Abstract
Megastigmus transvaalensis Hussey (Hymenoptera: Torymidae) parasitizes drupes of Rhus genus plants in Africa and Schinus (Anacardiaceae) in South America. This exotic wasp damages Schinus terebinthifolia Raddi drupes in native forests and ecological restoration areas in Brazil. The objective of the present study was to investigate the precipitation, temperature and relative humidity effects on M. transvaalensis flight activity, and to determine the parasitism rate and sex ratio of this wasp on S. terebinthifolia plants. The study was conducted with yellow sticky traps and S. terebinthifolia drupes collected in an ecological restoration area, from August 2014 to September 2015, in the Sorocaba municipality, São Paulo state, Brazil. Megastigmus transvaalensis populations were negatively correlated with maximum and minimum temperatures and precipitation, with population peaks at the end of May 2015, with 927 insects per evaluation (48.8 adults per trap). The M. transvaalensis sex ratio was higher in the laboratory (0.42) than in the field (0.08). The parasitism rate of S. terebinthifolia drupes by M. transvaalensis ranged from zero to 36.3% under natural environmental conditions. Megastigmus transvaalensis can be monitored with yellow sticky traps. Damage by M. transvaalensis in S. terebinthifolia drupes may decrease the germination of the seeds and the establishment of this plant in native and restoration ecological areas.
Similar content being viewed by others
Introduction
It is important to determine the geographic origin of the organisms to assess their impact on the ecosystems1,2. Schinus terebinthifolia Raddi, 1820 (Sapindales: Anacardiaceae), native to Argentina, Brazil and Paraguay3 presents pioneering behavior, attractiveness for avifauna, good development in low fertility soils and it is used in land recovering of degraded areas in Brazil4. This plant adapts to different environmental conditions with high competitiveness and easy cultivation5.
Schinus terebinthifolia occurs from Pernambuco state to southern Brazil on sandy and clayey soils and is also reported in temperate regions6. Its drupes, collected in native areas, are an income source in the Espírito Santo state, Brazil7. This plant was introduced into more than 20 countries, including Australia, Bermuda, Fiji, Mauritius, Micronesia, New Caledonia, Reunion Island, South Africa, and Tahiti3 and the Bahamas and Virgin Islands in the Caribean8 with fruits used for ornamental purposes and in the spice trade9,10. In 1980, S. terebinthifolia was introduced into Florida, USA, proving to be highly invasive11 and spreading to Arizona, California, Hawaii, and Texas8. Schinus terebinthifolia has an allelopathic effect, preventing seed germination of native species12,13,14 and causing environmental impacts11.
In Florida, the Department of Agriculture and Consumer Services classified S. terebinthifolia as very harmful and the USA has banned the sale of this plant8,11. The uncontrolled increase of the area occupied by S. terebinthifolia led to studies with the parasitoids of their drupes15,16 as candidates for the biological control of this plant, but none were effective in the USA17.
In 1988, Megastigmus transvaalensis Hussey, 1956 (Hymenoptera: Torymidae), unknown in the USA, was recovered and reared from S. terebinthifolia drupes collected in Palm Beach County8. This phytophagous wasp, native to South Africa, may reduce S. terebinthifolia germination18.
Megastigmus transvaalensis parasitizes drupes of Rhus angustifolia L. and Rhus laevigata L. (Sapindales: Anacardeaceae) and S. terebinthifolia and Schinus molle L. (Sapindales: Anacardeaceae) in South Africa19, Schinus polygamus (Cav.) Cabrera in Chile20 and S. terebinthifolia in Paraná21 and São Paulo state, Brazil22. The M. transvaalensis embryonic period is four to five days with its larval stage lasting from 20 to 25 d23 with pupation inside the fruits, where it can remain in diapause for months with its adults emerging during S. terebinthifolia flowering and fruit formation stages24.
In Brazil, unlike the USA, S. terebinthifolia is important mainly in riparian forest recovery and dune stability programs and projects25, where M. transvaalensis may limit the development of this plant22.
Integrated pest management depends on insect population monitoring, mainly peaks and relationships with abiotic factors26,27. Capture with traps favors abundance, sampling, population dynamics and monitoring studies of insect pests28,29,30.
The objectives of this study were to investigate the precipitation, temperature and relative humidity effects on M. transvaalensis flight activity, and to determine the parasitism rate and sex ratio of this wasp on S. terebinthifolia plants.
Results
Numbers of M. transvaalensis adults
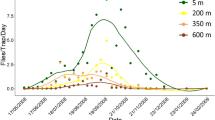
The number of M. transvaalensis adults caught in the sticky traps, varied with the seasons, with 3,415 insects captured, being 279 females and 3,136 males. The number of M. transvaalensis adults during the summer, when the rains were more constant (690 mm) and the minimum and maximum temperatures remained around 20.5 and 26 °C, was about two individuals per trap. This number increased from the 18th evaluation (May 6, 2015), during the fall, when rains were less intense (138 mm) and the minimum and maximum temperatures were 17 and 22 °C, respectively. The M. transvaalensis population peak of 927 insects in the 19th evaluation (May 20, 2015) in autumn, coincided with lower precipitation (13 mm) and temperature (20.1 °C). Megastigmus transvaalensis were not captured in the third (October 10, 2014) and 14th (March 11, 2015) evaluations in 19 sticky traps on S. terebinthifolia plants.
