Abstract
We synthesized multiple-cation Rb(MAFA)PbI3 perovskite single crystals for the first time. The effect of Rb+ substitution was systemically investigated, and the addition of 1.5 M 5% RbI was the optimum condition to obtain high-quality Rb(MAFA)PbI3 single crystals. Lattice shrinkage occurred in the Rb(MAFA)PbI3 single crystal because of the small ionic radius of Rb+, resulting in blue-shifted absorption and photoluminescence (PL) peaks. The 1.5 M 5% RbI-added (MAFA)PbI3 single crystal showed the longest carrier lifetime of 18.35 ns, exhibiting the highest photoresponse than other crystals. We believe that this work will provide a basic insight into the mixed-cation perovskite single crystals for the future optoelectronic applications.
Similar content being viewed by others
Introduction
Hybrid organic–inorganic perovskites with an ABX3 structure (where A is a monovalent cation such as methylammonium (CH3NH3+; MA) and formamidinium (CH3(NH2)2+; FA); B and X refer to divalent cations (Pb2+, Sn2+) and halogen anions (I−, Br−, Cl−), respectively) have been considered as promising optoelectronic materials because of their excellent optical and electrical properties1,2,3,4,5. However, these perovskites suffer from low stability in air because of the inherent instability of their organic cations6,7, which limits their practical applications. To overcome this limitation, compositional engineering of these perovskites has been intensively studied6,8,9,10. For example, Zhang et al. obtained better crystal quality and enhanced device performance by adding 10% FA+ to MAPbI3 solar cells11. Wang et al. also obtained similar results when they mixed MA+ and FA+ cations in a ratio of 7:39. Recently, Saliba et al. added Cs cations to MAFA perovskites and achieved great solar cell performance with improved thermal stability12. Zhang et al. also investigated the effect of incorporating Cs+ in perovskites and demonstrated that triple-cation solar cells exhibit better performance than do single-cation solar cells in terms of thermal and humidity stabilities13. More recently, Rb has been introduced as the fourth candidate for multiple-cation perovskites14,15. Interestingly, based on the Goldschmidt tolerance factor, perovskite structures cannot be formed with Rb+ because of its small ionic radius (152 pm)14. However, with the incorporation of small amounts Rb+, high photovoltaic efficiency and low hysteresis can be achieved because Rb+ can significantly suppress the yellow phase in perovskite films14,16,17,18,19. Despite these rapid improvements in device performance, the use of polycrystalline perovskites is still limited because of their grain boundary issues. Shao et al. reported that grain boundaries acts as ion migration channels, resulting in current hysteresis in perovskite optoelectronic devices20. Moreover, Wang et al. studied the morphology-dependent degradation of polycrystalline perovskite films and found that the grain boundaries in perovskites accelerate their degradation process because of the diffusion of moisture through them21. This limitation can be overcome using perovskite single crystals, which offer the following advantages: the absence of grain boundaries, low trap density, and excellent air stability2,22,23. However, the effect of mixed cations on single crystals remains nearly unexplored. To the best of our knowledge, there has been no report on triple-cation perovskite single crystals, especially those with Rb+ incorporation.
In this study, we synthesized and characterized multiple-cation Rb(MAFA)PbI3 perovskite single crystals for the first time. X-ray diffraction (XRD) and time-of-flight secondary ion mass spectroscopy (ToF-SIMS) analyses confirmed the incorporation of Rb+ ions into the perovskite single crystal. We found that an Rb concentration of 1.5 M (5%) was optimum to obtain Rb(MAFA)PbI3 single crystals with high Rb ratios. The effect of the addition of Rb+ on the perovskite single crystal was systemically studied via UV-Vis absorption and photoluminescence (PL) spectroscopy. Finally, based on the time-resolved PL (TRPL) and photocurrent data, we proved that the addition of Rb+ increased the carrier lifetime and photoresponse of the perovskite single crystal. We believe that this work will provide a basic insight into the structure of mixed-cation perovskite single crystals for future applications.
