Abstract
As post-transcriptional regulators, microRNAs (miRNAs) play an important role in growth and reproductive processes. So far, there is limited information regarding crustacean miRNAs. To explore the potential role of miRNAs in the gonadal development of the prawn Macrobrachium rosenbergii, we constructed seven small RNA libraries from ovarian and testicular tissues at various stages using somatic tissue as the control. A total of 1,954 known and 129 novel miRNAs were retrieved. By comparing differentially expressed miRNAs (DEMs) between testes and ovaries, forty-one miRNAs were identified with sex-biased expression patterns, including 17 ovary-biased and 24 testis-biased patterns. Furthermore, the putative target genes of the sex-biased miRNAs, such as cyclin L1, mitogen-activated protein kinase 7 (MAPK 7), heat shock protein (HSP), and zinc finger protein, were significantly enriched in many reproduction-related pathways including the Gonadotropin-releasing hormone (GnRH) pathway, glycolysis, gluconeogenesis pathway, ovarian steroidogenesis, estrogen signaling pathway, MAPK pathway, Wnt pathway, and insulin signaling pathway, implicating potential regulatory roles of miRNAs in reproduction. These data aid in the further investigation of the mechanism of gonadal development and reproductive regulation mediated by miRNA in M. rosenbergii.
Similar content being viewed by others
Introduction
MicroRNAs (miRNAs) are small single-stranded non-coding RNA molecules. As key post-transcriptional regulators, miRNAs in animals are capable of controlling gene expression mostly through interaction with the 3′ untranslated region (3′UTR) of the target mRNA for degradation or translational inhibition1. A large number of protein-coding genes (PCGs) have been revealed to be targeted by miRNAs. It is interesting to note that a single miRNA molecule can target thousands of mRNAs, and that each mRNA could have recognition sequences for multiple miRNAs as well. More than one third of the human genome has been determined to be regulated by miRNAs2,3.
miRNAs are involved in the regulation of a variety of life processes4,5. Recent evidence has shown that miRNAs play an important role in the development of reproductive systems and the differentiation of germ cells6,7,8. Statistical, genome-wide analysis of miRNAs discovered many differentially expressed miRNAs (DEMs) during gonadal development in mammals, including mouse (Mus musculus)9,10, pig (Sus scrofa)11, cattle (Bos Taurus)12, and sheep (Ovis aries)13. Sex-biased miRNAs may be directly involved in the differential expression of many target genes, which contribute to different sexual traits during gonadal development14. In Acipenser schrenckii, 37 miRNAs were detected exclusively in the gonad and 148 miRNAs exhibited sex-biased expression, including 83 female-biased and 65 male-biased miRNAs. Based on these DEMs, twenty-five putative gametogenesis-related target genes were predicted, including anti-Mullerian hormone (Amh), winged helix/forkhead transcription factor (Foxl2), and Vasa15. Subsequent studies also revealed many DEMs in sea urchin (Strongylocentrotus nudus)16, tilapia (Oreochromis niloticus)17, and common carp (Cyprinus carpio)18, suggesting that miRNAs involved in gonadal development are mediated by their differential expression of sexual dimorphism.
Gonadal development involves a series of complex regulatory processes at transcriptional and post-transcriptional levels. Although efforts have been made to study this regulation in Decapoda crustaceans, only a few studies have focused on post-transcriptional gene regulation. Furthermore, miRNAs related to gonadal development have only been reported in the Chinese mitten crab (Eriocheir sinensis)8,19 and swimming crab (Portunus trituberculatus)7. In E. sinensis, miR-2 and miR-133 were demonstrated to down regulate cyclin B gene expression during meiotic oocyte maturation19. Moreover, some miRNAs (let-7c, miR-21-5p, and miR-17-3p) displayed different expression patterns at various stages of testicular development and may be involved in spermatogenesis8. In P. trituberculatus, 44 ovarian and 31 testicular miRNAs were preferentially expressed7. Furthermore, the expression of miR-7 and miR-87 was significantly increased and they were predicted to target cell division cycle 4 (Cdc4) and cyclin-dependent kinase 2 (Cdk2)7, both of which are critical for controlling the G1-to-S-phase transition during the cell cycle20,21. In the giant freshwater prawn Macrobrachium rosenbergii, however, there is limited information currently available regarding miRNAs during gonadal development.