The correlation matrix presenting the transformed data showed a linear inverse relationship between the variable number of insects (N_Insects) and explanatory variables, minimum (T_Min) (r = −0.50) and maximum (T_Max) (r = −0.59) temperatures, precipitation (r = −0.35) and low correlation with relative humidity (r = 0.15) and drupes damaged (r = 0.08) (Fig. 1). Differences between the independent and dependent variables presented more significant effects from each other. Minimum (P = 0.0013) and maximum (P = 0.0034) temperatures influenced the dependent variables.
Linear correlation matrix between the response variable insects number (N_insects) and the explanatory variables minimum temperature (T_Min), maximum temperature (T_Max), relative humidity (RH), accumulated precipitation (Prec_mm) and damaged drupes (N_damages). Sorocaba, São Paulo state, Brazil. August 29, 2014 to September 9, 2015. Captions: N_Insects = insects number; RH = relative humidity; N_Damages = damages number; Prec_mm = precipitation; T_Min = minimum temperature; and T_Max = maximum temperature.
The distribution of the database was normal with a value of W = 0.4835 showing that the samples were derived from the same distribution, with non-significant results (P = 0.9438) and indicating a good fit for the model. The database was compatible and could be used to interpret and discuss the variables. The hypothesis that population variability is similar (variance homogeneity), that is, that there are differences between the M. transvaalensis populations, was rejected.
The main component analysis showed a relationship with the response variable and can be evaluated with the PC1 and PC2 axes, since they have an eigenvalue of more than one, 43.93 and 26.32%, respectively, of the data set variation (Fig. 2). The PCA retained all factors with eigenvalues greater than 1 (Table 1), showing that the variables belong to certain axes. Broken-stick axes 1 and 2 explain most of the variability, with a steep decrease (14.51%) from axis 3. The variability ratio was low for axes 4 (8.72%), 5 (5.93%) and 6 (0.06%) (Table 1 and Fig. 2). The correlation between the variables and the PC 1 axis was high and inversely proportional to the minimum (r = −0.93) and maximum (r = −0.89) temperatures, moderate for precipitation (r = −0.65) and low for drupes damage (r = −0.13) and relative humidity (r = −0.03) (Fig. 3). The PC1 axis showed that the variable number of insects (N_Insects) and the explanatory minimum (T_min) and maximum (T_max) temperatures and precipitation (Prec_mm) are more strongly related to the number of M. transvaalensis individuals captured. The adjusted coefficient of determination (R2 adj. = 0.2882; n = 27) showed how the regression analysis line fits the data set (Fig. 4). Low R2 adj. values do not always mean bad models, since the relationship between the analyzed variables and the normality of the data and the value of F (F = 31.062) should be considered. This is necessary to determine the linear relationship between the dependent and independent (Table 2) variables. The PC 2 axis was not significant (P = 0.8149).
Principal components of main component analysis (PCA) containing the explanatory variables minimum temperature (T_Min), maximum temperature (T_Max), relative humidity (RH), accumulated precipitation (Prec_mm) and damaged drupes (N_Damages). Sorocaba, São Paulo state, Brazil. August 29, 2014 to September 9, 2015. Captions: sit = evaluations number.
Residual analysis obtained from the scores related to the simple linear regression equation, characterized by the comparison between the values of the response variable insects number (N_insects) of Megastigmus transvaalensis (Hymenoptera: Torymidae) and the explanatory variables minimum temperature (T_Min), temperature (T_Max), relative humidity (RH), accumulated precipitation (Prec_mm) and Schinus terebinthifolia damaged drupes (Anacardiaceae) (N_Damage).
The number of M. transvaalensis individuals captured in the field varied with temperature and precipitation. Population peaks of this insect were higher from May to August 2015, mainly in late May and early June, with inversely proportional correlation with minimum and maximum temperatures (transition from autumn to winter) and precipitation.
Relative humidity did not affect M. transvaalensis population peaks in May or June 2015, that is, did not show any association with the variable response relative humidity (P = 0.1885). The drupe damage explanatory variable showed no relation with the number of insects captured (P = 0.0506).
Parasitism rate
The damaged S. terebinthifolia drupe numbers were higher in September (15.6%), November (36.3%) and December (19.3%) in 2014 (Fig. 5). Differences in the number of drupes produced per plant may explain variation in the damage in those parasitized by M. transvaalensis (F = 33.75, df = 36.54, P = 1.187e-06).
Sex ratio
The M. transvaalensis adult numbers emerged in the laboratory was 1,161, with 39.6 males and 28.6 females per evaluation. In the field, the M. transvaalensis adult numbers caught in the sticky traps was 3,415, with 116.4 males and 10.0 females per evaluation. The sex ratio in the laboratory was higher (0.42) than in the field (0.08) (Table 3). The number of adults caught in the field traps was similar between collections (F = 1.973, df = 37.15, P = 0.1684), but it varied in the laboratory (F = 7.015, df = 15.09, P = 0.0181) (Fig. 5). Megastigmus transvaalensis males showed phenotypic variation (Fig. 6a–f).
