Abstract
Allopregnanolone (ALLO) is a neurosteroid produced in the brain, but so far, no study has explored its link with itching. Herein, we used a diet-induced atopic dermatitis mouse model to examine whether exogenously administered and endogenously produced ALLO contribute to inducing scratching. Systemic administration of ALLO elicited robust scratching in the atopic dermatitis model, while it did not affect spontaneous and pruritogen-induced scratching in normal mice. ALLO caused scratching when administered intracisternally, but not when administered intrathecally or intradermally, suggesting the involvement of supraspinal mechanisms. Pharmacological analyses suggested that both γ-aminobutyric acid type A receptor activation and serotonin type 3 receptor inhibition were involved in ALLO-induced scratching. We next examined whether endogenously produced ALLO is involved in ethanol-induced scratching in atopic dermatitis mice, because ethanol administration increases ALLO in rodent brain. Acute ethanol administration increased brain ALLO levels, which coincided with increased scratching. Pre-treatment with finasteride, a synthetic ALLO inhibitor, suppressed ethanol-induced scratching and ALLO production in the brain. Collectively, our results demonstrated for the first time that ALLO administration caused marked scratching in atopic dermatitis mice, and ethanol-induced scratching may be mediated through endogenously produced brain ALLO.
Similar content being viewed by others
Introduction
Itch (or pruritus) is an unpleasant sensation inducing the desire to scratch. Atopic dermatitis is a common chronic skin disease, and pruritus is a cardinal symptom of this disease, which markedly reduces the quality of life of the patient1. Although several pathogenic mechanisms in the periphery and spinal cord have been postulated to be involved in atopic dermatitis itch2,3,4,5, supraspinal (i.e. brain) mechanisms may also play an important role. It is known that clinically, emotional stress, sleep, and alcohol intake often trigger or enhance pruritus in atopic dermatitis6, and these factors seem to primarily affect brain function. Therefore, unique brain mechanisms of itch may be involved in atopic dermatitis; however, its molecular basis remains largely unclear.
We previously reported a unique, diet-induced chronic mouse model of atopic dermatitis. HR-1 hairless mice fed a special diet (named HR-AD) develop atopic dermatitis-like skin inflammation7,8. Interestingly, in this model, administration of certain central nervous system (CNS) drugs such as ethanol and barbiturates, markedly increased scratching9,10. Barbiturate-induced scratching was replicated in another chronic dermatitis model NC/Nga mice but not in the histamine-induced acute itch model using normal healthy mice10, suggesting that such enhancement of scratching is characteristic of chronic disease conditions. Further, we have shown that the CNS drug-induced scratching could be attributed, at least partly, to a synergistic effects on multiple targets including γ-aminobutyric acid type A (GABAA) receptors, N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors, and L-type voltage-dependent calcium channels (L-VDCC)9,10; however, whether endogenous substances acting on these targets contribute to pruritus remains to be studied.
3α,5α-Tetrahydroprogesterone, also called allopregnanolone (ALLO), is one of the neurosteroids that are synthesized de novo in the brain or reach the brain from peripheral steroidogenic organs, such as adrenals and gonads11. Neurosteroids not only act on gene expression via intracellular steroid hormone receptors, but also rapidly alter neuronal excitability by acting through various membrane receptors12,13. Specifically, ALLO has been shown to positively modulate GABAA receptors, thereby producing barbiturate-like neurobehavioral effects such as anxiolytic, anticonvulsant, and sedative/hypnotic actions14,15. It has also been reported that similar to ethanol and barbiturates, ALLO inhibits L-VDCC16. Since ALLO has some similar pharmacological properties to those of ethanol and barbiturates, we hypothesized that it is involved in pruritus in atopic dermatitis.
In the present study, to prove this hypothesis, we first investigated whether ALLO administration causes scratching in the diet-induced atopic dermatitis model. Second, we also determined whether endogenously produced ALLO is involved in ethanol-induced scratching in the same model, because acute ethanol administration has been shown to increase brain ALLO levels in rodents17,18,19.
Results
Systemic administration of ALLO elicits robust scratching in special diet-fed atopic dermatitis model, while it did not affect spontaneous and pruritogen-induced scratching in normal mice
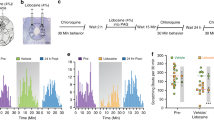
Consistent with our previous results7,8, special diet-fed hairless mice exhibited red scaly skin resembling that observed in human atopic dermatitis (Fig. 1a, right panel), whereas normal diet-fed mice had no gross abnormalities (Fig. 1a, left panel). When ALLO (5 and 10 mg/kg) was administered intraperitoneally (i.p.) to normal mice, no significant change occurred in the cumulative duration of hindlimb scratching (Fig. 1b). In contrast, in the special diet-fed atopic dermatitis mouse model, the scratching duration was dose-dependently increased by ALLO administration, with a significant effect observed at 10 mg/kg (Fig. 1b). The mice administered ALLO frequently scratched their face and ears (Supplemental video-1), although sometimes also their back and trunk. The time course of scratching showed that ALLO-induced scratching started within 10 min after administration, peaked at 10–20 min, and had almost subsided at 30 min (Fig. 1c); thus, this scratching response was probably mediated by a non-genomic action.