The prawn M. rosenbergii is a commercially valuable decapod species that is widely cultured in southern and southeastern Asia as well as in the western Pacific22,23. During the last decade, there has been significant development in farming technology of M. rosenbergii in China, with an average annual farming expansion rate of 15.28%24. Although this increased productivity and technology has achieved great progress, there have been numerous developmental problems. Furthermore, annual production is not very stable due to the limitation of breeding and farming technology, such as inbreeding and disease24. Therefore, it is necessary to focus on the mechanism of gonadal development for breeding and farming technology optimization in M. rosenbergii.
By taking advantage of high-throughput next-generation sequencing (NGS) technology, we screened miRNAs in from seven different M. rosenbergii miRNA libraries. During this process we were able to identify novel miRNAs and DEMs for the first time in M. rosenbergii. We further conducted miRNA expression profile analysis and the functional annotation of DEMs by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) to explore potential roles of miRNAs in gonadal development. The results from this study provide valuable information regarding the regulatory roles of miRNAs in gonadal development and reproductive control in M. rosenbergii.
Results
Identification of miRNAs from M. rosenbergii
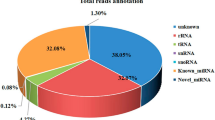
We constructed seven cDNA libraries of small RNAs from early-middle (ET) and late (LT) developing testis; early (EO), middle (MO) and late developing (LO) ovaries; and male (MS) and female (FS) somata. A total of 19,316,857; 30,914,458; 51,441,365; 31,871,854; 29,986,842; 29,092,334; and 25,530,458 raw reads were initially generated from the seven libraries, respectively. After filtering out low quality sequences, there were 17,837,427; 26,153,111; 41,516,524; 27,583,544; 26,887,205; 24,940,628; and 22,053,365 clean reads remaining in the corresponding libraries. By comparing the small RNA sequences with those sequences in the Rfam, non-coding RNAs in all of the libraries were categorized into rRNA, snRNA, tRNA, known miRNA and those without annotation (Table 1). As shown in Table 1, most of the small RNAs were unannotated with a range from 10,003,331 to 34,476,353 sequences, implying that small RNA information is still underdeveloped for decapod species. After removing the repeat reads, known and novel miRNAs were retrieved from each library as shown in Table 2. A total of 1,954 known and 129 novel miRNAs were obtained. The highest number of the known miRNAs was found in the ET library, while three ovary libraries generated the highest number of novel miRNAs, displaying 49, 46, and 44 novel miRNAs, respectively. To get a rudimentary understanding for the distribution of the miRNA among seven libraries, we further analyzed the data using box-whisker plot (Fig. 1).
Length distribution of small RNAs
The length distribution of the high-quality reads varied through markedly different bimodal patterns in size distribution (Fig. 2). One peak for the 22 nucleotide (nt) size class represents the typical miRNA of Dicer-processed product. Other peaks for 27 nt or 32 nt mostly represent longer piwi-interacting RNAs (piRNAs), which are endogenous small non-coding RNA molecules. As Fig. 2 showed, in EO, MO, and LO, the peak represented by the longest size class (27–29 nt) was much higher than for the peaks of the 21–23 nt size class, indicating that piRNAs were more abundant in M. rosenbergii ovary. On the other hand, the amount of miRNAs and piRNAs were not significantly different in ET and LT. In comparison with the status in gonad, the length distributions were quite different in MS and FS. MiRNAs were more abundant with a percentage of approximately 30% in MS and FS. Additionally, the length of piRNAs ranged from 31 to 33 nt, which was longer than that those of the gonads.
Length distribution of clean reads. The nucleotide length of miRNAs is shown on the X-axis; the percentage of different nucleotide lengths in the sized miRNAs among the total miRNA population is shown on the Y-axis. ET, early-middle testis; LT, late testis; EO, early ovary; MO, middle ovary; LO, late ovary; MS, male somata; and FS, female somata.
Nucleotide bias of miRNAs
The nucleotide bias of miRNAs at each position was analyzed (Supplementary Fig. S1). The results showing that U and A were the dominant nucleotides in all seven libraries. Interestingly, there were relatively fewer U on bases 2–4 and A was generally distributed on the 10th base. This is because the localization of the 10th base is generally the cleavage site of the miRNA when its target gene is sheared. Analysis on the first nucleotide bias of miRNAs showed that U was the predominantly common first nucleotide between the lengths of 15–25 nt, with its proportion exceeding 50% (Supplementary Fig. S2). Nevertheless, A was the dominant first nucleotide of 26–27 nt, and its proportion ranged from 75% to 100%.