Results and Discussion
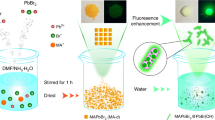
The Rb(MAFA)PbI3 perovskite single crystals were grown using an inverse temperature crystallization (ITC) method24. The structure of Rb(MAFA)PbI3 and the detailed growth process are illustrated in Fig. 1a. (MAFA)PbI3 has a cubic structure at room temperature23,25, and smaller Rb+ ions randomly replace the organic cations26, MA+, and FA+, reducing its cubic volume. Generally, the solubility of common precursor salts increases with an increase in temperature. Perovskite salts, on the other hand, show reduced solubilities in solvents such as γ-butyrolactone (GBL) at elevated temperatures24. Hence, we synthesized Rb(MAFA)PbI3 single crystals with different Rb ratios using a GBL-based ITC method. The filtrates of 1 M MAPbI3 and FAPbI3 solutions were mixed in a 1:1 ratio. To this mixture, 1 and 1.5 M RbI solutions (5, 10, and 15%) were added (see Supplementary Information for details). After storing the mixed solution in a convection oven at 130 °C for 6 h, the dodecahedral Rb(MAFA)PbI3 single crystals were grown, as shown in Fig. 1b. The shape of single crystal highly depends on the growth solution; both dodecahedral- and cubic-shaped single crystal of the same composition can be grown by varying the growth solution condition27. The size of the crystals depended on the RbI content. The average crystal size was found to be 3–5 mm. We further proved the absence of grain boundaries in the Rb(MAFA)PbI3 single crystals via scanning electron microscopy (SEM) (Figure S1). As mentioned earlier, the absence of grain boundaries is one of the most important advantages of single crystalline perovskites over polycrystalline perovskites.
In order to investigate the effect of the Rb ratio on the structure of the Rb(MAFA)PbI3 single crystals, we carried out a powder XRD analysis on them. The diffraction peak at 13.9° in Fig. 2a corresponds to the (001) plane of the cubic perovskite structure25, which means that the (MAFA)PbI3 single crystal had a cubic phase before the addition of Rb+. We analyzed the changes in the XRD patterns of the single crystal as a function of the RbI concentration. However, no additional peak was observed after the addition of Rb+. This indicates that the Rb(MAFA)PbI3 single crystal retained the original cubic phase of (MAFA)PbI3 even after the addition of Rb+. For more details, we zoomed the (001) plane in Fig. 2b, and it was found that the diffraction peak shifted to higher angles depending on the Rb ratio. We fitted the diffraction peak at 13.9° to Lorentzian function, and the average FWHM value of Rb(MAFA)PbI3 perovskite crystals was about 0.11°, which is sharper than that of the perovskite film26. Generally, FWHM of XRD profiles gives information about crystal quality of materials28; thus, we assumed that the single crystalline perovskites showed sharper FWHM than that of polycrystalline perovskites as a result of no grain boundaries. Further, we compared the FWHM values with the Rb+ addition, and the 1.5 M 5% RbI-added single crystal showed slightly broader FWHM of 0.13°. Considering the small size of Rb+, the broader FWHM can also be understood by the effect of crystal distortion in Rb(MAFA)PbI3 single crystals. We plotted the shifted peak values as a function of the RbI content (Fig. 2c) and observed a significant peak shift when 5% RbI was added (blue arrow). Recently, Shi et al. have reported similar XRD peak shifts for Rb+-incorporated FAPbBr3 films. They reported that the diffraction peak shifted toward wider angles because of the Rb+-induced crystal distortion due to the small ionic radius (152 pm) of Rb+ (compared to that of FA+ (253 pm))29. In other words, the diffraction peak shift increased with an increase in the Rb+ content. Thus, by monitoring the shifted peak values in Fig. 2c, we assumed that the addition of 5% RbI was optimum. Further, we compared the other diffraction peaks and observed the remaining PbI2 peak at 25.9° from the 1 M 5% RbI-added crystal, as shown in Fig. 2d. Although the addition of 5% RbI was optimum the addition of 1.5 M RbI resulted in better crystal quality than that obtained when 1 M RbI was added. Hence, it can be stated that the addition of 1.5 M 5% RbI was optimum to synthesize high-quality Rb(MAFA)PbI3 single crystals with a high Rb+ concentration.
