (2001).
Torres, M. A., West, A. J. & Li, G. Sulphide oxidation and carbonate dissolution as a source of CO2 over geological timescales. Nature 507, 346, https://doi.org/10.1038/nature13030 (2014).
Torres, M. A., Moosdorf, N., Hartmann, J., Adkins, J. F. & West, A. J. Glacial weathering, sulfide oxidation, and global carbon cycle feedbacks. Proceedings of the National Academy of Sciences, https://doi.org/10.1073/pnas.1702953114 (2017).
Emberson, R., Galy, A. & Hovius, N. Weathering of reactive mineral phases in landslides acts as a source of carbon dioxide in mountain belts. Journal of Geophysical Research: Earth Surface, https://doi.org/10.1029/2018JF004672 (accepted).
Ishikawa, N. F. et al. Sources of dissolved inorganic carbon in two small streams with different bedrock geology: Insights from carbon isotopes. Radiocarbon 57, 439–448, https://doi.org/10.2458/azu_rc.57.18348 (2015).
Stuiver, M. & Polach, H. A. Discussion reporting of C-14 data. Radiocarbon 19, 355–363, https://doi.org/10.1017/S0033822200003672 (1977).
Liu, J. T., Hung, J.-J. & Huang, Y.-W. Partition of suspended and riverbed sediments related to the salt-wedge in the lower reaches of a small mountainous river. Marine Geology 264, 152–164, https://doi.org/10.1016/j.margeo.2009.05.005 (2009).
Breitenbach, S. F. M. & Bernasconi, S. M. Carbon and oxygen isotope analysis of small carbonate samples (20 to 100 µg) with a GasBench II preparation device. Rapid Communications in Mass Spectrometry 25, 1910–1914, https://doi.org/10.1002/rcm.5052 (2011).
Wacker, L. et al. A versatile gas interface for routine radiocarbon analysis with a gas ion source. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 294, 315–319, https://doi.org/10.1016/j.nimb.2012.02.009 (2013).
Stenström, K. E., Skog, G., Georgiadou, E., Genberg, J. & Johansson, A. A guide to radiocarbon units and calculations. 17 (2011).
Chung, C.-H., You, C.-F. & Chu, H.-Y. Weathering sources in the Gaoping (Kaoping) river catchments, southwestern Taiwan: Insights from major elements, Sr isotopes, and rare earth elements. Journal of Marine Systems 76, 433–443, https://doi.org/10.1016/j.jmarsys.2007.09.013 (2009).
Ishikawa, M. & Ichikuni, M. Uptake of sodium and potassium by calcite. Chemical Geology 42, 137–146, https://doi.org/10.1016/0009-2541(84)90010-X (1984).
Zheng, L.-W. et al. Isotopic evidence for the influence of typhoons and submarine canyons on the sourcing and transport behavior of biospheric organic carbon to the deep sea. Earth and Planetary Science Letters 465, 103–111, https://doi.org/10.1016/j.epsl.2017.02.037 (2017).
Hilton, R. G., Galy, A., Hovius, N., Horng, M.-J. & Chen, H. The isotopic composition of particulate organic carbon in mountain rivers of Taiwan. Geochimica et Cosmochimica Acta 74, 3164–3181, https://doi.org/10.1016/j.gca.2010.03.004 (2010).
Aquilina, L. et al. Long-Term Effects of High Nitrogen Loads on Cation and Carbon Riverine Export in Agricultural Catchments. Environmental Science & Technology 46, 9447–9455, https://doi.org/10.1021/es301715t (2012).
Aquilina, L. et al. Autotrophic denitrification supported by biotite dissolution in crystalline aquifers (1): New insights from short-term batch experiments. Science of The Total Environment 619–620, 842–853, https://doi.org/10.1016/j.scitotenv.2017.11.079 (2018).
Ho, C.-S. An introduction to the geology of Taiwan: explanatory text of the geologic map of Taiwan. 2nd edn., 192 (Central Geological Survey, Ministry of Economic Affairs ROC, Taipei, Taiwan, 1988).
Pauwels, H., Ayraud-Vergnaud, V., Aquilina, L. & Molénat, J. The fate of nitrogen and sulfur in hard-rock aquifers as shown by sulfate-isotope tracing. Applied Geochemistry 25, 105–115, https://doi.org/10.1016/j.apgeochem.2009.11.001 (2010).
Lyons, W. B., Carey, A. E., Hicks, D. M. & Nezat, C. A. Chemical weathering in high-sediment-yielding watersheds, New Zealand. Journal of Geophysical Research: Earth Surface 110, https://doi.org/10.1029/2003JF000088 (2005).
Liu, Y.-C. et al. Boron sources and transport mechanisms in river waters collected from southwestern Taiwan: Isotopic evidence. Journal of Asian Earth Sciences 58, 16–23, https://doi.org/10.1016/j.jseaes.2012.07.008 (2012).
Hilton, R. G., Gaillardet, J., Calmels, D. & Birck, J.-L. Geological respiration of a mountain belt revealed by the trace element rhenium. Earth and Planetary Science Letters 403, 27–36, https://doi.org/10.1016/j.epsl.2014.06.021 (2014).
Torres, M. A. et al. The acid and alkalinity budgets of weathering in the Andes–Amazon system: Insights into the erosional control of global biogeochemical cycles. Earth and Planetary Science Letters 450, 381–391, https://doi.org/10.1016/j.epsl.2016.06.012 (2016).