GO and KEGG annotation
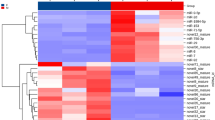
We obtained DEMs by comparing the expression of miRNAs between different libraries. By comparing the differentially expressed miRNAs (DEMs) between testes and ovaries, forty-one miRNAs were identified with sex-biased expression patterns, including 17 ovary-biased and 24 testis-biased miRNAs (Fig. 3). For instance, miR-194-5p and miR-148a-5p demonstrated higher expression in the ET library, suggesting they could play important roles in early testicular development. In contrast, miR-24-3p was up-regulated in ovary, especially in LO the library, indicating that miR-24-3p may be involved in ovarian maturation. Subsequently, Gene ontology (GO) annotation was performed to elucidate the function of the target genes identified as being potentially regulated by DEMs. A total of 253 potential target genes were enriched between the ovaries and testes. Amongst the three categories of biological process, cellular component, and molecular function, the most enriched GO terms were “transcription, DNA-templated”, “cytoplasm”, and “poly (A) RNA binding”, respectively (Supplementary Fig. S3A). Additionally, many predicted target genes were involved in gonadal development, such as cyclin L1, mitogen-activated protein kinase 7 (MAPK 7), heat shock protein (HSP), and zinc finger protein (Supplementary Table S1).
Heat-map of 41 differentially expressed miRNAs between ovary and testis. The heat-map was constructed based on the relative expression level of miRNA (TPM fold change > 2, P < 0.05). The color indicates the log2-fold change from high (red) to low (blue), as indicated by the color scale. The name of miRNAs and the libraries to which they belong are shown on the right side. The novel miRNAs have been revised as MRO-novel-**, the others are known miRNAs. ET, early-middle testis; LT, late testis; EO, early ovary; MO, middle ovary; LO, late ovary; MRO, Macrobrachium rosenbergii; aae, Aedes aegypti; aca, Anolis carolinensis; bbe, Branchiostoma belcheri; bmo, Bombyx mori; bta, Bos taurus; ccr, Cyprinus carpio; cfa, Canis familiaris; cgr, Cricetulus griseus; cin, Ciona intestinalis; cqu, Culex quinquefasciatus; dme, Drosophila melanogaster; dpu, Daphnia pulex; dre, Danio rerio; efu, Eptesicus fuscus; gga, Gallus gallus; ggo, Gorilla gorilla; hme, Heliconius melpomene; ipu, Ictalurus punctatus; lmi, Locusta migratoria; mdo, Monodelphis domestica; mml, Macaca mulatta; nvi, Nasonia vitripennis. oha, Ophiophagus Hannah; rno, Rattus norvegicus.
Furthermore, the predicted genes were submitted to KEGG analysis. The results showed that target genes were assigned into 141 signaling pathways. As shown in Fig. 4, glycolysis, gluconeogenesis and glucagon signaling pathways were the main metabolic pathways identified in this study (P < 0.05), indicating that these signaling pathways might play an important role in gonadal development. In addition, many predicted pathways were also involved in gonadal development, such as the MAPK pathway, GnRH signaling pathway, and insulin signaling pathway (P < 0.05). In addition to the pathways showed in Fig. 4, the Wnt signaling pathway, ovarian steroidogenesis, and estrogen signaling pathway were associated with gonadal development as well (Supplementary Table S2).
Top 20 significantly enriched KEGG pathways of putative target genes between testes and ovaries. KEGG pathways with P < 0.05 were considered significantly enriched. The color indicates a p-value from small (orange) to large (red). The smaller the p-value, the more vivid the red color. Each point corresponds to a pathway, and the larger the point is, the more genes are enriched in the pathway.
GO enrichment analysis different gonadal development stages (between ET and LT, EO and MO, MO and LO, respectively) showed that the main GO terms at the level of biological processes were “transcription, DNA-templated” and “regulation of transcription, DNA-templated”. At the level of cellular components, “cytoplasm” and “nucleus” were the main terms. At the molecular function level, “metal ion binding”, “ATP binding” and “zinc ion binding” were the dominant terms (Supplementary Fig. 3B–D). The KEGG results indicated that two signaling pathways “transcriptional misregulation in cancers” and “cGMP-PKG signaling pathway” were shared the most between the three groups (Supplementary Fig. 4A–C).
