Abstract
Western hemisphere goats have European, African and Central Asian origins, and some local or rare breeds are reported to be adapted to their environments and economically important. By-in-large these genetic resources have not been quantified. Using 50 K SNP genotypes of 244 animals from 12 goat populations in United States, Costa Rica, Brazil and Argentina, we evaluated the genetic diversity, population structure and selective sweeps documenting goat migration to the “New World”. Our findings suggest the concept of breed, particularly among “locally adapted” breeds, is not a meaningful way to characterize goat populations. The USA Spanish goats were found to be an important genetic reservoir, sharing genomic composition with the wild ancestor and with specialized breeds (e.g. Angora, Lamancha and Saanen). Results suggest goats in the Americas have substantial genetic diversity to use in selection and promote environmental adaptation or product driven specialization. These findings highlight the importance of maintaining goat conservation programs and suggest an awaiting reservoir of genetic diversity for breeding and research while simultaneously discarding concerns about breed designations.
Similar content being viewed by others
Introduction
Unlike other livestock species, goats are unique in terms of their function and environments where they are utilized. Their body size, levels of production, dietary preferences, and low cost of investment make them a pliable species for livestock producers to use1,2. Globally, goats tend to be raised in low input production systems and generally lack high levels of artificial selection, suggesting their genetic composition may be less structured than other species3.
Goat domestication occurred in the Fertile Cresent4 from 9,900 to 10,500 YBP. The Bezoar ibex (Capra aegagrus) is thought to be the only living wild progenitor of the goat5. Upon domestication, goats accompanied human migration and trade, thereby developing subpopulations and breeds differentiated by various selection factors and genetic drift6.
During the colonization of the western hemisphere, settlers brought goats potentially from the Iberian Peninsula and west Africa7. These populations have become well adapted to low input agricultural environments typically found in northeastern Brazil, west Texas, and southern Argentina (Patagonia)8,9, creating locally adapted breeds. Further, multiple waves of importation to the western hemisphere have occurred and included product-specialized breeds, such as dairy (e.g., Saanen), fiber (Angora) and meat (Boer). However, western hemisphere breeding lags behind other livestock species, in part due to their low economic return10.
In general, local goat breeds may be largely panmictic, due to multiple importation waves, unsupervised crossbreeding and the lack of strong artificial selection. In this work, we used genotypic data (50 K SNP) from 12 goat breeds found in the Americas, augmented by genotypes from South Africa, Iran, Morocco and Bezoar ibex (Table 1) to: characterize western hemisphere goat diversity, understand genetic structure, and identify genomic regions under selection in these animals.
Results
Genetic diversity and admixture
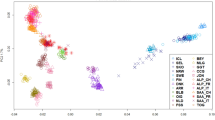
Biological function of the tested populations appeared to be responsible for the observed differences in the principal components analysis (PCA – Fig. 1 and Supplementary Fig. S1). The eigenvalues (Supplementary Fig. S2) showed five as a reasonable number of components to be evaluated (explain 76.8% of the variation). Five distinct groups were identified: meat (Boer), Brazilian (Moxoto and Caninde), dairy (Saanen and LaMancha), fiber (Angora) and the remaining populations in a neutral clustering position. Angora populations showed a dispersed pattern where; the admixed Argentinean (AR) population was placed closer to the graph’s origin, while South African (SA) Angora with higher inbreeding levels were the most distant from the origin, and USA Angora were in an intermediate position.
Bezoar ibex had the highest inbreeding coefficient (0.36). Brazilian breeds had the highest number of monomorphic SNPs and inbreeding coefficients (12%, 0.24 and 6%, 0.22 for Caninde and Moxoto, respectively) from the New World samples. Compared to Angora_USA, Angora_SA had a higher inbreeding coefficient (0.14 vs 0.23, respectively). The Spanish breed had the lowest inbreeding coefficient (0.01), while Saanen_CR and C. Neuquino_AR also had low inbreeding levels (Table 1).
Cross validation error of ADMIXTURE11 indicated K = 9 as the optimal number of populations (Supplementary Fig. S3). Seven populations were substantially admixed at K = 9 (Fig. 2). High proportions of the Angora_USA cluster (81.6% of assignment to this cluster) were found in C. aegagrus (48.1%), Iran (42.2%), Spanish_USA (26.2%), Morocco (12.1%) and all Argentinean breeds (11.6%). The dairy breed cluster (Lamancha_USA, 92.3%; and Saanen_CR, 80.5%) was observed in Spanish_USA (31.0%) and C. aegagrus (30.4%). The cluster that represented 80.2% of genomic composition of C. Llanos_AR was observed in Spanish_USA (11.0%) and other nondescript Argentinean breeds (Table 1). The Moroccan goat cluster (77.4%) was observed in some Argentinean breeds (Formosena – 39.7%, Riojano – 26.6%, Neuquino – 21.3% and Pampeana – 15.3%), as well as in Spanish_USA (16.2%) and Iran (12.3%). As these result suggest, Spanish_USA was found to be highly admixed and when K was increased (10 to 16) no specific cluster for Spanish_USA was identified (Supplementary Fig. S4). Local breeds from Argentina were grouped together or shared the same clusters in the PCA and ADMIXTURE results, with the exception of C. Llanos (Supplementary Fig. S4).