Induction of scratching behaviour by intraperitoneal (i.p.) administration of allopregnanolone (ALLO) to atopic dermatitis-induced mice. (a) Mouse fed normal diet (left panel, Normal) or special diet (right panel, Atopic) for 12 weeks. (b) Vehicle (20% castor oil in saline) or ALLO (5 and 10 mg/kg) was i.p. administered to normal and atopic dermatitis-induced mice, and cumulative duration of scratching was measured for 1 h. Each column represents mean ± S.E.M. of six animals. **P < 0.01, vs. vehicle (designated as 0 mg/kg), one-way ANOVA with Dunnett’s multiple comparison test. (c) Time course of the cumulative duration of scratching when vehicle or 10 mg/kg ALLO was administered to atopic dermatitis-induced mice. Each column represents the mean ± S.E.M. of six animals. *P < 0.05 and ***P < 0.001, vs. vehicle, two-way ANOVA with Bonferroni’s multiple comparison test.
We next examined whether ALLO administration also increases pruritogen-induced acute scratching in normal mice. However, systemic administration of ALLO (10 mg/kg, i.p.) did not significantly affect histamine- or chloroquine-induced scratching (Fig. 2).
No effect of allopregnanolone (ALLO) on pruritogen-induced scratching in normal mice. Vehicle (saline) or ALLO (10 mg/kg, i.p.) was administered to normal mice, and 10 min later, saline (50 µL/site), histamine (Hist, 250 µg/50 µL/site) or chloroquine (CQ, 200 µg/50 µL/site) was i.d. injected to the nape of the neck. Immediately after i.d. injection, the number of scratching bouts on the injection site was measured for 1 h. Each column represents the mean ± S.E.M. of four animals. *P < 0.05, one-way ANOVA with Bonferroni’s multiple comparison test.
ALLO acts supraspinally to induce scratching
To determine the site of action of ALLO-induced scratching, a low dose of ALLO was locally (intracisternal [i.ci.], intrathecal [i.t.], or intradermal [i.d.]) injected to the atopic dermatitis-induced mice. The i.ci. injection of ALLO (2.5 and 5 µg/site) dose-dependently and significantly induced scratching (Fig. 3c), which was comparable to that induced by i.p. administration (Fig. 1b). Time course and manner of scratching after i.ci. injection of ALLO (Fig. 3d and Supplemental video-2) was very similar to that of i.p. administration (Fig. 1c and Supplemental video-1). On the other hand, i.t. and i.d. injections at the same dose did not cause significant scratching (Fig. 3e–h).
Induction of scratching behaviour by an intracisternal (i.ci.) injection of low-dose allopregnanolone (ALLO) to atopic dermatitis-induced mice. (a) Schematic illustration of i.ci. injection. (b) Gross distribution of 1% Evans blue solution i.ci. injected at 10 µL. (c,e,g) Vehicle (20% castor oil in saline, 10 µL) or ALLO (2.5 and 5 µg/10 µL in each site) was i.ci. (c), intrathecally (i.t.) (e), or intradermally (i.d.) (g) injected into atopic dermatitis-induced mice, and from immediately (for i.ci. and i.d.) or 5 min (for i.t.) after injection, the cumulative duration of scratching was measured for 1 h. Each column represents the mean ± S.E.M. of five animals. **P < 0.01, vs. vehicle (designated as 0 µg/site), one-way ANOVA with Dunnett’s multiple comparison test. (d,e,h) Time course of the cumulative duration of scratching when vehicle or 5 µg/site ALLO was i.ci. (d), i.t. (f), or i.d. (h) administered to atopic dermatitis-induced mice. Each column represents the mean ± S.E.M. of five animals. ***P < 0.001, vs. vehicle, two-way ANOVA with Bonferroni’s multiple comparison test.
ALLO-induced scratching is suppressed by GABAA receptor antagonist and serotonin 5-HT3 receptor agonist
Next, we pharmacologically analysed the mechanism underlying ALLO-induced scratching. Opioid receptor antagonists, such as naloxone and naltrexone, have been demonstrated to suppress various types of pruritus20,21,22. Thus, we first examined the effect of naltrexone on ALLO-induced scratching. However, naltrexone, even at the high dose (10 mg/kg, i.p.), did not suppress scratching (Fig. 4a), suggesting that ALLO-induced scratching is not a common opioid-mediated itch response.
Effects of several drugs on allopregnanolone (ALLO)-induced scratching in atopic dermatitis-induced mice. ALLO was i.p. administered at 10 mg/kg. (a) Vehicle (saline) or naltrexone (NTX, 10 mg/kg) was i.p. administered 30 min before ALLO administration. (b) Vehicle (saline) or picrotoxin (2.5 or 5 mg/kg) was i.p. administered 5 min after ALLO administration. (c) Vehicle (0.5% Tween 80-containing saline) or Bay K 8644 (0.3 or 1 mg/kg) was i.p. administered 5 min after ALLO administration. (d) Vehicle (saline) or NMDA (4 nmol/10 µL/site) was i.ci. administered 5 min after ALLO administration. (e) Vehicle (saline) or 2-methyl-5-HT (10 or 30 µg/10 µL per site) was i.ci. administered 5 min after ALLO administration. Each column represents the mean ± S.E.M. of six (a), eight or nine (b), 16 or 17 (c), six or seven (d), and five to six (e) animals. *P < 0.05 and ***P < 0.001, vs. vehicle (Veh), unpaired Student’s t-test or one-way ANOVA with Dunnett’s multiple comparison test.