Discussion
Yellow sticky traps are efficient to monitor M. transvaalensis in an ecological restoration area, similar to reports for Psyllaephagus bliteus Riek, 1962 (Hymenoptera: Encyrtidae) population fluctuations in a Eucalyptus camaldulensis plantation in São Paulo state31, Brazil, Thaumastocoris peregrinus Carpintero & Dellapé, 2006 (Hemiptera: Thaumastocoridae) in Rio Grande do Sul state, Brazil32 and Leptocybe invasa Fisher & La Salle, 2004 (Hymenoptera: Eulophidae) and of its parasitoid Megastigmus sp. (Hymenoptera: Torymidae) in the field in Dandeli, India33. These traps are the most attractive to Hymenoptera insects34 due to the yellow color resembling the bright leaves preferred by insects of this order as oviposition sites, as they can also be confused by appearance, which concerns the greater nitrogen quantity in the plant sap34,35.
Megastigmus transvaalensis adult peaks, from early May to September 2015, coincided with minimum and maximum temperatures of around 17 and 23 °C, respectively, similar to the increase in the P. bliteus adult numbers caught in fall with minimum and maximum temperatures31. This inverse correlation of the M. transvaalensis populations with temperature can be explained by the direct effect on the development of this insect, as reported for L. invasa, with high survival at lower temperatures36. Temperature decreases reduce metabolic rate, embryonic development, larva and pupa stages and affect insect behavior37, being pecilothermic organisms that are sensitive to temperature changes and thermal fluctuations38.
The lack of impact of relative humidity on the M. transvaalensis population fluctuation suggests that the mean humidity remained stable during the study period, since variations in this parameter affected the fecundity, longevity, parasitism and progeny of Pediobius furvus Gahan, 1928 (Hymenoptera: Eulophidae)39 in Kenya and Cotesia flavipes Cameron, 1891 (Hymenoptera: Braconidae) in Ethiopia, Africa40.
The inverse correlation of M. transvaalensis adult numbers captured with precipitation was similar to that reported for the decrease of Trichogramma (Hymenoptera: Trichogrammatidae) species numbers collected with increasing precipitation in Piracicaba, São Paulo state, Brazil41. This may be related to environmental resistance with physical and biological factors reducing insect population growth and climatic variables such as temperature and seasonal rainfall affecting these population structures42. The precipitation impact on M. transvaalensis is due to the mechanical control of populations of these insects, regardless of their population density43.
The M. transvaalensis population peak coincided with the highest number of plants with drupe (n = 13) of S. terebinthifolia, showing that this may be related to this host development44. This is similar to the higher activity of Oomyzus sokolowskii Kurdjumov, 1912 (Hymenoptera: Eulophidae) with increasing host numbers. The absence of viable hosts for parasitism drives the insects to search for longer periods for adequate hosts45, that is, the M. transvaalensis population increase depends on food availability in the field.
The lack of correlation between M. transvaalensis adult numbers caught and damaged S. terebinthifolia drupes is due to these individuals emerging a few months after oviposition23 on the mature drupe that remained on the trees. The diapause period of Megastigmus wasps varies depending on the host and the number of adults emerged on food availability46. Hymenoptera parasitoids have different strategies to avoid adverse environmental conditions47, with variations in the development of immature stages, adult stage duration, male and female maturation and diapause28.
The lack of effect of S. terebinthifolia fruiting on the M. transvaalensis adult numbers collected in the field during the population peak of this insect (May 20, 2015 with 927 insects), when the damage to the drupes collected was lower, reinforces the hypothesis that adults of this insect emerged from the drupes present on the trees or from previous fruits fallen on the ground. Damage in September 2014 may be related to previous flowering and fruiting periods during February to April of the same year, and the estimate of M. transvaalensis damage may have only been based on individuals who emerged from previous fruiting, since this insect presents diapause. However, S. terebinthifolia may also flower from October to December48, explaining damage during November and December 2014 through February 2015, with the end of one cycle and the beginning of another.
A M. transvaalensis generation was observed at 12 months at the beginning of the evaluations in 2014, when drupes present on the plants were from previous fruiting. Therefore, there was a single flowering and complete fruiting period, which was observed in 2015 and that can be explained by the flowering and fruiting differing between plants and localities, due to the wide geographic distribution and the morphological characteristics of each individual present48. The lack of S. terebinthifolia drupe production in the final evaluation period in the 19 trees selected may have affected the emergence of subsequent M. transvaalensis generations, since this insect may produce more than one generation per year according to flower and fruit production of this plant24. Megastigmus sp. presented three generations from May to November corresponding to spring, summer and autumn in the province of Adana, southern Turkey49.