Population trees were constructed using Treemix software12 by running simulations of 0 to 20 migration events (applying three replications per migration event). Likelihood estimates indicated 6 to 9 migration events best fit the model (Supplementary Figs S5 and S6). According to the residual values of the model for six migration events (Supplementary Fig. S7), the relationship between only few pairs of populations (C. Formosena with Morocco; Lamancha with Spanish breed; Saanen_CR with C. Formosena; C. Formosena and C. Riojano with Brazilian breeds) are not well explained. Therefore, the tree with six migrations was chosen as the reference for analysis of the ancestral relationships of these goat populations (Fig. 3).
Selection signatures and gene annotation
Selection sweeps were conducted using the main groups (Boer/Meat, Dairy, Brazilian, Argentinean, Spanish and Angora) identified by the genetic structure analyses (PCA and ADMIXTURE). Pairwise Fst suggested high differentiation of Brazilian breeds, Angora_SA and Boer_USA (Supplementary Fig. S8). Spanish, Morocco and C. Neuquino_AR had low levels of genetic differentiation in relation to all the other populations. Fst analyses per marker were performed with various paired comparisons as shown in Supplementary Table S1. These comparisons showed various selection sweeps (Supplementary Tables S2 and S3).
Using only the South Africa and USA Angora populations, significant loci (above three standard deviations) on chromosomes 6, 7, 13, 18 and 25 (Fig. 4) were identified. Significant regions also were seen for dairy, meat, Argentinean and Spanish populations (Supplementary Figs S9–S12). The Brazilian breeds did not show any significant regions (Supplementary Fig. S13). An additional Fst comparison among specialized breed groups (Fiber, Meat and Milk) versus local breeds (Argentinean, Spanish and Brazilian) did not show any significant regions (Supplementary Fig. S14).
Smoothed Fst per SNP for comparison between Angora and Meat (Boer) and Milk (Saanen and LaMancha) specialized breeds and between Angora and all others goat breeds in the analyses. Red line: significant threshold of three standard deviations above the mean. Blue line: threshold of two standard deviations above the mean.
Selection signatures were also identified using a haplotype based approach (hapFLK)13. HapFLK analyses used the same breed groupings except that Bezoar ibex was used as the root. Five regions with reduced haplotype diversity were identified and considered selection signatures for these groups (Table 2).
HapFLK’s detection power significantly decreases when populations are too genetically distant from each other13. To improve the haplotype estimation process, five different hapFLK runs were performed (Fig. 5) grouping the samples by the populations in Table 1. Thereby seeking to overcome bias generated by genetic distance between the populations. By taking this approach, we observed sixteen regions with reduced haplotype diversity (Supplementary Table S4).
Genome scan for selection in five different scenarios of 16 populations of goat using a haplotype-based (hapFLK) test. 6 groups: Boer/Meat, Argentinean, Brazilian, Milk, Spanish and Angora breeds. 16 populations: Angora_AR, Angora_SA, Angora_USA, Iran, Boer_USA, Argentinean populations, Caninde_BR, Moxoto_BR, LaMancha_USA, Morocco, Saanen_CR, Spanish_USA. 12 populations: 16 populations minus Angora and Iran populations. Angora populations: Angora animals from South Africa, United States and Argentina. Argentinean populations: C. Formosena_AR, C. Llanos_ AR, C. Neuquino_AR, C. Pampeana_AR, C. Riojano_AR.
For each significant region detected in hapFLK analyses, population tree and haplotype clusters were plotted to identify the populations or group that had selection pressure in each region. A region on chromosome 3 (Fig. 6), for example, indicated a selection signal in Boer (Meat group). Three hapFLK runs (Groups, 16 populations and Angora, as described in Table 1) suggested a strong selection sweep signal in Angora_SA on chromosome 23 (Fig. 7).
Group trees (at left) generated using all available SNPs and only 351 SNPs surrounding the hapFLK peak in chromosome 3 analyzing the six groups. Haplotype clusters frequencies (at right) in the region of chromosome 3 for each group used in the test. AR: Argentinean breeds; BR: Brazilian breeds. This peak are used as example here, the others significant regions are showed in supplementary files.
A region on chromosome 6 observed in two hapFLK analyses (12 and 16 populations) highlighted a selection sweep signal in Caninde_BR and Moxoto_BR (Supplementary Figs S19, S23 and S28). C. Neuquino_AR also displays a soft selection sweep in this region, which was later confirmed when running hapFLK with only Spanish and Argentinean local breeds (Supplementary Fig. S36).
All other selected regions were evaluated and the selected populations in each region were determined (Supplementary Figs S15 to S37). The spectral decomposition of the signal in each region13 was also evaluated (Supplementary Fig. S38). Significant regions and gene annotation for each selected region are presented in Table 2 and Supplementary Tables S2 to S4. Several genes observed in the selected regions have been associated with various traits in other livestock species (pig, cattle or sheep) (Supplementary Table S5). Gene ontology of the significant regions for group comparison are shown in supplementary information (Supplementary Tables S7 and S8).
Discussion
A plausible path from the center of domestication and migration to the western hemisphere14 is presented in Fig. 3. Post-domestication dispersal of goats to the west was characterized by migrations routes through Europe (Danubian and Mediterranean corridors), and North Africa (by Sinai Penisula or Mediterranena sea) and later a south migration via eastern Africa14. The early partition of Boer and Angora supported the southern distribution via eastern Africa for Boer formation in South Africa and the Angora development in Turkey. The remaining breeds formed a branch of European-ancestry. The Spanish breed was placed closely to the beginning of this branch and had a drift parameter indicating little change from the populations found near the center of domestication, suggesting the genetic diversity conserved in this population. At a later point, Treemix suggested this branch segregated into dairy and South American breeds.