We next examined whether activation of GABAA receptors is required for ALLO-induced scratching, because ALLO is a potent, positive allosteric modulator of GABAA receptors14,15. When the GABAA receptor antagonist picrotoxin was administered i.p. 5 min after i.p. administration of ALLO, the scratching response was significantly suppressed depending on the dose of picrotoxin (Fig. 4b). This result supports the hypothesized role of GABAA receptors in ALLO-induced scratching, but we postulated the involvement of other mechanisms because we previously found that the selective GABAA receptor agonist muscimol failed to induce scratching in the same model10.
Similar to ethanol and barbiturates, ALLO inhibits the L-VDCC16, and our previous10 and unpublished results showed that both ethanol- and barbiturate-induced scratching in atopic dermatitis mice were significantly suppressed by co-administration of the L-VDCC agonist BAY K 8644 (0.3 and 1 mg/kg, i.p.). Thus, we evaluated the effect of the same dose of BAY K 8644 on ALLO-induced scratching, and found that BAY K 8644 tended to suppress ALLO-induced scratching, but the effect was not statistically significant (Fig. 4c).
Although there is no convincing evidence that ALLO acts on glutamate receptors, we previously found that an i.ci. injection of NMDA (4 nmol/site) significantly blocked ethanol-induced scratching in atopic dermatitis in mice9. Accordingly, we examined the effects of NMDA at the same dose on ALLO-induced scratching, but it did not suppress scratching (Fig. 4d).
Wetzel et al.23 have demonstrated that ALLO acts as a functional antagonist of serotonin 5-hydroxytryptamine type 3 (5-HT3) receptors. Thus, we examined the effect of the 5-HT3 receptor agonist 2-methyl-5-HT on ALLO-induced scratching. When 2-methyl-5-HT was i.ci. injected 5 min after ALLO administration, scratching behaviour was almost completely suppressed (Fig. 4e). On the other hand, the i.ci. injection of 2-methyl-5-HT, even at the high dose (30 µg/site), did not significantly affect locomotor activity (data not shown). This observation suggested that suppression of ALLO-induced scratching by 2-methyl-5-HT was not a simple side-effect of the drug.
Concomitant increase in scratching behaviour and brain ALLO levels after acute ethanol administration
It has been shown that acute administration of ethanol (1.35–4 g/kg) rapidly increases brain ALLO levels in rodents17,18,19. Further, we previously showed that oral administration of ethanol (2.4 g/kg) markedly increased scratching in atopic dermatitis in mice9. Based on these previous findings and the present results, we hypothesized that ALLO endogenously produced in the brain contributes to the ethanol-induced scratching. To test this hypothesis, we investigated the time-course of scratching behaviour and brain ALLO levels after administering ethanol (2.4 g/kg, orally [p.o.]) to the atopic dermatitis mouse model. Consistent with previous results9, the scratching behaviour was apparently increased for 0–30 min after ethanol administration (Fig. 5a). The brain ALLO levels were also significantly elevated at 10 min and remained high for up to 30 min after ethanol administration, compared with the basal levels (Fig. 5b).
Time course of scratching and brain allopregnanolone (ALLO) levels following ethanol administration. (a,b) Ethanol (2.4 g/kg) was p.o. administered to atopic dermatitis-induced mice and the cumulative duration of scratching (a) and the brain ALLO levels (b) were measured at indicated time points. Each column represented means ± S.E.M. of nine or 11 (a) and six or seven (b) animals. *P < 0.05 and ***P < 0.001, vs. before, one-way ANOVA with Dunnett’s multiple comparison test.
Pre-treatment with finasteride suppresses ethanol-induced scratching and brain ALLO production
Finally, we investigated whether the ethanol-induced increase in brain ALLO levels contributes to scratching. ALLO has been shown to be synthesized from progesterone via 5α-reductase type 1, which is the rate-limiting enzyme in ALLO production11. Thus, we examined the effects of the 5α-reductase inhibitor finasteride on the scratching response during 1 h and the brain ALLO levels at 10 min after ethanol administration. As a result, pre-treatment with finasteride significantly suppressed ethanol-induced scratching and ALLO production in the brain (Fig. 6).
Suppression of ethanol-induced scratching and brain allopregnanolone (ALLO) production in atopic dermatitis-induced mice pre-treated with the 5α-reductase inhibitor, finasteride. (a,b) Vehicle (20% β-cyclodextrin-containing saline) or finasteride (FIN, 50 mg/kg, i.p.) was twice administered 24 h and 1 h before administration of ethanol (2.4 g/kg, p.o.). (a) Cumulative duration of scratching during 1 h period after ethanol administration. (b) Brain ALLO levels 10 min after ethanol administration. Each column represents mean ± S.E.M. of (a) 16 or 17 and (b) four or five animals. *P < 0.05 and **P < 0.01, vs. vehicle (Veh), unpaired Student’s t-test.