Variability in the number of drupes damaged by M. transvaalensis, from zero to 36.3% and on S. terebinthifolia plants, 73.4%, is similar to that of this species with 1–55% in São Paulo state, Brazil, with a higher number of S. terebinthifolia plants in urban areas explaining that of damaged drupes (35.0 ± 15.8%) compared to restoration areas (15.8 ± 8.4%)22. Environmental conditions, age of the individuals sampled and S. terebinthifolia drupe production may explain damage variations between trees of this plant24.
The highest parasitism rate (36.3%) occurred during the spring 2014, under natural environmental conditions which presented temperatures of 16.7 to 27.6 °C, may be related to the previous S. terebinthifolia flowering periods, during October to November and February to April, which varied depending on the region as reported for this plant cultivated in the Botanical Garden of the Institute of Biosciences, Botucatu, São Paulo state, Brazil50. In Florida, USA, the parasitism peak was 31 and 76% during winter and spring, respectively, with temperatures different from those in Brazil24. In Argentina, M. transvaalensis parasitism on S. molle drupes of 6.29 to 26.84% occurred during autumn and winter fruiting51. In South Africa, this wasp damaged S. molle drupes throughout the entire summer rainy season52. Periods of greater parasitism and differences between sites may be related to S. terebinthifolia phenology. That is, periods of flowering and fruiting influence food availability and, consequently, the incidence of the wasp. These factors may affect the incidence of M. transvaalensis, similar to reports of significant variation in damage between trees and sites in Sorocaba, São Paulo state, Brazil22.
The greater emergence of M. transvaalensis females in the laboratory at 25 ± 2 °C, relative humidity of 60 ± 12% and photoperiod of 12:12 h (day: night) may be due to the controlled conditions. In the field, S. terebinthifolia drupes were naturally exposed, but with the emergence of M. transvaalensis, only when the conditions were favorable to this wasp. Megastigmus transvaalensis females can control their offspring sex at oviposition53,54 through environmental stimuli such as abiotic factors55,56. In addition, the greater number of males, captured on the sticky traps in the field than in the laboratory in all evaluations, can be attributed to arrhenotokous parthenogenesis57. The high or low sex ratio may be a response to environmental factors such as temperature and relative humidity with arrhenotokous parthenogenesis, Hymenoptera characteristics and linked to host size and age58,59. The sex ratio may vary with temperature as reported for Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae), with a higher female numbers at temperatures below 30 °C60. Megastigmus sp. sex ratio in laboratory was close to 1: 1 between 23 to 31 °C49.
Variations in body length, coloration, presence or absence of wing spots and size of the abdomen of M. transvaalensis males is similar to that reported for those emerged from S. polygamus drupes in Chile20. Chalcidoidea pigmentation varies between species of this superfamily and between sites and hosts, as reported for Megastigmus dorsalis Fabricius, 1978 (Hymenoptera: Torymidae) in Jordan61.
Conclusions
The number of M. transvaalensis individuals was negatively correlated with maximum and minimum temperatures and precipitation, presenting population peaks at the end of May 2015. The parasitism rate by M. transvaalensis in S. terebinthifolia drupes ranged from zero to 36.3%, in field conditions. Megastigmus transvaalensis sex ratio was higher in the laboratory (0.42) than in the field (0.08). Megastigmus transvaalensis can be monitored with yellow sticky traps and males of this exotic wasp presented phenotypic variation in Brazil.
Material and Methods
Study sites
The study was carried out in an ecological restoration area near Semideciduous Seasonal Forest (SSF) fragments in an experimental area of the Federal University of São Carlos, Campus Sorocaba, São Paulo state, Brazil (23°34′S and 47°31′W) with average altitude of 580 m and annual precipitation of 1,311.2 mm from August 29, 2014 to September 9, 2015. Schinus terebinthifolia is referred to in the early stages of this type of forest according to Resolution of the National Environmental Council (CONAMA) of the Brazilian Ministry of Environment n° 392 of June 25, 2007. The climate of the region is of type “Cfa” (temperate humid with summer), according to the climatic classification of Köppen-Geiger62, with average annual temperature of 21.4 °C, maximum of 30.1 °C and minimum of 12.2 °C. The predominant soil type is Red Latosol63.
Megastigmus transvaalensis adult sampling with sticky traps
The adult population survey of M. transvaalensis was carried out on 19 randomly selected, identified, georeferenced S. terebinthifolia plants with diameter at breast height (DBH) and tree height measured. Megastigmus transvaalensis was collected with traps consisting of yellow plastic cards (12 cm long x 10 cm wide) with adhesive on both sides and a capture area of 100 cm2 each31, discounting the area for the card identification. Each trap was installed on an S. terebinthifolia plant, attached to a plastic coated wire and fixed with a string at an approximate height of 2.50 meters.