South American breeds diverged in two branches (Argentinean and Brazilian), suggesting the progenitors were from both Spanish and Portuguese populations which were pivotal sources of genetics exported to the western hemisphere7. The close association of the Moroccan and Brazilian populations and migration events (Fig. 3) are consistent with information concerning trade flows among the Iberian Peninsula, North Africa, and the Canary and Cape Verde Islands15,16.
A weak genetic structure was observed (Fig. 1) for Spanish, Argentinean local breeds, Moroccan and Iranian populations which were closely placed in PCA and share genomic clusters, suggesting genetic drift and selection have not separated western hemisphere populations from old world progenitor groups. This finding differs markedly from other livestock species17,18,19,20.
Previous studies also showed a weak structuring of goat breeds7,8,21,22,23. Carvalho et al.23 reported the concept of breed for meat goats might not be relevant for goat production, reinforcing our perspective that many so called breeds are actually landraces at best and panmixia predominates in these genetic reservoirs. In addition, Lenstra et al.10 suggested pure genetic ancestry was not a prerequisite for goat breeds. The Spanish goat raised predominately in the southern USA seems to typify such an assessment24,25. Our results demonstrate how this population shared genomic components (>10%) with dairy breeds (Saanen and Lamancha), Argentinean, Morocco, Iran and C. aegagrus. It is known that no gene flow has occurred recently between the populations mentioned due to geographic distances, except to Lamancha. Therefore, this genomic sharing represents old (400–500 years ago) admixture events that remain conserved in the populations. Given these levels of admixture with old world populations suggests the Spanish breed is a genetic diversity reservoir in the western hemisphere.
Angora are unique in the sense that they are the only population in the western hemisphere originating from near the center of domestication. Their history is important, as South Africa and the USA have highly structured mohair industries, which has served to facilitate selection programs for fiber improvement and resulted in the two countries leading global mohair production. Angora in South Africa had higher levels of inbreeding, reflective of their national policy of not importing genetic resources26. Conversely the USA had imported South African Angora in the 1980’s which likely decreased inbreeding and is evident in the clustering analysis (Figs 1 and 2).
The Argentinean Angora were developed using imports of USA Angora26, thereby explaining their placement in the PCA (Fig. 1), ADMIXTURE clusters (Fig. 2) and Treemix (Fig. 3). In general, there are always strong migrations events between Angora breeds and C. Neuquino_AR to Angora_AR (as indicated by Treemix, Supplementary Fig. S6). Argentinean mohair production is located in northern Patagonia27 the same region where C. Neuquino are raised suggesting gene flow between the breeds as the Angora population was developed.
Brazilian goats were distinct based upon the number of monomorphic SNP, high inbreeding coefficients, mean Fst and pairwise Fst with all others populations. McManus et al.28 showed that Caninde and approximately 70% of Moxoto herds were concentrated in a specific region within a radius of 500 km from the breed’s geographic midpoint. Our results suggest these breeds had high genetic drift and founder effect coupled with inbreeding, which led to a relatively small population size, agreeing with the geographical distribution. In addition, most of the animals sampled are from two Conservation Nucleus where the acquisition of new animals is restricted.
Generally, genetic diversity measures suggested weak population structures, but this does not imply selection is totally absent from the breeds evaluated. Various genes within selected regions have functional roles that were notable in differentiating the populations (Table 2 and Supplementary Tables S5,S7 and S8).
Five significantly selected regions were observed in Moxoto and Caninde, the main breeds raised in Northeast Brazil noted for high temperatures and low humidity28. One selected region in chromosome 6 (32.5–37 Mb) were previously observed as a selection signature for hot and arid environments29. Another region in chromosome 6 (86.6–94.9 Mb) harbors two genes (PPEF2 and SHROOM3) previously associated with platelet distribution width, mean corpuscular volume and mean corpuscular hemoglobin concentration in swine30, which is also related to heat tolerance and parasite resistance31.
Angora populations showed five genes within selected regions that were associated with body size, average daily gain, longissimus muscle area and carcass weight (CCSER1, CPEB2, NMUR2, SPARC, DNMT3B),32,33,34,35,36,37. Angora goats have been intensely selected for increased mohair production while compromising body weight and potentially lowering their adaptability to sub-optimum conditions38,39,40. South Africa and United States populations shared selected regions (chromosome 17 and 6), which was previously observed as a selection signature for arid environment29 and crimp in wool41. The region on chromosome 17 (FGF2, IL2, IL7 and IL21) has been associated with cytokine receptors and cell proliferation42 suggesting regions involved with mohair production and environmental adaptability. Therefore, this region can be simultaneously linked to the selection for mohair production and for harsh environments, or also can be a genetic hitchhiking based on the selection for one of the traits.
South Africa and United States population had two distinct selected regions. These could be linked to different environmental constraints or different genetic solutions that can arise to achieve similar phenotypic selection goals5.
Angora goats have been bred for mohair production in the United States since the introduction of these animals from Turkey in 184943. The heritability estimates for fleece weight are medium to high (range 0.22 to 0.45 for greasy fleece weight24,44,45,46). Selection for fiber production among Angora_USA during 60’s and 2000’s was substantial24,43. These animals are able to continue producing mohair fiber even during periods of feed shortage or nutrient restriction47. Therefore, we expected to find a higher number of strong selection signatures in this breed than in local breeds that did not undergo any artificial selection. However, the number of selection signatures found was not very different from other genetic groups or populations. This could be related to the high polygenic nature of the fleece traits, which did not leave strong selection signals in the genome48. Moreover, this reinforced the different picture of goat genetic structure in comparison to other livestock species.