Quantitative real-time PCR analysis of sex-biased miRNAs
To validate the differential expression of sex-biased miRNAs identified by sequencing, we randomly selected five ovary-biased miRNAs (miR-146a, miR-100, miR-24-3p, miR-190, and MRO-novel-13) and three testis-biased miRNAs (miR-1a-3p, miR-8-3p, and miR-137b-3p) to analyze expression profiles using quantitative real-time PCR (qPCR). The qPCR results indicated that miR-146a, miR-100, miR-24-3p, miR-190, and MRO-novel-13 were up-regulated in ovary and miR-1a-3p, miR-8-3p, and miR-137b-3p were up-regulated in testis (Fig. 5), which was consistent with the sequencing results. Additionally, we selected some conservative and novel miRNAs to further characterize the DEMs between ovary and testis. MiR-100-5p, miR-9-5p, miR-21-5p, and MRO-novel-69 were highly expressed in ovary, whereas miR-1, miR-26a, MRO-novel-104, and MRO-novel-19 were highly expressed in testis. In general, the expression of DEMs was significantly different between the ovary and testis. For example, the expression level of miR-137-3p and miR-26a in the testis was four times and two times its concentration in the ovary, respectively. The resulting miRNA expression profiles were consistent with each other.
Expression patterns of sex-biased miRNAs confirmed by real-time PCR. The scales of the Y axis were indicated by Log2. The red column represent testis, while the blue column represents ovary. (A) Seven testis-biased miRNAs were highly expressed in the testis; (B) Nine ovary-biased miRNAs were highly expressed in the ovary. SnRNA was used as an internal reference. Bars represent the triplicate mean + SD from three individuals (n = 3). Asterisks indicate a significant difference (P < 0.05) between testis and ovary.
Discussion
Although the importance of miRNAs has been recognized in regulating gene expression during sexual differentiation and gonadal development7,8,25, there is little information available regarding the expression of miRNAs in the prawn M. rosenbergii. Thus, the main objective of this study was to identify conserved and novel miRNAs present in M. rosenbergii and to evaluate differential expression patterns during gonadal development. Therefore, a series of DEMs and novel miRNAs were identified from seven miRNA libraries, and their corresponding target genes were predicted. Elucidation of the differences in miRNA and their sex-biased expression between tissues and stages will be valuable in understanding the gene expression regulatory networks underlying gonadal development.
The results of miRNA analysis revealed that U and A were the dominant nucleotides. This phenomenon may be related to some of the function of miRNA, such as binding to target genes18. Despite two peaks for the length distribution of small RNAs present in all libraries, the distribution is varied. As our results showed, the length distribution of small RNAs was roughly the same at various stages in gonads, but another differently peak was observed in MS and FS libraries (Fig. 2). In Decapod species P. trituberculatus, the amount of miRNAs and piRNAs (25–27 nt) was roughly the same7. However, piRNAs, with lengths ranging from 27 to 29 nucleotides, were the main enriched small RNA in ovarian tissue in M. rosenbergii. In contrast, miRNA was the dominant small RNAs observed in somatic tissues. This was also verified in M. nipponense26, suggesting that the length distribution and percentage of different small RNAs displayed specificity among various tissues as well as among species. Interestingly, after analysis of differently expressed piRNAs between ovary and testis, we found up regulation of 327 piRNAs in testis, while no piRNAs were unregulated in ovary (Supplementary Table S3). These results corroborate the previous study that piRNAs can affect the process of sperm production27.
In total, we obtained 1,954 known and 129 novel miRNAs from seven libraries. Among the 2,083 miRNAs, we focused on DEMs for the purpose of this study. From these we identified 17 ovary-biased and 24 testis-biased miRNAs in terms of expression patterns. These miRNAs could play a vital role in gonadal development. For instance, miR-194-5p and miR-148a-5p were more expressed in the ET library, suggesting possible involvement in early testicular development. In contrast, miR-24-3p was up-regulated in ovary, especially in the LO library, indicating that miR-24-3p could promote ovarian maturation. In addition, cgr-miR-122 was abundant in the ET library. Furthermore, GO enrichment analysis indicated that its target gene was MAPK 7, a key member in the MAPK signaling pathway, which regulates cyclin D1 expression during ovarian development and is therefore required for cell cycle progression28. Additionally, the testis-biased miRNA miR-193, target F-box/WD repeat-containing protein 7 (FBW 7) and could therefor suppress cell proliferation through the G2/M cell cycle transition29. Another testis-biased miRNA miR-145 was predicted to target cytosolic phospholipase. Cytosolic phospholipase has been verified to function in the synthesis of prostanoid involved in ovarian development in Penaeus monodon30. In addition, prediction results showed that many target genes participate in gonadal development, such as cyclin L1, HSP, and zinc finger protein31,32,33. For instance, Hsp110 is required for progression of spermatogenesis, and is associated with germ cell apoptosis32. The early growth response (Egr) family of zinc finger-containing transcription factors includes 4 members34. The Egr4 transcript and protein were localized in the developing murine testis and displayed distinct intracellular localization patterns within specific cell populations of the testis35. Additionally, some putative genes were mapped to reproductive biological pathways, and the main pathways included glycolysis, gluconeogenesis and the glucagon signaling pathway. Previous studies have shown that inefficient glycolysis combined with an increase in amino acid catabolism could be incompatible with the need for a high level of metabolism to maintain the rate of cell division required to form a gonad and populate it with germ cells8. Furthermore, Glucagon-like peptide 1 could be a factor involved in control of the hypothalamic-pituitary-gonadal axis36. Other pathways, such as the MAPK signaling pathway11,37, Wnt signaling pathway38,39, GnRH signaling pathway40, insulin signaling pathway41, cAMP signaling pathway42, ovarian steroidogenesis43, and the estrogen signaling pathway44 were proven to have influence on gonadal development. It is known that GnRH is the main hypothalamic regulator of the reproductive system in mammals45. Similar to results from previous studies on GnRH signaling pathway, lysophospholipase, 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase classes I and II-like, and MAPK 7 were mapped to the GnRH signaling pathway with p-value of approximately 0.01 in this study. Therefore, these DEMs and target genes are potentially involved in gonadal development. Further research is needed to confirm their regulatory mechanism mediated by miRNAs.
