Abstract
Dexamethasone is widely used for postoperative nausea and vomiting (PONV) prophylaxis, but its effect on PONV prevention in paediatric patients is validated only in short minor surgical procedures. In this study, we aimed to determine whether a single dose of dexamethasone reduces PONV in highly invasive surgeries that require opioid-based postoperative analgesia. One hundred adolescents undergoing scoliosis correction surgery were randomized to receive intravenous dexamethasone 0.15 mg/kg (dexamethasone group) or saline (control group) at induction of anaesthesia. The primary outcome was the incidence of PONV in the 72 h postoperatively. Data for 98 patients were available for analysis. The 72-h incidence of PONV was significantly lower in the dexamethasone group than in the control group (62.5% vs 84.0%; RR 0.74, 95% CI 0.58–0.96, P = 0.02). During the first and second 24-h postoperative intervals, fewer patients in the dexamethasone group received rescue antiemetics. Visual analogue scale scores for nausea and pain were lower in the dexamethasone group than in the control group during the first 24 h postoperatively. Dexamethasone did not increase the number of adverse events. The results of this study showed that a single dose of dexamethasone was effective for reducing PONV after paediatric scoliosis correction surgery.
Similar content being viewed by others
Introduction
Scoliosis correction surgery has been described as the most invasive orthopaedic surgery performed in young persons1. This surgery is associated with severe postoperative pain that requires advanced pain management, which is typically opioid-based patient-controlled analgesia (PCA). However, the management of opioid-related complications, in particular postoperative nausea and vomiting (PONV), is still inadequate in the majority of patients1. Serious outcomes from anaesthesia are rare, but PONV is a major concern in surgical patients2. PONV can impair patient satisfaction, delay postoperative recovery, and increase medical costs3. PONV affects approximately one-third of surgical patients and up to 70% of high-risk patients4. For patients at a high risk of PONV, prophylactic use of antiemetics including corticosteroids is recommended in international consensus guidelines5. However, the evidence for prophylactic use of dexamethasone in paediatric patients is based only on minor surgical procedures, such as tonsillectomy and strabismus surgery6,7, in which PONV may be induced by volatile anaesthetics or intraoperative opioids. Although a multicentre, multinational survey demonstrated that the use of dexamethasone for PONV prophylaxis is common practice in paediatric scoliosis surgery8, the effect of dexamethasone on PONV prevention has not been validated for highly-invasive surgical procedures that require opioid-based PCA for postoperative analgesia, in which PONV can be prolonged by opioids.
The aim of this study was to determine whether dexamethasone reduces PONV in children and adolescents undergoing highly invasive surgery, such as posterior correction and spinal fusion surgery for adolescent idiopathic scoliosis (AIS).
Materials and Methods
Patient recruitment and randomization
This randomized, single-centre, double-blind, prospective, placebo-controlled clinical trial was approved by the ethical committee at Keio University School of Medicine on 23 February 2015 (protocol number 20140395). The study was registered on the University Hospital Medical Information Network (UMIN) Clinical Trials Registry on 19 March 2015 (UMIN000016847). The study was conducted in accordance with the ethical standards of the Declaration of Helsinki of 1975.
Patients aged 10–19 years with a diagnosis of AIS and scheduled for posterior correction and fusion surgery at Keio University Hospital from May 2015 onwards were eligible for participation. The exclusion criteria were use of corticosteroids within the month before surgery, use of an antiemetic in the 24 h before surgery, and a contraindication to the study drug. Written informed consent was obtained from the parents of the patients along with assent from the patients.
Before surgery, the patients were provided with instructions regarding use of the PCA device and the visual analogue scale (VAS) for nausea and pain (0–100 mm).
On the day of surgery, the patients were randomly assigned 1:1 to either of two study groups using a computer-generated random number table. Randomization was performed by an anaesthesiologist who was not involved in the trial. Participants and their parents, the surgeon, anaesthesiologists, nurses, and the investigator following the participant postoperatively were blinded to study group allocation.