Twenty-seven collections were performed approximately every 15 days when the traps were replaced with new ones, wrapped in transparent plastic film to avoid damaging the insects captured and placed into paper bags identified with the tree number and collection date. The traps were sent to the laboratory and stored at 0 °C until the M. transvaalensis adults were counted. Males and females of this wasp on both sides of the traps were counted using a stereoscopic microscope (Leica Microsystems TL3000 Ergo, Wetzlar, Germany) with 10X. The maximum and minimum temperature (°C), relative humidity (%) and precipitation (mm) values were obtained from the Meteorological Database for Education and Research - BDMEP Station N° 83851, Sorocaba, São Paulo state, Brazil (23° 29′ S and 47° 26′ W and 645 m altitude) of the National Institute of Meteorology (INMET). Readings were taken daily and the average maximum and minimum temperature, average relative humidity and accumulated precipitation, by evaluation date, were used.
Schinus terebinthifolia drupe sampling
Branches with S. terebinthifolia drupes were collected at random in the middle third of the 19 plants (three branches per tree) with a pruning shear connected to a 3-meter long aluminum pole. These branches were collected, packed into paper bags when the yellow traps were collected and replaced, taken to the laboratory and stored in a Biochemical Oxygen Demand (BOD) incubator at 18 ± 2 °C to reduce the drying rate of leaves and drupes.
One hundred S. terebinthifolia drupes were chosen at random from three branches per sample point (tree) to calculate the M. transvaalensis parasitism rate. The total number of vesicular outflow holes (after the emergence of M. transvaalensis adults) was also counted under a stereoscopic microscope with 10X.
Megastigmus transvaalensis sex ratio in laboratory and field
Twenty S. terebinthifolia drupes were randomly chosen from three branches/tree/evaluation and placed into 1,300 ml plastic containers with a lid, labeled and stored in BOD at 25 ± 2 °C, relative humidity of 60 ± 12% and photoperiod of 12:12 h (day: night) in the laboratory. Megastigmus transvaalensis males and females that emerged after approximately 15 days of incubation were counted, with females identified by the presence of the ovipositor at the extremity of the abdomen23. The M. transvaalensis sex ratio, sampled in the S. terebinthifolia drupes and those caught in the sticky traps were calculated.
Data analysis
Megastigmus transvaalensis adult number variation
The number of insects captured in the sticky traps was subjected to multiple linear regression analysis64 using the meteorological variables data and the number of drupes damaged.
Statistical analyses were performed with the R Studio® program at a significance level of 5% in variance analysis (ANOVA) and input of the variables in the regression model. The variation coefficient was tested between trees to determine damage levels between them. The data were standardized for base 10 logarithm to reduce/equalize the value range and aid in the interpretation. This transformation was performed to evaluate the variance homogeneity and data distribution normality. A correlation matrix between the variables was generated and the data submitted to Tukey range test65. Shapiro-Wilk (W) normality test was used to verify if the dataset had normal distribution66, if there were differences between the means and if the factors could influence the dependent variable. The homogeneity of the variances was evaluated with the Levene test67.
The relationship between the data set variables and the trend visualization of the set, that is, the reduction or elimination of the number of variables, was verified by main component analysis (PCA). The eigenvalues, associated with a component or factor in descending order versus the number of the component or factor, were displayed in a scree plot graph of the data set with the broken-stick model68.
The multiple linear regression analysis was performed with the scores obtained from the PC1 and PC2 axes, adopting the model: Yi = β0 + β1xi + €i, to i = 1, … n.
The explanatory variables with the greatest contribution or predictive power were calculated with the significant axis for regression analysis PC1 (P = 0.0052) and the adjustment statistics of the model.
Schinus terebinthifolia drupe parasitism rate by Megastigmus transvaalensis
The S. terebinthifolia drupe parasitism percentage was calculated including those presenting M. transvaalensis outlet holes categorized as damaged (DD) and those without such holes as undamaged (ND). The difference between the damaged drupe values per evaluation was verified by ANOVA.
Megastigmus transvaalensis sex ratio
The sex ratio (RS) of M. transvaalensis was calculated (RS = females number ÷ insects number), submitted to variance analysis (ANOVA) and compared using the F test with 5% probability.
References
Kambhampati, S., Black, I. V. W. C. & Rai, K. S. Geographic origin of the US and Brazilian Aedes albopictus inferred from allozyme analysis. Heredity 67, 85–94 (1991).
Haymer, D. S., He, M. & McInnis, D. O. Genetic marker analysis of spatial and temporal relationships among existing populations and new infestations of the Mediterranean fruit fly (Ceratitis capitata). Heredity 79, 302–309 (1997).
Mytinger, L. & Williamson, G. B. The invasion of Schinus into saline communities of Everglades National Park. Fla. Scien. 50, 7–12 (1987).
Panetta, F. D. & McKee, J. Recruitment of the invasive ornamental, Schinus terebinthifolius, is dependent upon frugivores. Aust. J. Ecol. 22, 432–438 (1997).
Spector, J. B. & Putz, F. E. Biomechanical plasticity facilitates invasion of maritime forests in the southern USA by Brazilian pepper (Schinus terebinthifolia). Biol. Invasions 8, 255–260 (2006).
Degáspari, C. H., Waszczynskyj, N. & Prado, M. R. M. Antimicrobial activity of Schinus terebinthifolius Raddi. Ciênc. Agrotec. 29, 617–622 (2005).