In conclusion, we provided the first report of known and novel miRNAs for M. rosenbergii gonad at different developmental stages. Based on the DEMs analysis, we identified 24 male-biased miRNAs and 17 female-biased miRNAs. These miRNAs may play an important role in gonadal development and reproduction. The GO and KEGG analysis of DEMs further revealed that some DEMs involved the process of gonadal development. The data obtained from our study could serve as a basis for further genetic mechanism research and contribute to valuable information for reproduction-control technology on M. rosenbergii.
Materials and Methods
Animals and sample collection
Healthy prawns were collected from a local fish market and transported back to our laboratory. The prawns were maintained at 26 ± 2 °C in a 30-L aerated aquarium for three days. Various samples, including ovaries, testes, and somatic tissue of female and male prawns were collected and immediately frozen in liquid nitrogen followed by storage at −80 °C. The developmental stages of the ovary and testis were determined according to the gonadal histological sections (Supplementary Fig. S5). Based on previous studies, ovarian development was divided into early, middle and late stages40,46, and testicular development was divided into early-middle and late stages47. Thus, seven samples including two samples of female and male somata, were prepared for the following RNA-Seq and qPCR experiments.
RNA isolation
Total RNA was extracted using Trizol reagent (Invitrogen, USA) and treated with RNase-free DNase I (TaKaRa, Japan) for removing genomic DNA according to the manufacturer’s instructions. The extracted RNA was run on 1.5% agarose gel and Bioanalyzer 2100 system (Agilent Technologies, USA) for checking RNA integrity. The RNA quantity and purity were analyzed by a Nanodrop2000c spectrophotometer to obtain the A260/280 and A260/230 ratios with values within 1.90–2.10 and 2.00–2.50, respectively.
Library construction and high-throughput sequencing
The libraries were constructed using NEB Next Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs, USA) following the manufacturer’s instructions. Small RNAs were first isolated from the total RNAs of each of the seven samples. Then, these small RNAs were ligated to an activated 3′ and 5′ adaptor in turn. Then, adaptor-ligated small RNAs were reversed to create cDNA constructs. Subsequently, the generated cDNA constructs were amplified by PCR using Long AmpTaq 2 × Master Mix and SR Primer for Illumina. PCR products were purified on an 8% polyacrylamide gel. Finally, the seven resulting libraries were sequenced at OE Biotech. Co., Ltd. (Shanghai, China) using Illumina Solexa technology.