Discussion
This study examined whether endogenous neurosteroid ALLO is involved in pruritus in an atopic dermatitis mouse model. We found that systemic administration of ALLO markedly increased spontaneous scratching in mice with atopic dermatitis, while it did not affect spontaneous and pruritogen-induced scratching in normal mice. Local injection studies showed that ALLO acted at the supraspinal level to cause scratching. Thus, our data showed that the brain ALLO content may contribute to inducing scratching in atopic dermatitis. Although ALLO is the best characterized neurosteroid and has been extensively studied in various CNS disorders24, to the best of our knowledge, this is the first study to show the possible link between brain ALLO and itch-like behaviour.
Because animals cannot describe their sensations, it is difficult to conclude that ALLO-induced hindlimb scratching is really caused by increased itch sensation; however, numerous studies support the idea that hindlimb scratching in mice essentially reflects itch sensation25,26. µ-Opioid antagonists relieve various types of pruritus and, thus, are useful to evaluate whether animal scratching is an itch-related behaviour; however, they are not always effective in human and animal itch cases27,28,29. In our atopic dermatitis model, although neither the cumulative duration nor the frequency of spontaneous scratching bouts constantly increased, the duration of one scratching bout was reproducibly prolonged, and was suppressed by naloxone21. On the other hand, ethanol- and barbiturate-induced scratching were partially and not significantly suppressed, respectively by naltrexone at the same dose used in the present study9,10. We presently found that ALLO-induced scratching was insensitive to naltrexone treatment. Therefore, we assumed that the marked scratching response induced by ethanol, barbiturates, or ALLO was essentially different from common opioid-mediated itch responses. Rather, since similar to ethanol and barbiturates, ALLO exerts a hypnotic effect30,31, the unique scratching phenomenon might resemble the unconscious nocturnal pruritus frequently observed in patients with atopic dermatitis32,33,34.
ALLO has been shown to act as a potent GABAA receptor agonist15 and circulating ALLO reached brain tissues, which correlated with its CNS effects31,35. In this study, we found that ALLO-induced scratching was completely blocked by an i.p. administration of the GABAA receptor antagonist picrotoxin. It is widely accepted that systemically administered picrotoxin primarily acts on GABAA receptors in the brain. Furthermore, our preliminary experiment showed that i.ci. administered picrotoxin similarly suppressed i.p. ALLO-induced scratching in another but similar diet-induced atopic dermatitis mouse model (data not shown). Therefore, ALLO-induced scratching could be attributed to activation of brain GABAA receptors. We previously showed that other GABAergic drugs such as ethanol and barbiturates also markedly enhanced scratching in atopic dermatitis in mice, which was suppressed by GABAA receptor antagonists9,10. On the other hand, benzodiazepines only slightly enhanced scratching, whereas the selective GABAA receptor agonist muscimol decreased scratching10. The discrepancy between the effects of these GABAergic substances on scratching has two possible explanations. The first is the possible involvement of additional targets other than GABAA receptors. Although benzodiazepines and muscimol act exclusively on GABAA receptors, ethanol, barbiturates, and ALLO have many additional targets13,36,37. Indeed, our previous results suggest that inhibition of NMDA glutamate receptors and L-VDCC may contribute to ethanol-induced scratching in atopic dermatitis in mice9, and that inhibition of AMPA glutamate receptors and L-VDCC could be involved in barbiturate-induced scratching10. On the other hand, we showed that co-application of the 5-HT3 receptor agonist almost completely blocked ALLO-induced scratching, although either an L-VDCC agonist or NMDA had no significant effect. The sum of the inhibitory rates of ALLO-induced scratching induced by the GABAA receptor antagonist and 5-HT3 receptor agonist was much greater than 100%. Therefore, some synergistic interaction between GABAA receptor activation and 5-HT3 receptor inhibition in the brain may be required for ALLO-induced scratching. It should also be noted that the scratching responses caused by ethanol, barbiturates, and ALLO have distinctively different underlying mechanisms other than activation of GABAA receptors.
Second, GABAA receptor heterogeneity may also be involved in ALLO-induced scratching. There are various subtypes of GABAA receptors with distinct functions and pharmacology38, but they can be divided into two subtypes based on the presence of γ or δ subunits. The γ- and δ-containing receptors are mainly located synaptically and extrasynaptically, respectively, and thereby contribute to phasic and tonic GABA-mediated inhibitory transmission, respectively39. Although benzodiazepines specifically bind to γ-containing receptors, ALLO, ethanol, and barbiturates act on both receptor subtypes37,39,40,41. Importantly, although muscimol is generally regarded as a GABAA receptor pan-agonist, its in vivo effects are reported to be mediated preferentially through a small population of δ-containing receptors42,43. Considering these between-drug differences in the effects on GABAA receptors, activation of both GABAA receptor subtypes may also be necessary to fully induce scratching in atopic dermatitis mice. However, further studies using γ or δ subunit knockout mice are warranted to test this hypothesis.