Boer have the largest body size of the studied populations and had strong selection signals for traits associated with size and muscularity similar to cattle and sheep20,49. Three regions identified in Boer (CHI2: 119.2–119.8, CHI3: 24.1–37.8 and CHI7: 46.3–64.7 Mb) have genes related to meat traits also found in Australian and Canadian Boer50. Another region selected on Boer (CHI13) harbors the bone morphogenetic protein 2 (BMP2) gene, which plays a role in skeletogenesis, osteoblastic differentiation and limb patterning51.
Ear structure in goats is variable with implications for adaptation to heat stress. A selection signature in chromosome 7 was identified in two hapFLK analyses. Brito et al.50 associated this region with ear size selection on Lamancha animals (short ears). Here, Boer (long ears), Caninde_BR (average ears) and Lamancha showed selective sweep on this region. Interestingly, these three breeds have contrasting ear phenotypes of Brito et al.50, validating this region as related to ear morphogenesis.
The Fst and hapFLK analysis showed different selected regions probably due to the known differences in these approaches5. The Fst approach is more sensitive to bias by genetic drift in populations52. The hapFLK is only slightly affected by migration and is not affected by bottlenecks5. Depending on the time scale of selection, the causative SNP eventually became fixed while genetic drift gradually reduces the signal-to-noise ratio53, which compromises the Fst approach. The two Brazilian breeds (high inbred and drifted populations), for example, did not have any selection sweep identified by Fst, while the hapFLK analyses identified five regions under selection.
Our use of different population sets increase the power of the hapFLK to identify selection signatures, agreeing with Fariello et al.13 The Argentinean breeds, for example, did not have any selection signature in the runs with groups and 16 populations. In the run with 12 populations, a first soft signal for C. Neuquino was detected. Then, in the run with only Spanish and Argentinean local breeds, the region in chromosome 6 was confirmed as selected on C. Neuquino (Fig. S36) and two other new selected regions appeared.
The comparison of the genome of the wild ancestor Bezoar ibex (Capra aegagrus) with the domestic goat (Capra hircus) suggested that the population bottleneck associated with the domestication process was not as severe as for other domesticated species48. Goat domestication occurred multiple times, which provided a high diversity to the species54. Goat populations presented seven mitochondrial haplogroups until Neolithic era. Modern goat populations, otherwise, have predominant mitochondrial haplogroup (haplogroup A) in the world, which also confirms this history of gene flow across different geographical regions4,55,56. A study using wild and domestic goats and sheep showed that the average relatedness was 0.859 and 0.823 for sheep and Asiatic mouflon, respectively, while the average relatedness was around 0.915 between the domestic goats, and 0.916 between Bezoar ibex5. Therefore, goat populations are more related to each other than are sheep populations.
Alberto et al.5 observed that the number of positive selections in goats were almost half of what was observed in sheep and goats had several spots with higher diversity in domestic populations than in the wild. Bezoar ibex showed lower nucleotide diversity than Iranian goats and higher inbreeding than Iranian and Moroccan goats5. Genetic load was higher in domestic sheep than in mouflon, while in goats the genetic load was significantly higher for wild individuals5. These authors concluded that Capra and Ovis genus showed opposite global patterns of genomic diversity reinforcing our observation of high goat diversity.
Goats in the western hemisphere have maintained substantial genetic diversity with comparable levels found in the species domestication center. A substantial part of genetic variation seen in Iran and Moroccan populations, as well as in C. aegagrus, was observed in the American populations evaluated. A similar pattern was observed with sheep and microsatellite data57. Therefore, despite being brought to Americas around 400 years ago, a strong genetic linkage is still present.
Breeds, by definition, are closed populations with restricted gene flow, phenotypic uniformity and likely a higher inbreeding than expected from outbred populations15. While this general definition is applicable for some goat breeds, such as Angora, Saanen and Boer, the majority of goat populations are better described as landraces58 rather than breeds. Goats worldwide are generally restricted to small herds with substantial regional/local germplasm exchange between herds and nonuniform approaches to artificial selection strategies15; therefore minimizing genetic distinctions between such populations.
Several goat diversity studies highlighted the high levels of polymorphisms and concluded that goats contain more polymorphic sites than other livestock species3,8,9,22,23. Lower levels of long range linkage disequilibrium than sheep and cattle has been observed21, which also supports the contention that the goats have not been under intense selection. In general, goats are raised without a specific product goal and without a strong breeding control, which contributes to these observations.
Our results reinforce the concept that breed is not an important discrimination criteria in goat genetic diversity, especially for meat type/local goats commonly used throughout the western hemisphere. Genetic linkage among local breeds to centers of domestication was surprising and suggested little genetic differentiation has occurred due to genetic drift and selection. Western hemisphere local breeds are reservoir of genetic diversity awaiting for genetic improvement and research endeavors. As such, the importance of conservation efforts for these genetic resources should be addressed within countries. Our findings represent an important step to address future breeding, conservation and management policies for a specie that is particularly relevant for the sustainability of marginal livestock producing regions of the world.