Sequence data analysis
The initial sequence data from Illumina Solexa sequencing were filtered to remove low quality reads and adaptor sequences. Sequences originating from protein-coding genes (PCGs) were also removed by blast against the reference sequences derived from multi-transcriptomic datasets of M. rosenbergii in GenBank (SRX760286, SRX760284, SRX859032, SRX097639, SRX097638, and SRX092198) to obtain the final clean reads. The distribution of small RNA read lengths was determined. Then the reserved reads were classified into ribosomal RNA (rRNA), small nuclear (snRNA), and transfer RNA (tRNA) by blasting the Rfam database (version 10.0, http://rfam.xfam.org/). As there was no whole-genome data available for M. rosenbergii, the remaining sequences were searched against the miRBase database (version 21.0, http://www.mirbase.org/) to identify conserved miRNAs. Meanwhile, potential novel miRNAs were analyzed by Mirdeep2 and RNAfold48. The sequences exceeding a length of 18 bp were submitted to Mirdeep2 and the mapping genome sequences (P < 0.05) remained for further analysis. The remained sequences were able to predict its secondary structure, to be considered a novel miRNA. Subsequently, base-bias was analyzed for all miRNAs. To identify miRNAs with significantly different expression between samples, miRNA expression levels were normalized as transcripts per million (TPM): Normalized expression = (mapped read count / total reads) × 1.0 × 106. Differentially expressed miRNAs (DEMs) analysis among libraries was carried out using Audic-Claverie49. The criteria for significant expression was defined as P < 0.05 and TPM > 2. The box-whisker plot allowed rough visualization of whether the data has symmetry and the degree of dispersion by using five statistics in the data: minimum value, the first quartile (25%), median (50%), third quartile (75%) and maximum value.
Target gene prediction and functional annotation of DEMs
The raw sequences from transcriptomes of M. rosenbergii were trimmed and the overlapping high-quality reads were assembled to create longer contigs. Protein coding regions, 5′UTR, and 3′UTR were determined using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). Then the 3′UTR extracted from the above transcriptomes of M. rosenbergii were employed to predict potential target genes of the DEMs with Miranda software (S ≥ 150, ΔG ≤ −30 kcal/mol)50. All putative target genes were categorized into functional classes using Gene Ontology (GO) enrichment analysis (http://www.geneontology.org/), in which gene numbers were calculated for each term, and then hyper geometric testing was used to identify significantly enriched GO terms and the putative targets. We used Benjamini & Hochberg for the corrected p-value. GO terms with corrected values of P < 0.05 were defined as significantly enriched. Subsequently, pathway analysis of predicted target mRNAs was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/pathway.html). KEGG pathways with P < 0.05 were considered significantly enriched.
Quantitative real-time PCR
To verify the reliability of sequencing results, qPCR was employed on the sixteen randomly selected miRNAs. Total RNA (500 ng) was reverse transcribed using miRcute reverse transcriptase kit (Tiangen, China) according to the manufacturer’s instructions. Each reverse transcribed reaction mixture (20 μL) contained 5 μL of RNA (100 ng/μL), 2 × miRNA RT Reaction Buffer, 2 μL miRNA RT Enzyme Mix, and 3 μL H2O, with cycle parameters were 42 °C for 60 s and 95 °C for 3 min. Each miRNA was amplified by specific forward primers (Table 3) and a universal reverse primer (Tiangen, China) for qPCR. The qPCR was conducted using a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad). Each PCR reaction mixture (20 μL) contained 10 μL of 2 × miRcute Plus miRNA Premix, 1 μL of cDNA, 0.4 μL of forward and reverse primers, and 8.2 μL ddH2O. The PCR cycle parameters as follow, 95 °C for 15 min, 40 cycles of 94 °C for 20 s and 60 °C for 34 s. The miRNA expression levels were calculated using the 2−△△CT method. Each sample was run in triplicate. SnRNA was amplified as an internal control51. The statistical significance was measured with one-way ANOVA by SPSS (version 20.0) and the differences were considered to be significant if P < 0.05.
Data Availability
The datasets generated and analyzed during this are available from the corresponding author on reasonable request.
References
Kim, V. N., Han, J. J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10(2), 126–139 (2009).
Miranda, K. C. et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 126(6), 1203–1217 (2006).
Zhu, L. Y. et al. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci. Rep. 5, 16732 (2015).
Ryazansky, S. S., Mikhaleva, E. A. & Olenkina, O. V. Essential functions of microRNAs in animal reproductive organs. Mol. Biol. 48(3), 319–331 (2014).
Desvignes, T. et al. microRNA nomenclature: A view incorporating genetic origins, biosynthetic pathways, and sequence variants. Trends Genet. 31(11), 613–626 (2015).
Hossain, M. M., Sohel, M. M., Schellander, K. & Tesfaye, D. Characterization and importance of microRNAs in mammalian gonadal functions. Cell Tissue Res. 349(3), 679–690 (2012).
Meng, X. L., Zhang, X. H., Li, J. & Liu, P. Identification and comparative profiling of ovarian and testicular microRNAs in the swimming crab Portunus trituberculatus. Gene. 640, 6–13 (2017).
He, L. et al. Profiling microRNAs in the testis during sexual maturation stages in Eriocheir sinensis. Anim. Reprod. Sci. 162, 52–61 (2015).