Study intervention
All patients received intravenous propofol 2.5 mg/kg, fentanyl 4 μg/kg, and rocuronium 0.6 mg/kg for induction of anaesthesia. At this time, the patients also received intravenous dexamethasone 0.15 mg/kg in 5 ml of 0.9% normal saline (dexamethasone group; n = 50) or volume-equivalent 0.9% normal saline (control group; n = 50). The study drugs were prepared by a pharmacist who was not involved in the study. The patients, parents, and health care providers including the anaesthesiologists and nurses in the operating room, intensive care unit and on the floor remained unaware of the group assignment for an individual subject. After tracheal intubation, anaesthesia was maintained with propofol (adjusted to maintain a bispectral index of 40–60), intermittent administration of fentanyl, and infusion of remifentanil. Motor-evoked potentials were monitored after administration of sugammadex with the patient in the prone position. After emergence from anaesthesia, the trachea was extubated, and the patient was transferred to the intensive care unit.
Postoperative analgesia consisted of intravenous PCA with fentanyl (0.2 μg/kg/h as a background infusion and 0.4 μg/kg as a bolus dose, with a lockout interval of 10 min), infusion of ketamine 0.1 mg/kg/h, and administration of a 25-mg diclofenac sodium suppository every 6 h. In addition, IV flurbiprofen 50 mg was administered upon patients’ request. Metoclopramide 10 mg was administered intravenously to treat nausea on patient request. Intravenous PCA was continued for at least 3 days postoperatively unless otherwise specified.
At 24, 48, and 72 h after surgery, all patients were asked by trained ward nurses blinded to study group assignment to complete a VAS sheet describing the worst levels of nausea and pain experienced during the preceding interval.
Outcome measures
The primary outcome measure was the incidence of PONV in the 72 h after surgery. Nausea was defined as a subjective feeling of a desire to vomit without the presence of expulsive muscular movements. Vomiting was defined as the involuntary, forceful expulsion of the contents of stomach. The patient was considered to have nausea when the VAS score for nausea was more than 0 and to have PONV if nausea or vomiting had occurred or rescue metoclopramide had been administered. Retching was included in vomiting.
The secondary outcomes included the incidence of PONV, vomiting, use of rescue metoclopramide, VAS scores for nausea and pain in the 0–24, 24–48, and 48–72 h after surgery, number of PCA doses requested by the patient and total amount of fentanyl administered in the 72 h postoperatively, amount of blood loss in the 24 hours after surgery, and incidence of surgical site infection in the month following surgery.
Power calculation and statistical analysis
A power analysis was performed using a power of 80% and an α of 0.05 (two-sided). Our retrospective observations showed that 26 (79%) of 33 patients experienced PONV within 72 h after surgery. Thus, we assumed that the incidence of PONV in the 72 h after surgery in the control group would be approximately 80%. We considered that a 30% reduction in incidence of PONV would be clinically relevant. The power analysis showed that 46 patients were needed in each study group. Fifty patients were enrolled in each group to allow for possible dropouts.
The data were analysed by a statistician who was not involved in the study or data collection, based on the intention-to-treat population, i.e., all patients who were randomized, received the study drug, and underwent surgery. Categorical data are presented as frequencies and continuous data are summarised as the mean (standard deviation or standard error) or median [interquartile range]. Continuous parametric and non-parametric data were compared using the Student’s t-test and Mann-Whitney U test, respectively. Categorical data were compared using Fisher’s exact test. For sensitivity analysis, the incidence of PONV, vomiting, use of rescue metoclopramide, VAS scores for nausea and pain at each time-point were estimated by the generalised linear mixed model (GLMM), to obtain point estimates and 95% confidence limits. The correlation structure was assumed as Toeplitz, autoregressive, or compound-symmetry structures were used in order if convergence was not obtained. The statistical analyses were performed using SPSS software version 22 (IBM Corp., Armonk, NY, USA) and SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were 2-sided. A P-value of <0.05 was considered statistically significant.
Results
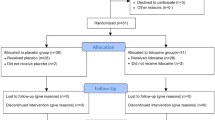
One hundred and eight patients underwent scoliosis correction surgery at our institution from May 2015 to August 2017. The first participant was enrolled on May 18, 2015. Eight patients refused to participate in the study, leaving 100 patients who underwent surgery for enrolment. Two patients in the dexamethasone group were subsequently excluded because of a protocol violation (the study drugs were not administered), leaving data for 98 patients (48 in the dexamethasone group and 50 in the control group) available for analysis (Fig. 1). The patient characteristics and intraoperative variables are shown in Table 1. Although two patients in the control group had a history of gastrointestinal surgery (appendectomy and intussusception surgery), no patients had gastrointestinal problems at the time of our study.