Powder XRD analysis of the Rb(MAFA)PbI3 single crystals. (a) XRD patterns of the Rb(MAFA)PbI3 perovskite single crystals with different Rb ratios. (b) XRD peak shifts in the region of the (001) diffraction and (c) shifted peak values with the increasing RbI concentration. (d) XRD spectra of Rb(MAFA)PbI3 near 25.9° attributed to the (020) diffraction of PbI2.
To demonstrate the Rb+ substitution, we carried out the ToF-SIMS analysis of the single crystals with various RbI concentrations. We selected I− (126.9) and Pb+ (207.9) for comparison and monitored the emitted Rb+ (84.9) signals from the Rb(MAFA)PbI3 single crystals as a function of the RbI content. Figure 3a shows the ToF-SIMS depth profiles of the secondary ions emitted from the Rb(MAFA)PbI3 single crystals. As shown in Fig. 3a, the intensity of the Rb+ signal changed significantly with an increase in the Rb ratio. We also plotted the changes in the intensity of the emitted I−, Pb+, and Rb+ ions as a function of the RbI content at a sputtering depth of 300 nm (Fig. 3b). We found that the intensity of both I− and Pb+ did not change; however, that of Rb+ increased gradually with an increase in the RbI content. In particular, the 1.5 M 5% RbI-added single crystal showed the largest number of Rb cations. However, at higher RbI contents of 10% or 15%, the Rb cation substitution ratio decreased significantly (Fig. S2). This indicates that the addition of 1.5 M 5% RbI is the optimum condition to obtain Rb(MAFA)PbI3 single crystals with high Rb+ content. This is consistent with the XRD results shown in Fig. 2.
We further investigated the effect of the RbI content on the optical properties of the Rb(MAFA)PbI3 single crystals. Linear absorption and PL spectra of the single crystals with various Rb contents are shown in Fig. 4a,b. Here, we have not shown the data for 15% RbI addition because of the small size and low yield of the corresponding single crystal (Fig. S3). Basically, in the absence of Rb, the (MAFA)PbI3 perovskite single crystal showed an optical band edge and a PL peak at around 870 and 800 nm, respectively. The above-band gap PL characteristics of perovskite single crystals have been reported2,30; however, the origin of this effect is still under debate. After the addition of Rb+ into the (MAFA)PbI3 single crystal, the absorption and PL peaks slightly blue-shifted by about 5.17 and 6.29 nm, respectively. According to previous reports, metal-halide-metal bonds, which are related to the electronic band structure of perovskites, are indirectly affected by the lattice shrinkage due to Rb+ substitution, leading to a down-shifted valence band maximum29. As a result, the Rb(MAFA)PbI3 perovskite single crystals showed blue-shifted absorption and PL peaks. The expanded absorption spectrum to the near-infrared region is needed to be addressed because the solar cell efficiency would be improved owing to a significant amount of solar energy in this wavelength range. The average thickness of Rb(MAFA)PbI3 crystals was 1.48 mm, and the optical band edges were located at approximately 865 nm for all crystals.
The TRPL measurements of the single crystals with various Rb ratios were also carried out. Figure 4c shows the normalized PL decay curves of the Rb(MAFA)PbI3 single crystals with various Rb ratios. We extracted two decay components fitted to a bi-exponential function and calculated the average lifetime of the crystals (see Supplementary Information for details). The (MAFA)PbI3 perovskite single crystal showed an average carrier lifetime of 13.01 ns (Table 1). In contrast, the 5% RbI-added single crystal showed a longer carrier lifetime than the other crystals. The 1.5 M 5% RbI-added (MAFA)PbI3 single crystal showed the longest carrier lifetime of 18.35 ns. We expect that Rb+-based perovskite single crystals have low trap-state densities and long carrier diffusion lengths through the remarkably increased lifetime in the TRPL results. From these results, it can be stated that the addition of Rb+ increases the carrier lifetime of perovskite single crystals, which is beneficial for future applications.