Ro, S., Song, R., Park, C. H., Sanders, K. M. & Yan, W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA. 13(12), 2366–2380 (2007).
Mishima, T. et al. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction. 136(6), 811–822 (2008).
Li, M. W. M., Mruk, D. D. & Cheng, C. Y. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 15(4), 159 (2009).
Huang, J. et al. Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int. J. Biol. Sci. 7(7), 1016–1026 (2011).
Mcbride, D. et al. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 144(2), 221 (2012).
Liu, S. et al. MicroRNAs show diverse and dynamic expression patterns in multiple tissues of Bombyx mori. BMC Genomics. 11(1), 85 (2010).
Zhang, X., Yuan, L., Li, L., Jiang, H. & Chen, J. Conservation, sex-biased expression and functional annotation of microRNAs in the gonad of Amur sturgeon (Acipenser schrenckii). Comp. Biochem. Physiol, Part D 18, 54–61 (2016).
Mi, X., Wei, Z., Zhou, Z. & Liu, X. L. Identification and profiling of sex-biased microRNAs from sea urchin Strongylocentrotus nudus gonad by Solexa deep sequencing. Comp. Biochem. Physiol., Part D: Genomics Proteomics. 10(1), 1–8 (2014).
Eshel, O. et al. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus). BMC Genomics. 15(1), 774 (2014).
Wang, F. et al. Identification and profiling of Cyprinus carpio microRNAs during ovary differentiation by deep sequencing. BMC Genomics. 18(1), 333 (2017).
Song, Y. N., Shi, L. L., Liu, Z. Q. & Qiu, G. F. Global analysis of the ovarian microRNA transcriptome: implication for miR-2 and miR-133 regulation of oocyte meiosis in the Chinese mitten crab, Eriocheir sinensis (Crustacea:Decapoda). BMC Genomics. 15(1), 547 (2014).
Schnackenberg, B. J., Palazzo, R. E. & Marzluff, W. F. Cyclin E/Cdk2 is required for sperm maturation, but not DNA replication, in early sea urchin embryos. Genesis. 45(5), 282–291 (2010).
Mathias, N. et al. Cdc53p acts in concert with Cdc4p and Cdc34p to control the G1-to-S-phase transition and identifies a conserved family of proteins. Mol. Cell. Biol. 16(12), 6634–6643 (1996).
Bonami, J. R. & Widada, J. S. Viral diseases of the giant fresh water prawn Macrobrachium rosenbergii: A review. J. Invertebr. Pathol. 106(1), 131–142 (2011).
Jiang, X. H. & Qiu, G. F. Female‐only sex‐linked amplified fragment length polymorphism markers support ZW/ZZ sex determination in the giant freshwater prawn Macrobrachium rosenbergii. Anim. Genet. 44(6), 782–785 (2013).
Fu, H. T., Jiang, S. F. & Xiong, Y. W. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (Macrobrachium nipponense) in China. Aquacult. Res. 43(7), 993–998 (2012).
Real, F. M. et al. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol. Reprod. 89(4), 78 (2001).
Jin, S. H. et al. Integrated analysis of microRNA and mRNA expression profiles during the sex-differentiation sensitive period in oriental river prawn, Macrobrachium nipponense. Sci. Rep. 7(1), 12011 (2017).
Mao, J. & Zhang, X. S. The piRNA pathway and spermatogenesis: Advances in studies (In chinese). Int. J. Androl. 22(10), 923–927 (2016).
Mulloy, R., Salinas, S., Philips, A. & Hipskind, R. A. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene. 22(35), 5387–5398 (2003).
Li, Y. et al. FBW7 suppress cell proliferation and G2/M cell cycle transition via promoting γ-catenin K63-linked ubiquitylation. Biochem. Biophys. Res. Commun. 497(2), 473–479 (2018).
Wimuttisuk, W. et al. Insights into the Prostanoid Pathway in the Ovary Development of the Penaeid Shrimp Penaeus monodon. PLoS One. 8(10), e76934 (2013).
Liu, T. H. et al. Identification and characterization of the BmCyclin L1-BmCDK11A/B complex in relation to cell cycle regulation. Cell Cycle. 16(9), 861–868 (2017).
Held, T. et al. Hspa4l-deficient mice display increased incidence of male infertility and hydronephrosis development. Mol. Cell. Biol. 26(21), 8099 (2006).
O’Donovan, K. J., Tourtellotte, W. G., Millbrandt, J. & Baraban, J. M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 22(4), 167–173 (1999).