Methods
Samples
We genotyped 244 animals with Illumina Goat 50 K SNP BeadChip (53,347 SNPs)59 plus 124 genotypes from previous studies. The dataset consisted of 12 breeds (specialized and local breeds) raised in the western hemisphere (Table 1). Populations sampled in the USA were: Spanish, Lamancha, Boer, and Angora; and were derived from National Animal Germplasm Program’s genetic resource collection. Two Brazilian and five Argentinean local breeds were sampled also from the germplasm conservation efforts of each country. Twenty-eight Saanen were sampled in Costa Rica. Seventy-eight animals from Angora populations (Argentinean and South Africa) were added to the dataset26.
Samples with call rate <0.90 were removed. SNP with call rate <0.90 or with MAF = 0.0 were removed and only autosomes SNPs were used. The final number of SNPs after quality control was 48,442 SNP.
In order to remove highly related animals within breeds, a genomic relationship matrix for each breed was calculated using SNP & Variation Suite v8.7 (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com). One animal of each pair with a genomic relationship higher than 0.25 was removed, reducing the dataset to 267 animals.
Raw data (50 K SNP chip) of NextGen consortium (http://projects.ensembl.org/nextgen/) consisting of 7 samples from Capra aegagrus, 9 samples from Iran population and 30 samples from Moroccan goat population were also used. The filtering criteria for this data were less stringent as the objective was to use them as the root and outgroup (reference population) in different analyses. The autosomes SNP were filtered based on SNP call rate (<0.8) and sample call rate (<0.9), yielding 49,051 SNPs. The two datasets were merged, resulting in a final dataset with 313 animals and 48,203 SNPs (Table 1).
No samples were collected for this study; rather they were collected as part of other programs not associated with this study. Therefore, an institutional animal care and use committee license specific for this study was not necessary. All methods were carried out in accordance with guidelines and regulations of each country.
Genetic diversity
Three analyses (principal components, ADMIXTURE and Treemix analyses) were applied to evaluate the genetic diversity in these goat populations. For these analyses, a stringent filtering criteria were applied to avoid bias related to linked markers. SNP with call rate lower than 0.95 and MAF < 0.05 were removed. Moreover, LD pruning was applied using a window size of 50 SNPs, an increment of 5 and r2 >0.5 (CHM method), resulting in 46,214 SNPs.
Principal component analysis (PCA) was carried out in SNP & Variation Suite v8.7 (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com) and the plotting of the first three components were performed in R 3.4.2 using the scatterplot3D package. The parameters for PCA analysis were set to find the first 10 components, normalizing each marker data by their actual standard deviation, using an additive model and outlier removal up to 5 times, which was considered as more than 6 standard deviations, from 5 components.
Genetic relationships among breeds and the level of admixture were evaluated through a model-based clustering algorithm implemented in the software ADMIXTURE v. 1.3.0. The cross-validation procedure (10-fold) was executed to estimate prediction errors for each K value (from 2 to 22). The value of K that minimizes the estimated prediction error represented the best predictive accuracy. Individual coefficients of membership to each K cluster produced by ADMIXTURE were visualized using the on-line CLUMPAK server with the feature DISTRUCT for many K’s.
The tree-based approach was used to reconstruct historical relationships between the analyzed populations and to test for the presence of gene flow using the TreeMix software12. The program was run on the dataset with animals classified in 17 populations, using Capra aegagrus as the root. A variable number of migration events (M) from 0 to 20 were tested and the log-likelihood was used to determine the most predictive model.
Selection signatures
The selective sweeps were identified by two different approaches, Fst and hapFLK statistics. The main groups identified in genetic structure analyses were used further in the selection signatures. The populations were grouped as: Fiber specialized (Angora populations from South Africa and USA), Meat specialized (Boer), Milk specialized (Saanen and LaMancha), Spanish breed, Brazilian local breeds (Moxoto and Caninde), Argentinean local breeds (Criolla Formosena, Criollo de los Llanos, Criollo Neuquino, Colorada Pampeana and Criollo Riojano). Angora_AR was not used due to their recent formation and crossbreeding observed in the previous analyses. Moroccan population was not used for selection signature detection as this was not an objective of this study. Capra aegagrus and Iran populations were used in selection signature detection as a reference group (outgroups).
The two tests used are able to detect different selection signature. The FST indicates a difference among groups of individuals in each marker that could be caused by different selection events. FST test detects highly differentiated alleles, where positive selection in a given genome region causes exaggerated frequency differences between populations50. The hapFLK is a haplotype FLK based test that identifies selection signatures among hierarchically structure populations13. It differs from Fst in order that takes into account the hierarchical structure of the sampling, allowing genetic drift to differ for each population5.
Each group had three Fst results from the comparison with: all other groups combined, all specialized breeds (milk, meat and fiber) and the meat and milk specialized breeds. For Brazilian breeds, since they were placed in different clusters in the ADMIXTURE results, we chose to run the Fst analysis for each breed separately as well. Fst comparison between breeds group and wild populations (C. aegagrus and Iran population) were also performed.
The Fst values were smoothed using the smoothing tool of SNP & Variation Suite v8.7 (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com) considering the mean asymmetric method. Smoothing process consider a moving average of a certain number of markers. This process is an approximate method when looking for regions where selection is apparent over multiple markers, rather than one-off high values50. The number of SNP to be included in the smoothing window in each comparison were determined based on the number of monomorphic SNP in each group and aiming a false discovery rate lower than 0.5 according to Ramey et al.60 (Supplementary Table S6). For each comparison, smoothed Fst values greater than the average plus three standard deviations were considered to be under selection.