The present study suggested that both GABAA receptor activation and 5-HT3 receptor inhibition contributed to ALLO-induced scratching. This conclusion is highly consistent with pharmacological studies on ALLO12, but seems inconsistent with reports on the role of these receptors in certain itch conditions. Several studies have shown that GABAA receptors in the spinal cord and central nucleus of the amygdala have an inhibitory role in itch signalling and the resultant scratching behaviour44,45,46. Moreover, activation of 5-HT3 receptors has been reported to contribute to certain types of pruritus47,48,49,50. However, this discrepancy could be explained by the following differences between these previous results and our present findings. (1) ALLO-induced scratching was not mediated through spinal GABAA receptors, although the effects on the receptors in the central nucleus of the amygdala could not be excluded. (2) In the previous studies, muscimol was used as an agonist for GABAA receptors to inhibit scratching responses45,46. On the other hand, ALLO caused scratching probably by pharmacological properties that differed from those of muscimol (as described above), whereas muscimol similarly reduced scratching in our model10. (3) Although 5-HT3 antagonists are highly effective against opioid-induced pruritus in humans47,48, ALLO-induced scratching is intrinsically independent on opioid receptors. (4) Activation of 5-HT3 receptors in the skin or spinal cord is considered to contribute to induction of scratching in pruritogen-induced acute and cholestatic itch models49,50. On the other hand, in our atopic dermatitis model, ALLO inhibited this receptor at the supraspinal level, and eventually likely contributed to the induction of scratching. (5) Inhibition of scratching by muscimol was observed in both acute and chronic itch conditions45,46, whereas ALLO-induced scratching was observed only in chronic dermatitis in mice. Therefore, considering these differences, it is reasonable that ALLO could induce scratching in certain chronic dermatitis conditions through an unknown mechanism.
In the present study, we found a concomitant increase in scratching behaviour and brain ALLO levels after acute ethanol administration, which was suppressed by pre-treatment with the ALLO synthetic inhibitor finasteride. Furthermore, our preliminary experiment showed that the increased levels of ALLO after ethanol administration were comparable to that after an i.p. administration of 10 mg/kg ALLO (data not shown). These results support our hypothesis that endogenously produced ALLO partly contributed to ethanol-induced scratching in the atopic dermatitis mouse model. It has been reported that the two enzymes responsible for ALLO synthesis (i.e., 5α-reductase type I and 3α-hydroxysteroid dehydrogenase) are colocalized in the cortical, hippocampal, and olfactory bulb glutamatergic principal neurons and in some output neurons of the amygdala and thalamus51. However, the brain region contributing to scratching induction remains to be determined and needs further investigation. Furthermore, a number of studies have shown that the expression levels of these enzymes and ALLO concentrations are altered under various physiological and disease conditions14. Therefore, it would be interesting to determine whether fluctuation of ALLO in some specific brain regions contributes to scratching behaviour in various physiological situations such as sleep, stress, and anxiety, which are all known to often exacerbate pruritus in pathological conditions6,52.
ALLO-induced enhancement of scratching was observed only in chronic atopic dermatitis mice, which is consistent with our previous findings using ethanol9 or barbiturates10. Therefore, such enhancement of scratching may be associated with highly chronic disease conditions. The present study showing that the same dose of ALLO induced scratching in atopic but not normal mice suggests that this difference is attributed likely to some differences of responsivity to ALLO (e.g., the expression or function of the related receptors, or the existence of unique nervous system under chronic disease conditions) only rather than the brain content of ALLO.
In conclusion, this study demonstrated that brain ALLO induced marked scratching in atopic dermatitis in mice, and suggested that ethanol-induced scratching is mediated partly through endogenously produced ALLO in the brain. Therefore, brain ALLO may be involved in pruritus in atopic dermatitis. However, as yet there is no direct evidence that endogenous brain ALLO contributes to naturally occurring itch, we need further studies using another chronic itch model that shows spontaneous scratching. In addition to ALLO, a variety of neurosteroids are present in the brain such as tetrahydro-deoxycorticosterone and pregnenolone sulphate, which exert similar and opposite actions to ALLO, respectively12. Further investigation of the roles of brain neurosteroids in pruritus may enhance the understanding of supraspinal mechanisms of chronic itch and could lead to the development of novel therapeutic approaches to intractable pruritus in chronic diseases including atopic dermatitis.
Methods
Animals
Four-week-old, female hairless mice (Hos: HR-1) were purchased from Hoshino Laboratory Animals (Saitama, Japan) and were maintained in plastic cages with free access to food and water and housed at 22 ± 1 °C on a 12-h light/dark cycle. All animal studies were approved by the Ethics Committee of Animal Research of Kyoto Pharmaceutical University (Approval numbers: 16–12–054 and 17–035), and were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006).
Diet-induced atopic dermatitis model
As reported previously7, hairless mice were fed a special diet (HR-AD diet; Norsan, Yokohama, Japan) for 8–12 weeks to fully induce atopic dermatitis-like symptoms and then they were used within 1 week. The disease signs were confirmed by gross observation, measurement of skin hydration, and transepidermal water loss using a Corneometer® CM825 and Tewameter® TM210 (both from Courage + Khazaka Electronic, Cologne, Germany), respectively. Mice fed a standard laboratory diet (MF; Oriental Yeast Industry, Tokyo, Japan) were used as the negative control.