Lastly, we have carried out additional experiments to disclose the practical effect with Rb+ addition as shown in Fig. S4. We studied photocurrent properties of the Rb(MAFA)PbI3 perovskite single crystals. For the photocurrent measurement, the 100-nm-thick Au electrodes were thermally evaporated on the top of the Rb(MAFA)PbI3 crystals with an average channel length of 622 μm. The device was characterized using a 689 nm excitation source, and we found that the 1.5 M 5% RbI-added (MAFA)PbI3 single crystal exhibited the highest photoresponsivity than other crystals. This result indicates that the addition of Rb+ also has a positive effect on the electrical properties of perovskite single crystals.
Conclusions
We successfully synthesized multiple-cation Rb(MAFA)PbI3 perovskite single crystals for the first time. The effect of Rb+ substitution on the properties of the perovskite single crystals was systemically investigated using XRD and ToF-SIMS measurements. It was found that the addition of 1.5 M 5% RbI was the optimum condition for the formation of high-quality Rb(MAFA)PbI3 single crystals. Lattice shrinkage occurred in the Rb(MAFA)PbI3 single crystal because of the small ionic radius of Rb+. As a result, blue-shifted absorption and PL peaks were observed upon Rb+ substitution. The 1.5 M 5% RbI-added (MAFA)PbI3 perovskite single crystal showed the longest carrier lifetime of 18.35 ns, with the highest photoresponsivity compared to other crystals. We believe that the fundamental understanding and optimization of the Rb+-incorporated perovskite single crystals will introduce a new platform for the development of multiple cation single crystals for future optoelectronic applications.
Change history
13 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-33296-8
References
Green, M. A., Ho-Baillie, A. & Snaith, H. J. The Emergence of Perovskite Solar Cells. Nat. Photon. 8(7), 506–514 (2014).
Dong, Q. et al. Electron-hole Diffusion Lengths >175 μm in Solution-grown CH3NH3PbI3 Single Crystals. Science 347(6225), 967–970 (2015).
Xing, G. et al. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 342(6156), 344–347 (2013).
Stranks, S. D. et al. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 342(6156), 341–344 (2013).
Wehrenfennig, C., Eperon, G. E., Johnston, M. B., Snaith, H. J. & Herz, L. M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 26(10), 1584–1589 (2014).
Poorkazem, K. & Kelly, T. L. Compositional Engineering To Improve the Stability of Lead Halide Perovskites: A Comparative Study of Cationic and Anionic Dopants. ACS Appl. Energy Mater. 1(1), 181–190 (2018).
Wang, F. et al. Organic Cation-Dependent Degradation Mechanism of Organotin Halide Perovskites. Adv. Funct. Mater. 26(20), 3417–3423 (2016).
Jeon, N. J. et al. Compositional Engineering of Perovskite Materials for High-performance Solar Cells. Nature 517(7535), 476–480 (2015).
Wang, C. et al. Compositional and Morphological Engineering of Mixed Cation Perovskite Films for Highly Efficient Planar and Flexible Solar Cells with Reduced Hysteresis. Nano Energy 35, 223–232 (2017).
Bush, K. A. et al. Compositional Engineering for Efficient Wide Band Gap Perovskites with Improved Stability to Photoinduced Phase Segregation. ACS Energy Lett. 3(2), 428–435 (2018).
Zhang, Y., Grancini, G., Feng, Y., Asiri, A. M. & Nazeeruddin, M. K. Optimization of Stable Quasi-Cubic FAxMA1–xPbI3 Perovskite Structure for Solar Cells with Efficiency beyond 20%. ACS Energy Lett. 2(4), 802–806 (2017).
Saliba, M. et al. Cesium-containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 9(6), 1989–1997 (2016).
Zhang, R. et al. Theoretical Lifetime Extraction and Experimental Demonstration of Stable Cesium-containing Tri-cation Perovskite Solar Cells with High Efficiency. Electrochim. Acta 265, 98–106 (2018).
Saliba, M. et al. Incorporation of Rubidium Cations into Perovskite Solar Cells Improves Photovoltaic Performance. Science 354(6309), 206–209 (2016).