The hapFLK analyses were performed with five population sets (Table 1). First, the six groups were used in order to have the same comparison pattern of the Fst analyses. Then, we moved to evaluation of the populations separately. As this method uses the haplotype estimation, the population groups could be causing some noise and bias in this process. One run used all populations (16), another only with Angora populations, other with the remaining 12 populations and the last one using just Argentinean local breeds and Spanish animals. The C. aegagrus was used as the outgroup in all runs. These different population sets were applied because, according to Fariello et al.13, little power is expected from analyses based on genetic differentiation if populations are too distant. Therefore, Angora populations were set apart since they are too distant and could be lowering the detection power of the analysis. Moreover, only Argentinean local breeds and Spanish was evaluated together to have these closely related populations in a specific run.
The hapFLK analysis involves first the generation of a genome wide Reynolds distance matrix to estimate the hierarchical population structure within each population set. To determine the number of haplotype clusters (K) to be used further, several runs of fastPHASE were performed to register the likelihoods. The point where the increase in number of clusters represent a small increase in log-likelihood was selected as the K to use in the hapFLK analysis. Then, the five hapFLK analyses (6 groups, 16 populations, Angora, 12 populations and Argentinean + Spanish) were run using K equal 70, 80, 35, 60 and 20, respectively. The hapFLK statistic was computed as the average across 20 expectation maximization (EM) runs to fit the LD model (–nfit = 20).
The hapFLK software was run chromosome by chromosome and the results merged to a single file. A python script (https://forge-dga.jouy.inra.fr/projects/hapflk) was used to estimate the hapFLK chi-squared density, standardize hapFLK values and calculate the corresponding p-values of hapFLK results. The plots were generated using the R packages ape and qqman. The significant regions (log p-value > 3) were identified and local population trees and haplotype clusters of each regions were plotted. The local population trees used only those SNPs located within the regions of signatures of selection identified to show the breeds undergoing selection.
Gene annotation
The regions identified in hapFLK methodology were applied in the Causal Variants Identification in Associated Regions (CAVIAR) software61. This statistical framework quantifies the probability of each variant to be causal while allowing an arbitrary number of causal variants. In this case, we allowed up to 10 associated variants in each region and selected only ones that showed p-value lower than 0.05. We used the eigen-decomposition based on the correlation matrix between SNPs selected for the analysis62.
The causal variants identified with CAVIAR and the significant SNP observed in Fst comparisons were used to identify genes in each region using the Genome Data Viewer in the NCBI platform (http://www.ncbi.nlm.nih.gov/). The genes were identified based on the Annotation Release 102 and ARS1 genome assembly.
The biological functions and pathways in which these genes are involved were assessed using PANTHER (http://www.pantherdb.org/) (Supplementary Tables S7 and S8). Thereafter, a search in the literature and in the Cattle, Pigs and Sheep QTL database (available online at http://www.animalgenome.org) was executed to identify phenotypes known to be affected by variation in the genes located in each significant genomic region.
Data Availability
The datasets generated and/or analyzed during the current study will be available in the Animal GRIN Repository from USDA (https://nrrc.ars.usda.gov/A-GRIN/).
References
Dubeuf, J.-P., Morand-Fehr, P. & Rubino, R. Situation, changes and future of goat industry around the world. Small Rumin. Res. 51, 165–173 (2004).
Dubeuf, J.-P. & Boyazoglu, J. An international panorama of goat selection and breeds. Livest. Sci. 120, 225–231 (2009).
Kijas, J. W. et al. Genetic diversity and investigation of polledness in divergent goat populations using 52 088 SNPs. Anim. Genet. 44, 325–335 (2013).
Naderi, S. et al. The goat domestication process inferred from large-scale mitochondrial DNA analysis of wild and domestic individuals. Proc. Natl. Acad. Sci. 105, 17659–17664 (2008).
Alberto, F. J. et al. Convergent genomic signatures of domestication in sheep and goats. Nat. Commun. 9, 813, https://doi.org/10.1038/s41467-018-03206-y (2018).
Tresset, A. & Vigne, J.-D. Last hunter-gatherers and first farmers of Europe. C. R. Biol. 334, 182–189 (2011).
Amills, M. et al. Mitochondrial DNA diversity and origins of South and central American goats. Anim. Genet. 40, 315–322 (2009).
Benjelloun, B. et al. Characterizing neutral genomic diversity and selection signatures in indigenous populations of Moroccan goats (Capra hircus) using WGSdata. Front. Genet. 6, 107, https://doi.org/10.3389/fgene.2015.00107 (2015).
Nicoloso, L. et al. Genetic diversity of Italian goat breeds assessed with a medium-density SNP chip. Genet. Sel. Evol. 47, 1–10 (2015).
Lenstra, J. A. et al. Microsatellite diversity of the Nordic type of goats in relation to breed conservation: how relevant is pure ancestry? J. Anim. Breed. Genet. 134, 78–84 (2017).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 19, 1655–1664 (2009).
Pickrell, J. K. & Pritchard, J. K. Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data. PLOS Genet. 8, e1002967, https://doi.org/10.1371/journal.pgen.1002967 (2012).
Fariello, M. I., Boitard, S., Naya, H., SanCristobal, M. & Servin, B. Detecting signatures of selection through haplotype differentiation among hierarchically structured populations. Genetics 193, 929–941 (2013).
Amills, M., Capote, J. & Tosser-Klopp, G. Goat domestication and breeding: A jigsaw of historical, biological and molecular data with missing pieces. Anim. Genet. 48(6), 631–644 (2017).