The incidence of PONV in the 72 h after surgery was significantly lower in the dexamethasone group than in the control group (62.5% vs 84.0%; number needed to treat 4.7, relative risk (RR) 0.74, 95% confidence interval (CI) 0.58–0.96, P = 0.02; Table 2). The frequency of PONV decreased with time, but remained high even after 48 h, particularly in the control group. Significantly fewer patients in the dexamethasone group experienced PONV during the first and second 24-h intervals than those in the control group (RR 0.27, 95% CI 0.11–0.67 and RR 0.40, 95% CI 0.18–0.91; P < 0.01 and P = 0.03, respectively). There were no significant differences between the study groups in the incidence of PONV during the 48–72 h after surgery (29.6% vs 48.0%, RR 0.54, 95% CI 0.24–1.24, P = 0.15).
Significantly fewer patients in the dexamethasone group required metoclopramide during the first and second 24-h periods than those in the control group (RR 0.34, 95% CI 0.15–0.78 and RR 0.34, 95% CI 0.14–0.88; P < 0.01 and P = 0.03, respectively). The estimation of VAS score for nausea was significantly lower in the dexamethasone group than in the control group during the first 24 h postoperatively (difference in estimation 16.8 mm, 95% CI 4.6–29.0 mm, P = < 0.01), but not thereafter. There was no significant difference in the incidence of vomiting between the two groups during the study period. No difference was found in the beginning of the diet (1.1 postoperative day in the dexamethasone group and 1.2 postoperative day in the control group, p = 0.29).
Although PCA requirements and the cumulative dose of fentanyl administered in the 72 h after surgery were similar between the two groups, the mean VAS score for pain during the first 24 h postoperatively was significantly lower in the dexamethasone group than in the control group (difference in estimation 14.8 mm, 95% CI 4.0–25.6 mm, P < 0.01). In addition, the total dose of patient-requested flurbiprofen administered during the first 72 h after surgery was significantly reduced in the dexamethasone group (45.8 mg, 95% CI: 23.9–67.8 mg) than in the control group (117.0 mg, 95% CI: 75.9–158.1 mg) (p = 0.021). Five (10.0%) patients in the control group and four (8.3%) in the dexamethasone group discontinued intravenous PCA within 72 h because of severe PONV (Table 3). Intravenous PCA was terminated within 72 h in one (2.0%) patient in the control group and five (10.4%) in the dexamethasone group by the attending orthopaedic surgeon on the grounds that further pain relief was unnecessary. The amount of blood loss in the 24 h following surgery was not different between the two groups and no surgical site infections were reported during the month after surgery.
Discussion
In this randomized, single-centre, double-blind, prospective, placebo-controlled trial targeted to children and adolescents, a single dose of dexamethasone 0.15 mg/kg administered at induction of anaesthesia reduced the incidence and severity of PONV as well as on-demand use of metoclopramide after posterior correction and spinal fusion surgery for AIS. Further, this dose of dexamethasone improved early postoperative pain scores without an increase in adverse events. To our knowledge, this is the first study to assess the prophylactic effect of dexamethasone on PONV in paediatric patients undergoing a highly invasive surgical procedure that requires intravenous PCA for postoperative pain management.
Intravenous PCA with opioids is generally used for management of postoperative pain in patients with AIS who undergo scoliosis correction surgery, but is often accompanied by opioid-related side effects, such as PONV9. These patients are at particularly high risk of PONV because of the high proportion of girls10 and the long operating time, which are considered as major risk factors for PONV in paediatric patients11. More than 80% of the patients in this study who did not receive dexamethasone developed PONV, as reported previously12,13. Patients who experience PONV on PCA often refuse to continue bolus doses for pain relief, so adequate pain control relies on prevention of PONV, particularly in patients known to have multiple risk factors for PONV.