Cesário, L. F. & Gaglianone, M. C. Floral biology and reproductive phenology of Schinus terebinthifolius Raddi (Anacardiaceae) in the restinga of northern Rio de Janeiro State. Acta Bot. Bras. 22, 828–833 (2008).
Habeck, D. H., Bennett, F. D. & Grissell, E. E. First record of a phytophagous seed chalcid from Brazilian peppertree in Florida. Fla. Entomol. 72, 378–379 (1989).
Mack, R. N. The commercial seed trade: an early disperser of weeds in the United States. Econ. Bot. 45, 257–273 (1991).
Guerra, M. J. M., Barreiro, M. L., Rodriguez, Z. M. & Rubaicaba, Y. Actividad antimicrobiana de um extracto fluido al 80% de Schinus terebinthifolius Raddi (Copal). Rev. Cub. Plant. Medic. 5, 23–5 (2000).
Medal, J. C. et al. Host specificity of Heteroperreyia hubrichi Malaise (Hymenoptera: Pergidae), a potential biological control agent of Brazilian Peppertree (Schinus terebinthifolius Raddi). Biol. Control 14, 60–65 (1999).
Gogue, G. J., Hurst, C. J. & Bancroft, L. Growth inhibition by Schinus terebinthifolius. HortScience 9, 301 (1974).
Morgan, E. C. & Overholt, W. A. Potential allelopathic effects of Brazilian pepper (Schinus terebinthifolius Raddi, Anacardiaceae) aqueous extracts upon germination and growth of selected Florida native plants. J. Torrey Bot. Soc. 132, 11–15 (2005).
Donnelly, M. J., Green, D. M. & Walters, L. J. Allelopathic effects of fruits of the Brazilian pepper Schinus terebinthifolius on growth, leaf production and biomass of seedlings of the red mangrove Rhizophora mangle and the black mangrove Avicennia germinans. J. Exp. Mar. Biol. Ecol. 357, 149–156 (2008).
Cassani, J. R. Arthropods on Brazilian peppertree, Schinus terebinthifolius (Anacardiaceae), in south Florida. Fla. Entomol. 69, 184–196 (1986).
Cassani, J. R., Maloney, D. R., Habeck, D. H. & Bennett, F. D. New insect records on Brazilian peppertree Schinus terebinthifolius (Anacardiaceae), in south Florida. Fla. Entomol. 72, 714–716 (1989).
McKay, F. et al. Natural enemies of Brazilian peppertree (Schinus terebinthifolius: Anacardiaceae) from Argentina: their possible use for biological control in the USA. Fla. Entomol. 92, 292–303 (2009).
Scheffer, S. J. & Grissell, E. E. Tracing the geographical origin of Megastigmus transvaalensis (Hymenoptera: Torymidae): an African wasp feeding on a South American plant in North America. Mol. Ecol. 12, 415–42 (2003).
Grissell, E. E. & Prinsloo, G. L. Seed-feeding species of Megastigmus (Hymenoptera: Torymidae) associated with Anacardiaceae. J. Hymenopt. Res. 10, 271–279 (2001).
Fernandes, D. R. R., Salas, C., Rothmamnn, S., Lara, R. I. R. & Perioto, N. W. Megastigmus transvaalensis (Hymenoptera: Torymidae) on Schinus polygamus (Anacardiaceae): a new native host for this invasive seed-feeding species. Idesia 32, 119–122 (2014).
Perioto, N. W. First record of the genus Megastigmus Dalman, 1820 (Hymenoptera: Torymidae) and first record for the subfamily Megastigminae from Brazil. Arq. Inst. Biol. 64, 115–116 (1999).
Ferreira-Filho, P. J. et al. The exotic wasp Megastigmus transvaalensis (Hymenoptera: Torymidae): first record and damage on the Brazilian peppertree, Schinus terebinthifolius drupes, in São Paulo, Brazil. An. Acad. Bras. Ciênc. 87, 2091–2095 (2015).
Milliron, H. E. Taxonomic and biological investigations in the genus Megastigmus with particular reference to the taxonomy of the Nearctic species (Hymenoptera: Chalcidoidea; Callimomidae). Am. Midl. Nat. 41, 257–420 (1949).
Wheeler, G. S., Massey, L. M. & Endries, M. The Brazilian peppertree drupe feeder Megastigmus transvaalensis (Hymenoptera: Torymidae): Florida distribution and impact. Biol. Control 22, 139–148 (2001).
Falkenberg, D. B. Aspects of the flora and secondary vegetation in the restinga from Santa Catarina State, south Brazil. Insula 28, 1–30 (1999).
Southwood, T. R. E., Brown, V. K. & Reader, P. M. Ecological methods with particular reference to study of insect diversities in succession. Biological Journal of the Linnean Society 327–48 (1979).
Zanuncio, J. C., Alves, J. B., Santos, G. P. & Campos, W. O. Monitoring and population dynamics of Lepidoptera associated with Eucalyptus: VI. Belo Oriente region, Minas Gerais, Brazil. Pesqui. Agropecu. Bras. 28, 1121–1127 (1993).