Drug preparation
The reagents were prepared as follows: ALLO (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in a mixture of castor oil (Cremophor® EL; Sigma-Aldrich) and physiological saline (1:4, v/v). Chloroquine diphosphate, histamine dihydrochloride, naloxone hydrochloride, picrotoxin, NMDA (all from Sigma-Aldrich), and 2-methyl-5-HT (both from Wako Pure Chemical Industries, Osaka, Japan) were dissolved in physiological saline. (−)-BAY K 8644 (Sigma-Aldrich) was suspended in physiological saline containing 0.5% (v/v) Tween 80. Ethanol (Wako Pure Chemical Industries) was diluted in purified water. Finasteride (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in 20% (w/v) β-cyclodextrin-containing physiological saline. Each drug was prepared immediately before use.
Drug administration
The i.ci. injection was performed in conscious animals, as described by Ueda et al.53 with slight modifications. Briefly, the head of each mouse was gently bent and a J-shaped needle (the 27-gauge stainless needle was curved 40 degrees at 3.5 mm from the tip) was inserted into the cleft between the occiput (Fig. 3a). Then, 10 µL aqueous solution was gradually injected into the cisternal magna. Using 1% Evans blue solution, we confirmed that the solution was primarily distributed in the area surrounding the cisternal magna and the ventral surface of the brain stem (Fig. 3b). The i.t. injection was performed under brief isoflurane (2%) anaesthesia, induced with 10 µL injected via lumbar puncture according to a modified method54. For the i.d. injection, 10 µL of the solution was injected into the nape of the neck using a 29-gauge butterfly needle, while p.o. administration was performed using a gavage needle (#5202; Fuchigami Kikai, Kyoto, Japan).
Analysis of scratching behaviour
Hindlimb scratching in mice is thought to be associated with itch sensation25,26. In the present study, all hindlimb scratching behaviours were analysed regardless of scratching sites, except for the i.d. injection, and only scratching directed to the area around the injection site was analysed.
Prior to observing scratching, the mice were acclimatized for a minimum of 10 min in an observation chamber. Immediately after drug administration, the scratching behaviour was recorded and then analysed by playing back the videotape as reported previously7. Briefly, the cumulative duration of scratching was determined by instructing the observer to touch the switch for the duration of the scratching behaviour, using an in-house counter. The time detectable by this instrument was 0.1 s. Exceptionally, pruritogen (histamine or chloroquine)-induced scratching response was evaluated by measuring the number of scratching bouts on the injected site.
Determination of brain ALLO levels
Immediately after euthanasia by cervical dislocation, whole brain samples were collected and stored at −80 °C until extraction. The obtained brain samples were homogenized in 10 volumes of ethyl acetate, the supernatants were evaporated under nitrogen stream, and then stored at −80 °C until the analysis. The amount of ALLO was determined using a commercially available enzyme-linked immune assay kit (ELISA, DirectX® Allopregnanolone immunoassay kit; Arbor Assays, Ann Arbor, MI, USA) according to the manufacture’s instructions. The data were normalized to the wet tissue weight.
Experimental procedures
In the experiment examining the effect of systemic administration of ALLO on spontaneous scratching, ALLO (5 and 10 mg/kg) was administered i.p. to normal mice and the atopic dermatitis model mice and then the scratching behaviour was recorded for 1 h. Histamine (250 µg/50 µL/site) or chloroquine (200 µg/50 µL/site) was i.d. injected 10 min after administration of ALLO (10 mg/kg, i.p.) and then scratching behaviour was recorded for 1 h. In the experiment examining the effects of local injection of ALLO on scratching, low doses of ALLO (2.5 and 5 µg/10 µL/site) were injected i.ci., i.t., or i.d. into atopic dermatitis-induced mice, and from immediately (for i.ci. and i.d.) or 5 min (for i.t. to recover from isoflurane anesthesia) after injection, the scratching behaviour was recorded for 1 h. For the co-administration with ALLO (10 mg/kg, i.p.) in atopic dermatitis-induced mice, the dose, route, and timing of administration were as follows. Naltrexone (10 mg/kg) was i.p. administered 30 min before ALLO administration. Picrotoxin (2.5 and 5 mg/kg) or BAY K 8644 (0.3 and 1 mg/kg) was i.p. administered 5 min after ALLO administration. NMDA (4 nmol/site) or 2-methyl-5-HT (10 and 30 µg/site) was i.ci. injected 5 min after ALLO administration, because these drugs cannot possibly pass through the blood-brain barrier. In these experiments, scratching behaviour was recorded for 1 h after administration of the latter drug. For determining the time course of scratching and the brain ALLO levels after ethanol administration, ethanol (2.4 g/kg) was p.o. administered to atopic dermatitis mice, and then the scratching behaviour was recorded for 1 h. Furthermore, the brain samples of another group of mice were collected at 10 and 30 min after ethanol administration. In the experiment examining the effects of finasteride on ethanol-induced scratching and brain ALLO production in atopic dermatitis mice, finasteride (50 mg/kg) was i.p. administered twice at 24 and 1 h before ethanol administration (2.4 g/kg, p.o.). Following the ethanol administration, scratching behaviour was recorded for 1 h and in another sets of mice the brain samples were collected at 10 min after ethanol administration. The control group received the vehicle of each drug. All selected doses were based on the results of our previous9,10, preliminary in vivo experiments, and those of in vitro and in vivo experiments reported by others16,23,55; the doses of ALLO by systemic (5 and 10 mg/kg, i.p.) or local (2.5 and 5 µg/site, i.ci.) administration have shown to produce antiepileptic or sedative/hypnotic effects in mice and rats56,57,58.