Manunza, A. et al. A genome-wide perspective about the diversity and demographic history of seven Spanish goat breeds. Genet. Sel. Evol. 48, 52, https://doi.org/10.1186/s12711-016-0229-6 (2016).
Pereira, F. et al. Tracing the History of Goat Pastoralism: New Clues from Mitochondrial and Y Chromosome DNA in North Africa. Mol. Biol. Evol. 26, 2765–2773 (2009).
Bovine HapMap Consortium. et al. Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science. 324, 528–32 (2009).
McTavish, E. J., Decker, J. E., Schnabel, R. D., Taylor, J. F. & Hillis, D. M. New World cattle show ancestry from multiple independent domestication events. Proc. Natl. Acad. Sci. USA 110, E1398–406 (2013).
Demirci, S. et al. Mitochondrial DNA Diversity of Modern, Ancient and Wild Sheep (Ovis gmelinii anatolica) from Turkey: New Insights on the Evolutionary History of Sheep. PLoS One 8, e81952, https://doi.org/10.1371/journal.pone.0081952 (2013).
Kijas, J. W. et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol 10, e1001258, https://doi.org/10.1371/journal.pbio.1001258 (2012).
Brito, L. F. et al. Characterization of linkage disequilibrium, consistency of gametic phase and admixture in Australian and Canadian goats. BMC Genet. 16, 67, https://doi.org/10.1186/s12863-015-0220-1 (2015).
Burren, A. et al. Genetic diversity analyses reveal first insights into breed-specific selection signatures within Swiss goat breeds. Anim. Genet. 47, 727–739 (2016).
Carvalho, G. M. C., Paiva, S. R., Araújo, A. M., Mariante, A. & Blackburn, H. D. Genetic structure of goat breeds from Brazil and the United States: Implications for conservation and breeding programs. J Anim Sci. 93(10), 4629–4636 (2015).
Shelton, M. Reproduction and Breeding of Goats. J. Dairy Sci. 61, 994–1010 (1978).
Cameron, M. R. et al. Growth and slaughter traits of Boer x Spanish, Boer x Angora, and Spanish goats consuming a concentrate-based diet. J. Anim. Sci. 79, 1423–1430 (2001).
Visser, C. et al. Genetic Diversity and Population Structure in South African, French and Argentinian Angora Goats from Genome-Wide SNP Data. PLoS One 11(5), e0154353, https://doi.org/10.1371/journal.pone.0154353 (2016).
Abad, M. et al. Breeding Scheme for Angora Goat Production in North Patagonia. http://www.wcgalp.org/system/files/proceedings/2002/breeding-scheme-angora-goat-production-north-patagonia.pdf (2002).
McManus, C. et al. Distribution of Goat Breeds in Brazil and Their Relationship With Environmental Controls. Biosci. J. 30, 1819–1836 (2014).
Kim, E. S. et al. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heredity. 116, 255–264 (2016).
Zhang, Z. et al. Genome-Wide Association Study Reveals Constant and Specific Loci for Hematological Traits at Three Time Stages in a White Duroc × Erhualian F2 Resource Population. PLoS One 8(5), e63665, https://doi.org/10.1371/journal.pone.0063665 (2013).
McManus, C. M. et al. Heat tolerance in Brazilian sheep: physiological and blood parameters. Trop. Anim. Health Prod. 41, 95–101 (2009).
Song, Y. et al. Genome-wide association study reveals the PLAG1 gene for Knuckle, Biceps and Shank weight in Simmental beef cattle. PLoS One 11(12), e0168316, https://doi.org/10.1371/journal.pone.0168316 (2016).
Abo-Ismail, M. K. et al. Single nucleotide polymorphisms for feed efficiency and performance in crossbred beef cattle. BMC Genet. 15, 14, https://doi.org/10.1186/1471-2156-15-14 (2014).
Wu, X. et al. Genome wide association studies for body conformation traits in the Chinese Holstein cattle population. BMC Genomics 14, 897, https://doi.org/10.1186/1471-2164-14-897 (2013).
Do, D. N. et al. Genome-Wide Association Study Reveals Genetic Architecture of Eating Behavior in Pigs and Its Implications for Humans Obesity by Comparative Mapping. PLoS One 8, e71509, https://doi.org/10.1371/journal.pone.0071509 (2013).
Fan, B. et al. Large-scale association study for structural soundness and leg locomotion traits in the pig. Genet. Sel. Evol. 41, 14, https://doi.org/10.1186/1297-9686-41-14 (2009).
Liu, X. et al. Novel single nucleotide polymorphisms of the bovine methyltransferase 3b gene and their association with meat quality traits in beef cattle. Genet. Mol. Res. 11, 2569–2577 (2012).
Snyman, M. A. & Olivier, J. J. Genetic parameters for body weight, fleece weight and fibre diameter in South African Angora goats. Livest. Prod. Sci. 47, 1–6 (1996).
Snyman, M. et al. Evaluation of a genetically fine mohair producing herd. Small Rumin. Res. 43, 105–113 (2002).
Visser, C. & Van Marle-Köster, E. Strategies for the genetic improvement of South African Angora goats. Small Rumin. Res. 121, 89–95 (2014).
Wang, Z. et al. Genome-wide association study for wool production traits in a Chinese merino sheep population. PLoS One 9, 3–10 (2014).
Raballo, R. et al. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 20, 5012–23 (2000).