Wolda, H. Insect seasonality: Why? Ann. Rev. Ecol. Evol. Syst. 19, 1–18 (1988).
Bragança, M. A. L., de Souza, O. & Zanuncio, J. C. Environmental heterogeneity as a strategy for pest management in Eucalyptus plantations. For. Ecol. Manage. 102, 9–12 (1998).
Zanuncio, J. C., Zanuncio, T. V., Freitas, F. A. & Pratissoli, D. Population density of Lepidoptera in a plantation of Eucalyptus urophylla in the State of Minas Gerais, Brazil. Animal Biology 53, 17–26 (2003).
Ferreira-Filho, P. J., Wilcken, C. F., Oliveira, N. C., Pogetto, M. H. F. A. D. & Lima, A. C. V. Population dinamics of red gum lerp psyllid, Glycaspis brimblecombei (Moore, 1964) (Hemiptera: Psyllidae) and its parasitoid, Psyllaephagus bliteus (Hymenoptera: Encyrtidae), in Eucalyptus camaldulensis plantation. Ciên. Rural 38, 2109–2114 (2008).
Smaniotto, M. A., Cunha, U. S., Finkenauer, E. & Garcia, M. S. Effect of color of adhesive traps in the monitoring of Thaumastocoris peregrinus Carpintero & Dellapé (Hemiptera: Thaumastocoridae) in field. Ciên. Florestal 27, 799–805 (2017).
Vastrad, A. S., Kumari, K. & Viraktamath, S. Monitoring Eucalyptus gall wasp, Leptocybe invasa Fisher and La Salle (Hymenoptera: Eulophidae) using yellow sticky trap in eucalyptus plantation. Karnataka J. Agricul. Scienc. 23, 215–216 (2010).
Prokopy, R. J. & Owens, E. D. Visual detection of plants by herbivorous insects. Ann. Rev. Entomol. 28, 337–364 (1983).
Mooney, H. A. & Gulmon, S. L. Constraints on leaf structure and function in reference to herbivory. Biosci. 32, 198–206 (1982).
Torres, J. B., Musolin, D. L. & Zanuncio, J. C. Thermal requirements and parasitism capacity of Trissolcus brochymenae (Ashmead) (Hymenoptera: Scelionidae) under constant and fluctuating temperatures, and assessment of development in field conditions. Biocontrol Sci.Technol. 12, 583–593 (2002).
Samson, P. R. & Blood, P. R. B. Biology and temperature relationships of Chrysopa sp., Micromus tasmaniae and Nabis capsiformes. Entomol. Exp. Appl. 25, 253–259 (1979).
Ju, R. T., Zhu, H. Y., Gao, L., Zhou, X. H. & Li, B. Increases in both temperature means and extremes likely facilitate invasive herbivore outbreaks. Nat. Commun. 5, 15715 (2015).
Duale, A. H. Effect of temperature and relative humidity on the biology of the stem borer parasitoid Pediobius furvus (Gahan) (Hymenoptera: Eulophidae) for the management of stem borers. Ann. Entomol. Soc. Am. 34, 1–5 (2005).
Emana, G. D. Comparative studies of the influence of relative humidity and temperature on the longevity and fecundity of the parasitoid, Cotesia flavipes. J. Insec. Sci. 19, 1–7 (2007).
Querino, R. B. & Zucchi, R. A. Trichogramma Species (Hymenoptera: Trichogrammatidae) collected in suction trap in forest reserve. Neotrop. Entomol. 33, 451–455 (2004).
Levings, S. C. & Windsor, D. M. Litter arthropod populations in a tropical deciduous forest: Relationships between years and arthropod groups. J. Anim. Ecol. 54, 61–69 (1985).
Boinski, S. & Fowler, N. Seasonal patterns in a tropical lowland forest. Biotropica 21, 223–233 (1989).
Mendel, Z., Protasov, A., Fisher, N. & La Salle, J. Taxonomy and biology of Leptocybe invasa gen. & sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on. Eucalyptus. Aust. J. Entomol. 43, 101–113 (2004).
Silva-Torres, C. S. A., Barros, R. & Torres, J. B. Effect of age, photoperiod and host availability on the parasitism behavior of Oomyzus sokolowskii Kurdjumov (Hymenoptera: Eulophidae). Neotrop. Entomol. 38, 512–519 (2009).
Annila, E. Diapause and population fluctuations in Megastigmus specularis Walley and Megastigmus spermotrophus Wachtl. (Hymenoptera: Torymidae). Ann. Entomol. Fenn. 48, 33–36 (1982).
Claret, J. & Carton, Y. Diapause in a tropical species, Cothonaspis boulardi (parasitic Hymenoptera). Oecologia 45, 32–34 (1980).
Liebsch, D. & Mikich, S. B. Reproductive phenology of plant species of Mixed Ombrophilous Forest in Paraná, Brazil. Rev. Bras. Bot. 32, 375–391 (2009).
Protasov, A., La Salle, J., Doganlar, M. & Mendel, Z. Occurrence of two local Megastigmus species parasitic on the Eucalyptus gall wasp Leptocybe invasa in Israel and Turkey. Phytoparasitica 36, 449–459 (2008).