Statistical analysis
Data were analysed using the GraphPad Prism (version 7.0; GraphPad Software, San Diego, CA, USA) and are presented as the means ± standard error of the mean (S.E.M.). Statistical differences were determined using unpaired Student’s t-test, a one-way analysis of variance (ANOVA) with Dunnet’s multiple comparison test or Bonferroni’s multiple comparison test, or two-way ANOVA with Bonferroni’s multiple comparison test. Differences were considered significant at P < 0.05.
References
Kido-Nakahara, M., Furue, M., Ulzii, D. & Nakahara, T. Itch in AtopicDermatitis. Immunol. Allergy Clin. North Am. 37, 113–122 (2017).
Nakashima, C., Otsuka, A. & Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp. Dermatol. 27, 327–331 (2018).
Rerknimitr, P., Otsuka, A., Nakashima, C. & Kabashima, K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 37, 14, https://doi.org/10.1186/s41232-017-0044-7 (2017).
Tsuda, M. Astrocytes in the spinal dorsal horn and chronic itch. Neurosci. Res. 126, 9–14 (2018).
Werfel, T. & Biedermann, T. Current novel approaches in systemic therapy of atopic dermatitis: specific inhibition of cutaneous Th2 polarized inflammation and itch. Curr. Opin. Allergy Clin. Immunol. 15, 446–52 (2015).
Ständer, S. & Steinhoff, M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp. Dermatol. 11, 12–24 (2002).
Fujii, M. et al. Atopic dermatitis-like pruritic skin inflammation caused by feeding a special diet to HR-1 hairless mice. Exp. Dermatol. 14, 460–468 (2005).
Fujii, M. et al. Dietary deficiencies of unsaturated fatty acids and starch cause atopic dermatitis-like pruritus in hairless mice. Exp. Dermatol. 24, 108–113 (2015).
Fujii, M. et al. Ethanol aggravates itch-related scratching in hairless mice developing atopic dermatitis. Eur. J. Pharmacol. 611, 92–99 (2009).
Fujii, M. et al. Barbiturates enhance itch-associated scratching in atopic dermatitis mice: a possible clue to understanding nocturnal pruritus in atopic dermatitis. Eur. J. Pharmacol. 836, 57–66 (2018).
Baulieu, E. E. Neurosteroids: a new function in the brain. Biol. Cell. 71, 3–10 (1991).
Dubrovsky, B. O. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 29, 169–192 (2005).
Rupprecht, R. & Holsboer, F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 22, 410–416 (1999).
Belelli, D. & Lambert, J. J. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 6, 565–575 (2005).
Majewska, M. D., Harrison, N. L., Schwartz, R. D., Barker, J. L. & Paul, S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 232, 1004–1007 (1986).
Earl, D. E. & Tietz, E. I. Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors. J. Pharmacol. Exp. Ther. 337, 301–311 (2011).
Gabriel, K. I., Cunningham, C. L. & Finn, D. A. Allopregnanolone does not influence ethanol-induced conditioned place preference in DBA/2J mice. Psychopharmacology (Berl). 176, 50–56 (2004).
Morrow, A. L. et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin. Exp. Res. 23, 1933–1940 (1999).
VanDoren, M. J. et al. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 20, 1982–1989 (2000).
Bernstein, J. E., Swift, R. M., Soltani, K. & Lorincz, A. L. Antipruritic effect of an opiate antagonist, naloxone hydrochloride. J. Invest. Dermatol. 78, 82–83 (1982).
Fujii, M., Nabe, T., Tomozawa, J. & Kohno, S. Involvement of skin barrier dysfunction in itch-related scratching in special diet-fed hairless mice. Eur. J. Pharmacol. 530, 152–156 (2006).
Yamaguchi, T. et al. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J. Dermatol. Sci. 25, 20–28 (2001).
Wetzel, C. H. et al. Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor. Mol. Endocrinol. 12, 1441–1451 (1998).
Reddy, D. S. & Estes, W. A. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 37, 543–561 (2016).
Carstens, E. & Kuraishi, Y. Animal models of itch: scratching away at the problem. Yosipovitch G., Greaves M. W., Fleischer A. B., McGlone F., Monticello (Eds), Itch: Basic Mechanisms and Therapy, Marcel Dekker, NY. 35–50 (2004).
LaMotte, R. H., Shimada, S. G. & Sikand, P. Mouse models of acute, chemical itch and pain in humans. Exp. Dermatol. 20, 778–782 (2011).
Kasutani, K. et al. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br. J. Pharmacol. 171, 5049–5058 (2014).
Pauli-Magnus, C. et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J. Am. Soc. Nephrol. 11, 514–519 (2000).
Siemens, W. et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database Syst. Rev. 11, CD008320 (2016).
Damianisch, K., Rupprecht, R. & Lancel, M. The influence of subchronic administration of the neurosteroid allopregnanolone on sleep in the rat. Neuropsychopharmacology. 25, 576–584 (2001).