Hilton, R. G. et al. Tropical-cyclone-driven erosion of the terrestrial biosphere from mountains. Nature Geoscience 1, 759–762, https://doi.org/10.1038/ngeo333 (2008).
Hilton, R. G. et al. Climatic and geomorphic controls on the erosion of terrestrial biomass from subtropical mountain forest. Global Biogeochemical Cycles 26 https://doi.org/10.1029/2012GB004314 (2012).
Kao, S. et al. Preservation of terrestrial organic carbon in marine sediments offshore Taiwan: mountain building and atmospheric carbon dioxide sequestration. Earth Surface Dynamics 2, 127, https://doi.org/10.5194/esurf-2-127-2014 (2014).
Michalopoulos, P. & Aller, R. C. Rapid clay mineral formation in Amazon delta sediments: Reverse weathering and oceanic elemental cycles. Science 270, 614, https://doi.org/10.1126/science.270.5236.614 (1995).
Evans, M. J., Derry, L. A. & France-Lanord, C. Degassing of metamorphic carbon dioxide from the Nepal Himalaya. Geochemistry, Geophysics, Geosystems 9, https://doi.org/10.1029/2007GC001796 (2008).
Gaillardet, J. & Galy, A. Himalaya - Carbon sink or source? Science 320, 1727, https://doi.org/10.1126/science.1159279 (2008).
Tsai, F., Hwang, J.-H., Chen, L.-C. & Lin, T.-H. Post-disaster assessment of landslides in southern Taiwan after 2009 Typhoon Morakot using remote sensing and spatial analysis. Natural Hazards and Earth System Sciences 10, 2179, https://doi.org/10.5194/nhess-10-2179-2010 (2010).
Blair, N. E. & Aller, R. C. The fate of terrestrial organic carbon in the marine environment. Annual Review of Marine Science 4, 401–423, https://doi.org/10.1146/annurev-marine-120709-142717 (2012).
Bröder, L. et al. Fate of terrigenous organic matter across the Laptev Sea from the mouth of the Lena River to the deep sea of the Arctic interior. Biogeosciences 13, 5003–5019, https://doi.org/10.5194/bg-13-5003-2016 (2016).
Maavara, T., Lauerwald, R., Regnier, P. & Van Cappellen, P. Global perturbation of organic carbon cycling by river damming. Nature Communications 8, 15347, https://doi.org/10.1038/ncomms15347 (2017).
Konhauser, K. O. et al. Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature 478, 369, https://doi.org/10.1038/nature10511 (2011).
Berner, R. A. Biogeochemical cycles of carbon and sulfur and their effect on atmospheric oxygen over Phanerozoic time. Palaeogeography, Palaeoclimatology, Palaeoecology 75, 97–122, https://doi.org/10.1016/0031-0182(89)90186-7 (1989).
Reinhard, C. T., Lalonde, S. V. & Lyons, T. W. Oxidative sulfide dissolution on the early Earth. Chemical Geology 362, 44–55, https://doi.org/10.1016/j.chemgeo.2013.10.006 (2013).
Chen, C.-H. Geologic Map of Taiwan (1:500,000) Central Geological Survey, Ministry of Economic Affairs, Taiwan R.O.C. (2000).
Acknowledgements
We thank the participants of the ETH PhD Student Taiwan Excursion for inspiring discussions and catalysing the initiation of this study. Valier Galy is thanked for stimulating discussions. Madalina Jaggi is thanked for measurement of DI13C. Fanny Leuenberger-West is thanked for measurement of dissolved ion concentrations. From National Sun Yat-sen University, we thank Yu-Shih Lin and group members for logistical support and Yuan-Pin Chang for support with sample export. The authors are indebted to three anonymous reviewers for their valuable input and to the editor Luc Aquilina for greatly improving this contribution. This work has been supported by SNF CAPS-LOCK2 Project (#200021_140850) and ETH Research Grant ETH-41 14-1.
Author information
Authors and Affiliations
Contributions
M.L., S.-L.W., T.I.E. and T.M.B. designed this study. S.-L.W., L.-H.C. and T.M.B conducted river sampling. N.H., L.M. and T.M.B. conducted laboratory analyses and L.W. and S.M.B. provided analytical assistance. M.L., S.-L.W., S.M.B., M.P., T.I.E. and T.M.B. contributed to data analysis and interpretation. All authors provided input and comments for this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blattmann, T.M., Wang, SL., Lupker, M. et al. Sulphuric acid-mediated weathering on Taiwan buffers geological atmospheric carbon sinks. Sci Rep 9, 2945 (2019). https://doi.org/10.1038/s41598-019-39272-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39272-5
This article is cited by
-
Microbial communities modulate chemical weathering and carbon dioxide cycling in an active orogen in Taiwan
Communications Earth & Environment (2024)
-
Isotopic evidence for sources of dissolved carbon and the role of organic matter respiration in the Fraser River basin, Canada
Biogeochemistry (2023)
-
Co-variation of silicate, carbonate and sulfide weathering drives CO2 release with erosion
Nature Geoscience (2021)
-
Tropical cyclones likely enhance chemical weathering but suppress atmospheric CO2 consumption in landslide-dominated catchments
Biogeochemistry (2021)
-
Mountains, erosion and the carbon cycle
Nature Reviews Earth & Environment (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.