Lupton, C. J. Prospects for expanded mohair and cashmere production and processing in the United States of America. J. Anim. Sci. 74, 1164–1172 (1996).
Gifford, D. R., Ponzoni, R. W., Lampe, R. J. & Burr, J. Phenotypic and genetic parameters of fleece traits and live weight in South Australian Angora goats. Small Rumin. Res. 4, 293–302 (1991).
Taddeo, H. R., Allain, D., Mueller, J., Rochambeau, H. & Manfredi, E. Genetic parameter estimates of production traits of Angora goats in Argentina. Small Rumin. Res. 28, 217–223 (1998).
Snyman, M. & Olivier, J. Repeatability and heritability of objective and subjective fleece traits and body weight in South African Angora goats. Small Rumin. Res. 34, 103–109 (1999).
Sahlu, T. et al. ASAS Centennial Paper: Impact of animal science research on United States goat production and predictions for the future. J. Anim. Sci. 87, 400–418 (2009).
Dong, Y. et al. Reference genome of wild goat (capra aegagrus) and sequencing of goat breeds provide insight into genic basis of goat domestication. BMC Genomics 16, 431, https://doi.org/10.1186/s12864-015-1606-1 (2015).
Xu, L. et al. Genomic Signatures Reveal New Evidences for Selection of Important Traits in Domestic Cattle. Mol. Biol. Evol. 32, 711–725 (2015).
Brito, L. F. et al. Genetic diversity and signatures of selection in various goat breeds revealed by genome-wide SNP markers. BMC Genomics 18, 229, https://doi.org/10.1186/s12864-017-3610-0 (2017).
Wang, Z., Yuan, L., Zuo, X., Racey, P. A. & Zhang, S. Variations in the sequences of BMP2 imply different mechanisms for the evolution of morphological diversity in vertebrates. Comp. Biochem. Physiol. - Part D Genomics Proteomics 4, 100–104 (2009).
Fariello, M.-I. et al. Selection Signatures in Worldwide Sheep Populations. PLoS One 9, e103813, https://doi.org/10.1371/journal.pone.0103813 (2014).
Schlötterer, C., Kofler, R., Versace, E., Tobler, R. & Franssen, S. U. Combining experimental evolution with next-generation sequencing: a powerful tool to study adaptation from standing genetic variation. Heredity. 114, 431, https://doi.org/10.1038/hdy.2014.86 (2014).
Daly, K. G. et al. Ancient goat genomes reveal mosaic domestication in the Fertile Crescent. Science 361, 85–88 (2018).
Ajmone-Marsan, P. et al. The characterization of goat genetic diversity: Towards a genomic approach. Small Rumin. Res. 121, 58–72 (2014).
Taberlet, P., Coissac, E., Pansu, J. & Pompanon, F. Conservation genetics of cattle, sheep, and goats. C. R. Biol. 334, 247–254 (2011).
Blackburn, H. D. et al. Genetic diversity of Ovis aries populations near domestication centers and in the New World. Genetica 139, 1169–1178 (2011).
FAO. In vivo conservation of animal genetic resources. FAO Animal Production and Health Guidelines. No. 14. http://www.fao.org/docrep/018/i3327e/i3327e.pdf (2013).
Tosser-Klopp, G. et al. Design and characterization of a 52K SNP chip for goats. PLoS One 9(1), e86227, https://doi.org/10.1371/journal.pone.0086227 (2014).
Ramey, H. et al. Detection of selective sweeps in cattle using genome-wide SNP data. BMC Genomics 14, 382, https://doi.org/10.1186/1471-2164-14-382 (2013).
Hormozdiari, F., Kostem, E., Kang, E. Y., Pasaniuc, B. & Eskin, E. Identifying causal variants at loci with multiple signals of association. Genetics 198, 497–508 (2014).
Rochus, C. M. et al. Revealing the selection history of adaptive loci using genome-wide scans for selection: an example from domestic sheep. BMC Genomics 19, 71, https://doi.org/10.1186/s12864-018-4447-x (2018).
Acknowledgements
To Coordination for the Improvement of Higher Education Personnel - Capes for providing scholarship for the first author. Portions of the data for this project were collected in the context of the project “Enhancement of Farmers Communities through Goats Utilization and Genetic Improvement” under the first call for proposals of the Funding Strategy for the Implementation of the Global Plan of Action for Animal Genetic Resources, for which financial support was provide by the Governments of Germany, Switzerland and Norway with in-kind support from Governments of Brazil (CNPq) and the United States of America. This study makes use of data generated by the NextGen Consortium. The European Union’s Seventh Framework Programme (FP7/2010–2014) provided funding for the project under grant agreement no 244356 – “NextGen”.
Author information
Authors and Affiliations
Contributions
T.P.P., C.M., D.A.F., M.I.C., C.A.M., A.M., S.R.P. and H.D.B. designed the study. M.L., L.C.E., M.I.C., E.J.A., A.M., O.F. and K.M.S. collected the samples and performed DNA extraction. C.M., C.A.M., S.R.P. and H.D.B. were responsible for genotyping. T.P.P., E.H.H. and D.A.F. analyzed the data. T.P.P., E.H.H., D.A.F., S.R.P. and H.D.B. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paim, T.d.P., Faria, D.A., Hay, E.H. et al. New world goat populations are a genetically diverse reservoir for future use. Sci Rep 9, 1476 (2019). https://doi.org/10.1038/s41598-019-38812-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38812-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.