Lancel, M. et al. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J. Pharmacol. Exp. Ther. 282, 1213–1218 (1997).
Ebata, T., Aizawa, H., Kamide, R. & Niimura, M. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br. J. Dermatol. 141, 82–86 (1999).
Fujita, H., Nagashima, M., Takeshita, Y. & Aihara, M. Correlation between nocturnal scratch behavior assessed by actigraphy and subjective/objective parameters in patients with atopic dermatitis. Eur. J. Dermatol. 24, 120–122 (2014).
Yosipovitch, G. et al. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int. J. Dermatol. 41, 212–216 (2002).
Zhu, D., Wang, M. D., Bäckström, T. & Wahlström, G. Evaluation and comparison of the pharmacokinetic and pharmacodynamic properties of allopregnanolone and pregnanolone at induction of anaesthesia in the male rat. Br. J. Anaesth. 86, 403–412 (2001).
Davies, M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci. 28, 263–274 (2003).
Löscher, W. & Rogawski, M. A. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 53, 12–25 (2012).
Olsen, R. W. & Sieghart, W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 56, 141–148 (2009).
Winsky-Sommerer, R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 29, 1779–1794 (2009).
Santhakumar, V., Wallner, M. & Otis, T. S. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 41, 211–221 (2007).
Saxena, N. C. & Macdonald, R. L. Assembly of GABAA receptor subunits: role of the delta subunit. J. Neurosci. 14, 7077–7086 (1994).
Chandra, D. et al. Prototypic GABA(A) receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology. 35, 999–1007 (2010).
Korpi, E. R. et al. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience. 109, 733–743 (2002).
Akiyama, T., Iodi Carstens, M. & Carstens, E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 6, e22665 (2011).
Cevikbas, F. et al. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 140, 454–464 (2017).
Chen, L., Wang, W., Tan, T., Han, H. & Dong, Z. GABA(A) Receptors in the Central Nucleus of the Amygdala Are Involved in Pain- and Itch-Related Responses. J. Pain. 17, 181–189 (2016).
Bonnet, M. P., Marret, E., Josserand, J. & Mercier, F. J. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: a quantitative systematic review. Br. J. Anaesth. 101, 311–319 (2008).
Kyriakides, K., Hussain, S. K. & Hobbs, G. J. Management of opioid-induced pruritus: a role for 5-HT3 antagonists? Br. J. Anaesth. 82, 439–441 (1999).
Ostadhadi, S., Kordjazy, N., Haj-Mirzaian, A., Mansouri, P. & Dehpour, A. R. 5-HT3 receptors antagonists reduce serotonin-induced scratching in mice. Fundam. Clin. Pharmacol. 29, 310–315 (2015).
Tian, B. et al. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci. Rep. 6, 36286 (2016).
Agís-Balboa, R. C. et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 103, 14602–14607 (2006).
Sanders, K. M. & Akiyama, T. The vicious cycle of itch and anxiety. Neurosci. Biobehav. Rev. 87, 17–26 (2018).
Ueda, H., Amano, H., Shiomi, H. & Takagi, H. Comparison of the analgesic effects of various opioid peptides by a newly devised intracisternal injection technique in conscious mice. Eur. J. Pharmacol. 56, 265–268 (1979).
Hylden, J. L. & Wilcox, G. L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 67, 313–316 (1980).
Kaufman, K. R., Tanchuck, M. A., Strong, M. N. & Finn, D. A. Replacement with GABAergic steroid precursors restores the acute ethanol withdrawal profile in adrenalectomy/gonadectomy mice. Neuroscience. 166, 5–14 (2010).
Belelli, D., Bolger, M. B. & Gee, K. W. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur. J. Pharmacol. 166, 325–329 (1989).
Bitran, D., Hilvers, R. J. & Kellogg, C. K. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 561, 157–161 (1991).
Singh, S. et al. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin-induced seizure model in mice. Pharmacol. Biochem. Behav. 94, 416–422 (2010).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP25870894 and JP16K09000 (both to M.F.) and the Kyoto Pharmaceutical University Fund for the Promotion of Scientific Research Grant (Grant Numbers 12-05 and 15-04, both to M.F.). We thank Miyako Mirin, Hoshi Watanabe, Takako Kamiya, Kyoko Fujii, Masahiro Adachi, Seita Nobuta, Ryosuke Miyagawa, and Kazunori Yoshikawa (Kyoto Pharmaceutical University) for experimental support.
Author information
Authors and Affiliations
Contributions
M.F. designed and conducted the research study, analysed the data and wrote the manuscript. S.O., E.A., T.N. and T.T., performed the research and also analysed the data. T.N. and S.O. contributed data interpretation and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujii, M., Ohgami, S., Asano, E. et al. Brain allopregnanolone induces marked scratching behaviour in diet-induced atopic dermatitis mouse model. Sci Rep 9, 2364 (2019). https://doi.org/10.1038/s41598-019-38858-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38858-3
This article is cited by
-
Anti-apoptotic effect of menaquinone-7 protects the brain of ovariectomized rats
Beni-Suef University Journal of Basic and Applied Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.