Carmello-Guerreiro, S. M. & Paoli, A. A. S. Ontogeny and structure of the pericarp of Schinus terebinthifolius Raddi (Anacardiaceae). Braz. Arch. Biol. Technol. 45, 73–79 (2002).
Román, N. L. E., Aquino, D. A., Ortiz, F. & Quispe, R. First record of Megastigmus transvaalensis (Hussey) (Hymenoptera: Chalcidoidea: Torymidae) in north-western Argentina. Effect on drupes of Schinus areira L. (Sapindales: Anacardiaceae). Acta Zool. Lilloana 58, 65–72 (2014).
Iponga, D. M., Cuda, J. P., Milton, S. J. & Richardson, D. M. Megastigmus wasp damage to seeds of Schinus molle, Peruvian pepper tree, across a rainfall gradient in South Africa: implications for invasiveness. Afr. Entomol. 16, 127–131 (2008).
Flanders, S. E. On the sexuality and sex ratios of hymenopterous populations. Am. Nat. 93, 489–494 (1965).
Gerber, H. S. & Klostermeyer, E. C. Sex control by bees: a voluntary act of egg fertilization during oviposition. Science 167, 82–84 (1970).
West, S. A. & Sheldon, B. C. Constraints in the evolution of sex ratio adjustment. Science 295, 1685–1688 (2002).
Peruquetti, R. C. & Del Lama, M. A. Sex allocation and sex-dependent selection for body size in Trypoxylon rogenhoferi Kohl (Hymenoptera: Sphecidae). Rev. Bras. Entomol. 47, 581–588 (2003).
Daane, K. M., Sime, K. R., Dahlsten, D. L., Andrews, J. J. W. & Zuparko, R. L. The biology of Psyllaephagus bliteus Riek (Hymenoptera: Encyrtidae), a parasitoid of the red gum lerp psyllid (Hemiptera: Psylloidea). Biol. Control 32, 228–235 (2005).
Urbaneja, A. et al. Effect of temperature on life history of Cirrospilus vittatus (Hymenoptera: Eulophidae), an ectoparasitoid of Phyllocnistis citrella (Lepidoptera: Gracillariidae). J. Econ. Entomol. 95, 250–255 (2002).
Lo Pinto, M., Salermo, G. & Wajnberg, E. Biology and behavior of Cirrospilus diallus and Cirrospilus pictus, parasitoids of Phyllocnistis citrella. BioControl 50, 921–935 (2005).
Fonseca, F. L., Kovaleski, A., Foresti, J. & Ringenberg, R. Development and thermal requirements of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on eggs of Bonagota cranaodes (Meyrick) (Lepidoptera: Tortricidae). Neotrop. Entomol. 34, 945–949 (2005).
Rizzo, M. C. & Askew, R. R. Hymenoptera Chalcidoidea inhabiting galls of Cynipidae in Jordan. Entomol. Fenn. 19, 218–227 (2008).
Alvares, C. A., Stape, J. L., Sentelhas, P. C., de Morais Gonçalves, J. L. & Sparovek, G. Köppen’s climate classification map for Brazil. Meteor. Zeitschrift 22, 711–728 (2013).
Silva, A. M. Natural potential of erosion in the county of Sorocaba, São Paulo, Brazil. Inter. J. Nat. Disast. Accid. Civil Infrastruct. 8, 5–13 (2008).
Tabachnick, B & Fidell, L. S. Using multivariate statistics (3a ed.). (New York: Harper Collins 1996).
Tukey, J. Comparing individuals means in the analysis of variance. Biometrics 5, 99–114 (1949).
Shapiro, S. S. & Wilk, M. B. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611 (1965).
Layard, M. W. J. Robust large-sample tests for homogeneicity of variance. Journal of the Am. Stat.Assoc. 68, 195–198 (1973).
Colwell, J. D., Suhet, A. R. & Van Raid, B. Statistical procedures for developing general soil fertility models for variable regions. Australia: CSIRO, 68p. (CSIRO. Division of Soils. Divis. Report, 93) (1988).
Acknowledgements
To “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)”, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and “Programa Cooperativo sobre Proteção Florestal/PROTEF do Instituto de Pesquisas e Estudos Florestais/IPEF” for financial support. Dr. Phillip John Villani (University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author information
Authors and Affiliations
Contributions
T.C.G. and P.J.F.F. designed the research; M.C.N.P., G.K.F.G. and J.C.G. performed the experiments; T.C.G., P.J.F.F., E.P.P., C.F.W. and J.C.Z. analyzed the data; T.C.G., P.J.F.F., M.C.N.P., G.K.F.G., W.T., M.V.M., E.P.P., J.C.G., A.G.C., C.F.W and J.C.Z., wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghiotto, T.C., do Nascimento Prado, M.C., Giuliani, G.K.F. et al. Megastigmus seed wasp damage on native Schinus terebinthifolia drupes in ecological restoration area in Brazil. Sci Rep 9, 2438 (2019). https://doi.org/10.1038/s41598-019-39129-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39129-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.