Dexamethasone is a synthetic glucocorticoid with anti-inflammatory and immunosuppressant properties. Although the mechanism of action remains unclear, dexamethasone is one of the agents most commonly used to prevent PONV14. The prophylactic effect of dexamethasone on PONV has been demonstrated in adults undergoing a wide range of surgical procedures14,15. In paediatric patients, it has been reported that dexamethasone 0.05–1.0 mg/kg administered at induction of anaesthesia reduced the incidence of PONV in the 24 h following common paediatric surgical procedures, such as tonsillectomy, correction of strabismus, and inguinal hernia repair6,7,16. A multicentre, multinational survey demonstrated that intravenous antiemetic prophylaxis is common practice for paediatric scoliosis surgery8. However, the prophylactic effect of dexamethasone on PONV in young patients undergoing highly invasive surgical procedures that require opioid-based PCA has not been clearly established. Dexamethasone has a long biological half-life (36–72 h)17, so may be particularly suitable for prevention of prolonged PONV caused by opioid-based PCA. In the present study, dexamethasone at a dose of 0.15 mg/kg, which is recommended in the latest guidelines5, reduced the overall incidence of PONV by 26% during the 72 h after surgery when compared with placebo. Moreover, it decreased the incidence of PONV and use of metoclopramide during the 0–24 h and 24–48 h postoperatively and the severity of nausea in the first 24 h after surgery. However, the incidence of vomiting and discontinuation of intravenous PCA because of severe PONV was similar between the two groups, suggesting that dexamethasone is more effective for reducing mild nausea than severe nausea or vomiting.
In addition to decreasing PONV, dexamethasone has been shown to reduce postoperative pain after several surgical procedures in both adults and children18,19. In the present study, the pain score during the first 24 h after surgery was significantly lower in the dexamethasone group than in the control group. Of note, in approximately 10% of the patients who received dexamethasone, intravenous PCA was terminated within 72 h after surgery because the attending orthopaedic surgeon considered that pain relief was no longer necessary. Use of multimodal analgesia and early discontinuation of intravenous PCA has been reported to reduce the length of hospital stay without an increase in pain scores20, so the analgesic properties of dexamethasone could also be of economic benefit in the perioperative management of patients undergoing posterior correction and spinal fusion surgery for AIS.
There are several limitations that should be borne in mind when interpreting the findings of this study. First, our sample size was not calculated to show differences in the secondary outcomes. Thus, it remains to be clarified whether dexamethasone 0.15 mg/kg reduces the incidence of PONV and use of metoclopramide in the late postoperative period (48–72 h). Second, the sample size was not large enough to determine the frequency of rare complications known to be associated with dexamethasone, such as hyperglycaemia and infection. We found no evidence of surgical site infection in this study. Although we did not examine the blood glucose level, it may be transiently elevated by a single dose of dexamethasone21,22. A recent meta-analysis and a large clinical trial did not find an increased risk of surgical site infection in patients with or without diabetes mellitus undergoing non-cardiac surgery who received a single dose of dexamethasone for PONV prophylaxis23,24. However, these trials and the studies involved in the meta-analysis did not aim to determine the safety of dexamethasone as a primary outcome. Therefore, a final judgment regarding the safety of dexamethasone should not be made until the results of an ongoing clinical trial of dexamethasone and surgical site infection (Perioperative Administration of Dexamethasone and Infection trial; ACTRN12614001226695) are available. Third, we did not determine whether the patients who received dexamethasone had an improved ability to ambulate and participate in physical therapy or whether they had a reduced length of hospital stay. Further investigations are needed to assess these potential additional benefits.
Conclusion
Dexamethasone 0.15 mg/kg administered at the time of induction of anaesthesia reduced PONV and improved postoperative pain without increasing adverse events in paediatric patients undergoing scoliosis correction surgery. These results may support routine use of a prophylactic dose of dexamethasone in these patients.
References
Rullander, A. C., Jonsson, H., Lundstrom, M. & Lindh, V. Young people’s experiences with scoliosis surgery: a survey of pain, nausea, and global satisfaction. Orthop Nurs 32, 327–333; quiz 334–325 (2013).
Macario, A., Weinger, M., Carney, S. & Kim, A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg 89, 652–658 (1999).
Parra-Sanchez, I. et al. A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Can J Anaesth 59, 366–375 (2012).
Gan, T. J. Postoperative nausea and vomiting–can it be eliminated? JAMA 287, 1233–1236 (2002).
Gan, T. J. et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118, 85–113 (2014).