Duong, T. et al. Rubidium Multication Perovskite with Optimized Bandgap for Perovskite-Silicon Tandem with over 26% Efficiency. Adv. Energy Mater. 7(14), 1700228 (2017).
Park, I. J. et al. Effect of Rubidium Incorporation on the Structural, Electrical, and Photovoltaic Properties of Methylammonium Lead Iodide-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 9(48), 41898–41905 (2017).
Zhang, M. et al. High-Efficiency Rubidium-Incorporated Perovskite Solar Cells by Gas Quenching. ACS Energy Lett. 2(2), 438–444 (2017).
Park, Y. H. et al. Inorganic Rubidium Cation as an Enhancer for Photovoltaic Performance and Moisture Stability of HC(NH2)2PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 27(16), 1605988 (2017).
Turren-Cruz, S.-H. et al. Enhanced Charge Carrier Mobility and Lifetime Suppress Hysteresis and Improve Efficiency in Planar Perovskite Solar Cells. Energy Environ. Sci. 11(1), 78–86 (2018).
Shao, Y. et al. Grain Boundary Dominated Ion Migration in Polycrystalline Organic–Inorganic Halide Perovskite Films. Energy Environ. Sci. 9(5), 1752–1759 (2016).
Wang, Q. et al. Scaling Behavior of Moisture-induced Grain Degradation in Polycrystalline Hybrid Perovskite Thin Films. Energy Environ. Sci. 10(2), 516–522 (2017).
Chen, Z. et al. Thin Single Crystal Perovskite Solar Cells to Harvest Below-bandgap Light Absorption. Nat. Commun. 8(1), 1890 (2017).
Li, W.-G., Rao, H.-S., Chen, B.-X., Wang, X.-D. & Kuang, D.-B. A Formamidinium–methylammonium Lead Iodide Perovskite Single Crystal Exhibiting Exceptional Optoelectronic Properties and Long-term Stability. J. Mater. Chem. A 5(36), 19431–19438 (2017).
Saidaminov, M. I. et al. High-quality Bulk Hybrid Perovskite Single Crystals within Minutes by Inverse Temperature Crystallization. Nat. Commun. 6, 7586 (2015).
Huang, Y. et al. The Intrinsic Properties of FA(1–x)MAxPbI3 Perovskite SingleCrystals. J. Mater. Chem. A, 5(18), 8537–8544 (2017).
J.-P. Correa-Banea et al. Homogenization of halide distribution and carrier dynamics in alloyed organic-inorganic perovskite. Arxiv (2018)
Meng-Huan, J., Chun-Fu, L., Pao-Yi, T. & Wei-Fang, S. Precise Facet Engineering of Perovskite Single Crystals by Ligand-Mediated Strategy. Crystal Growth & Design 17(11), 5945–5952 (2017).
Yabuzaki, J., Takahashi, Y., Adachi, H., Mori, Y. & Sasaki, T. High-quality crystal growth and characterization of organic nonlinear optical crystal: 4-dimethylamino-N-methyl-4-stilbazolium tosylate (DAST). Bull. Mater. Sci. 22, 11–13 (1999).
Shi, Y. et al. Rubidium Doping for Enhanced Performance of Highly Efficient Formamidinium-Based Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 10(11), 9849–9857 (2018).
Saouma, F. O., Park, D. Y., Kim, S. H., Jeong, M. S. & Jang, J. I. Multiphoton Absorption Coefficients of Organic–Inorganic Lead Halide Perovskites CH3NH3PbX3 (X=Cl, Br, I) Single Crystals. Chem. Mater. 29(16), 6876–6882 (2017).
Acknowledgements
This work was supported by IBS-R011-D1 and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2016R1A2B2015581).
Author information
Authors and Affiliations
Contributions
H.K. and H.R.B. conceived the presented idea and carried out all the experiments. H.K., H.R.B., and M.S.J. contributed to data analysis and manuscript preparation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H., Byun, H.R. & Jeong, M.S. Synthesis and Characterization of Multiple-Cation Rb(MAFA)PbI3 Perovskite Single Crystals. Sci Rep 9, 2022 (2019). https://doi.org/10.1038/s41598-019-38947-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38947-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.