Shin, H., Kwon, S., Song, H. & Lim, H. J. The transcription factor Egr3 is a putative component of the microtubule organizing center in mouse oocytes. PLoS One. 9(4), e94708 (2014).
Hogarth, C. A., Mitchell, D., Small, C. & Griswold, M. EGR4 displays both a cell- and intracellular-specific localization pattern in the developing murine testis. Dev. Dyn. 239(11), 3106–3114 (2010).
Beak, S. A. et al. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Invest. 101(6), 1334 (1998).
Brixey, R. et al. Examining the role of MAPK signalling in mouse gonad development. Genet. Res. 93, 434 (2011).
Kopczynski, J. et al. Oncogenic activation of the Wnt/β-Catenin signaling pathway in signet ring stromal cell tumor of the ovary. Appl. Immunohistochem. Mol. Morphol. 24(5), e28–33 (2016).
Jordan, B. K. et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am. J. Hum. Genet. 68(5), 1102–1109 (2001).
Du, Y. X., Ma, K. Y. & Qiu, G. F. Discovery of the genes in putative GnRH signaling pathway with focus on characterization of GnRH-like receptor transcripts in the brain and ovary of the oriental river prawn Macrobrachium nipponense. Aquaculture. 442, 1–11 (2015).
Pitetti, J. L. et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 27(5), 814–827 (2013).
Shimizu, H., Morio, T., Shimizu, H. D. & Urushihara, H. A mutation in the cAMP signaling pathway affects sexual development of Dictyostelium discoideum. Dev., Growth Differ. 39(2), 227–234 (1997).
Spears, N., Murray, A. A., Allison, V., Boland, N. I. & Gosden, R. G. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J. Reprod. Fertil. 113(1), 19–26 (1998).
Miao, N., Wang, X., Hou, Y., Feng, Y. & Gong, Y. Identification of male-biased microRNA-107 as a direct regulator for nuclear receptor subfamily 5 group A member 1 based on sexually dimorphic microRNA expression profiling from chicken embryonic gonads. Mol. Cell. Endocrinol. 429, 29–40 (2016).
Gur, G., Bonfil, D., Safarian, H., Naor, Z. & Yaron, Z. GnRH signaling pathways regulate differentially the tilapia gonadotropin subunit genes. Mol. Cell. Endocrinol. 189(1), 125–134 (2002).
Meeratana, P. & Sobhon, P. Classification of differentiating oocytes during ovarian cycle in the giant freshwater prawn, Macrobrachium rosenbergii de man. Aquaculture. 270(1), 249–258 (2007).
Chen, j., Liu, P., Li, Z., Chen, Y. & Qiu, G. F. The cloning of the cdk2 transcript and the localization of its expression during gametogenesis in the freshwater giant prawn, Macrobrachium rosenbergii. Mol. Biol. Rep. 40(8), 4781–4790 (2013).
Friedländer, M. R., Mackowiak, S. D., Li, N., Chen, W. & Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40(1), 37–52 (2012).
Tino, P. Basic properties and information theory of Audic-Claverie statistic for analyzing cDNA arrays. BMC Bioinf. 10(1), 310–310 (2009).
John, B. et al. Human MicroRNA targets. PLoS Biol. 2(11), e363 (2004).
Tan, T. T. et al. Deep parallel sequencing reveals conserved and novel miRNAs in gill and hepatopancreas of giant freshwater prawn. Fish Shellfish Immunol. 35(4), 1061–1069 (2013).
Acknowledgements
This work was supported by the National Key R&D Program of China (project number 2018YFD0900201), Natural Science Foundation of China (project number 31772841), Shanghai University Youth Teacher Training Program (project number ZZSHOU16007), the Shanghai Sailing Program (project number 17YF1408000) and Special Fund for the Development of Science and Technology of Shanghai Ocean University (project number A2-0203-17-100201).
Author information
Authors and Affiliations
Contributions
K.Y.M. and G.F.Q. supervised the project. K.Y.M. designed the experiments. X.L., B.Y.L. and L.X.Z. conducted the experiments. X.L. and J.B.F. conducted the data analyses. X.L. and K.Y.M. wrote the manuscript. K.Y.M. and G.F.Q. finalized the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Luo, BY., Feng, JB. et al. Identification and profiling of microRNAs during gonadal development in the giant freshwater prawn Macrobrachium rosenbergii. Sci Rep 9, 2406 (2019). https://doi.org/10.1038/s41598-019-38648-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38648-x
This article is cited by
-
Integrated mRNA and miRNA expression profile analysis of female and male gonads in Hyriopsis cumingii
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.