Steward, D. L., Grisel, J. & Meinzen-Derr, J. Steroids for improving recovery following tonsillectomy in children. Cochrane Database Syst Rev, CD003997 (2011).
Madan, R. et al. Prophylactic dexamethasone for postoperative nausea and vomiting in pediatric strabismus surgery: a dose ranging and safety evaluation study. Anesth Analg 100, 1622–1626 (2005).
Palmer, G. M., Pirakalathanan, P. & Skinner, A. V. A multi-centre multi-national survey of anaesthetists regarding the range of anaesthetic and surgical practices for paediatric scoliosis surgery. Anaesth Intensive Care 38, 1077–1084 (2010).
Dolin, S. J. & Cashman, J. N. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth 95, 584–591 (2005).
Konieczny, M. R., Senyurt, H. & Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop 7, 3–9 (2013).
Rose, J. B. & Watcha, M. F. Postoperative nausea and vomiting in paediatric patients. Br J Anaesth 83, 104–117 (1999).
Cassady, J. F. Jr., Lederhaas, G., Cancel, D. D., Cummings, R. J. & Loveless, E. A. A randomized comparison of the effects of continuous thoracic epidural analgesia and intravenous patient-controlled analgesia after posterior spinal fusion in adolescents. Reg Anesth Pain Med 25, 246–253 (2000).
Eschertzhuber, S. et al. Comparison of high- and low-dose intrathecal morphine for spinal fusion in children. Br J Anaesth 100, 538–543 (2008).
De Oliveira, G. S. Jr., Castro-Alves, L. J., Ahmad, S., Kendall, M. C. & McCarthy, R. J. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 116, 58–74 (2013).
Apfel, C. C. et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 350, 2441–2451 (2004).
Busoni, P., Crescioli, M., Agostino, R. & Sestini, G. Vomiting and common paediatric surgery. Paediatr Anaesth 10, 639–643 (2000).
Schimmer B. P, Parkel, K. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics (ed. Brunton LL) Ch. 59, 1594 (McGRAW-HILL, 2006).
Nielsen, R. V. et al. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: a randomized, blinded, placebo-controlled trial. Pain 156, 2538–2544 (2015).
Hermans, V., De Pooter, F., De Groote, F., De Hert, S. & Van der Linden, P. Effect of dexamethasone on nausea, vomiting, and pain in paediatric tonsillectomy. Br J Anaesth 109, 427–431 (2012).
Muhly, W. T. et al. Rapid Recovery Pathway After Spinal Fusion for Idiopathic Scoliosis. Pediatrics 137 (2016).
Tien, M. et al. The effect of anti-emetic doses of dexamethasone on postoperative blood glucose levels in non-diabetic and diabetic patients: a prospective randomised controlled study. Anaesthesia 71, 1037–1043 (2016).
Elston, M. S. et al. Duration of cortisol suppression following a single dose of dexamethasone in healthy volunteers: a randomised double-blind placebo-controlled trial. Anaesth Intensive Care 41, 596–601 (2013).
Toner, A. J., Ganeshanathan, V., Chan, M. T., Ho, K. M. & Corcoran, T. B. Safety of Perioperative Glucocorticoids in Elective Noncardiac Surgery: A Systematic Review and Meta-analysis. Anesthesiology 126, 234–248 (2017).
Corcoran, T. et al. Intraoperative dexamethasone does not increase the risk of postoperative wound infection: a propensity score-matched post hoc analysis of the ENIGMA-II trial (EnDEX). Br J Anaesth 118, 190–199 (2017).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the study. R.W., H.S., S.I., N.I., R.M., K.W. and H.M. designed the study. R.W., H.S., S.I., N.I. and R.M. recruited the patients and collected the data. Y.S. analysed data. H.S., K.W. and H.M. supervised the study. R.W. and H.S. wrote the main manuscript text. S.I., K.W. and Y.S. contributed to the writing of the manuscript. R.W. made a Figure, and S.I. drew up the Tables. All authors reviewed and approved the final version of the manuscript to be submitted.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wakamiya, R., Seki, H., Ideno, S. et al. Effects of prophylactic dexamethasone on postoperative nausea and vomiting in scoliosis correction surgery: a double-blind, randomized, placebo-controlled clinical trial. Sci Rep 9, 2119 (2019). https://doi.org/10.1038/s41598-019-38764-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38764-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.