Abstract
Rab small GTPases play key roles in intracellular membrane trafficking. Rab33a promotes axon outgrowth of cultured rat hippocampal neurons by mediating the anterograde axonal transport of Golgi-derived vesicles and the concomitant exocytosis of these vesicles at the growth cone. However, the functions of Rab33 in vivo are unclear. Here, we show that zebrafish rab33a and rab33ba are orthologs of mammalian Rab33a and Rab33b, respectively. They are expressed in the developing brain, including in neurons of the telencephalic dorsorostral cluster and the diencephalic ventrorostral cluster, which project axons to form the anterior and postoptic commissures, respectively. Although rab33a single mutant and rab33ba single mutant fish did not show remarkable defects, fish carrying the rab33a;rab33ba double mutations displayed dysgenesis of the anterior and postoptic commissures. Single-cell labeling in the telencephalic dorsorostral cluster demonstrated that the rab33a;rab33ba double mutation inhibits axonal extension in the anterior commissure. These results suggest that Rab33a and Rab33ba mediate axon outgrowth and the formation of the forebrain commissures in the zebrafish brain in a cooperative manner.
Similar content being viewed by others
Introduction
Axon outgrowth requires rapid expansion of the plasma membrane1. Axonal membrane expansion is mediated by multiple processes, including membrane synthesis at the rough endoplasmic reticulum and Golgi apparatus in the cell body, vesicular transport along the axonal shaft, and vesicular exocytosis at the growth cone1,2,3,4,5. Rab family proteins are key regulators of intracellular vesicular trafficking pathways6,7,8,9,10. They localize to specific membrane compartments and function as molecular switches that cycle between the GTP-bound active form and the GDP-bound inactive form6,7,8,9,10. The GTP-bound active form recruits and activates specific effectors such as sorting adaptors, tethering factors, kinases, phosphatases and motor proteins, thereby regulating the specificity and directionality of vesicular trafficking7,8,9,10.
Rab33a11 is expressed in cells, including neurons, lymphocytes, melanocytes and parotid acinar cells12,13,14,15. In cultured rat hippocampal neurons, Rab33a is localized to the Golgi apparatus and post-Golgi vesicles transported along axons14. With regard to the mechanism for axonal membrane expansion, our previous study with cultured hippocampal neurons demonstrated that Rab33a promotes anterograde axonal transport of the post-Golgi vesicles, which is associated with vesicular exocytosis at the growth cones and axon outgrowth14. Rab33a is also reported to be involved in the regulation of vesicular exocytosis in parotid acinar cells, PC12 cells and umbilical vein epithelial cells15,16,17. In addition, Rab33a interacts with singar1/RUFY318, which suppresses the formation of surplus axons in cultured hippocampal neurons19. However, the role of Rab33a in axon outgrowth in vivo remains unclear.
Rab33b is another Rab33 protein that is expressed ubiquitously in mouse tissues and is localized in the Golgi apparatus20. Rab33b interacts with Golgi proteins such as GM130, rabaptin-5 and rabex-521, and modulates autophagosome formation by interacting with Atg16L22. In addition, mutations in human RAB33B are found in patients with an autosomal recessive skeletal dysplasia, Smith–McCort dysplasia23,24,25. In zebrafish, three rab33 genes, rab33a, rab33ba and rab33bb, have been identified26. While rab33a and rab33ba are highly conserved in vertebrates, rab33bb is reported only in zebrafish.
In this study, we analyzed the functions of Rab33a and Rab33ba in the zebrafish forebrain, an easily accessible system for the analyses of axon tract formation in vivo27,28,29. We show that zebrafish rab33a and rab33ba are orthologs of mammalian Rab33a and Rab33b, respectively, and that rab33a and rab33ba mediate the outgrowth of forebrain commissural axons in the developing zebrafish brain.
Results
Zebrafish rab33a and rab33ba are orthologs of mammalian Rab33a and Rab33b, respectively
Zebrafish rab33a, rab33ba and rab33bb encode 236-, 239- and 227-amino acid (aa) proteins, respectively26 (Supplementary Fig. S1a). Zebrafish Rab33a has 74.9% identity with human RAB33A and 53.8% identity with human RAB33B, whereas zebrafish Rab33ba has 53.4% identity with human RAB33A and 66.3% identity with human RAB33B. On the other hand, zebrafish Rab33bb has 48.1% identity with human RAB33A and 47.3% identity with human RAB33B. Phylogenetic analysis revealed that zebrafish rab33 genes are clustered into separate groups (Supplementary Fig. S1b). Zebrafish rab33a and mammalian Rab33a belong to the same group, while zebrafish rab33ba and mammalian Rab33b belong to another group, which is distinct from the group of zebrafish rab33bb (Supplementary Fig. S1b).
Synteny analyses in human, mouse, chicken, Xenopus, zebrafish, medaka and fugu genomes showed that human, mouse, chicken and Xenopus rab33b share conserved synteny with zebrafish, medaka and fugu rab33ba (Supplementary Fig. S2c). In the case of rab33a, synteny was observed among human, mouse, chicken and Xenopus, but disrupted in fish (zebrafish, medaka and fugu) (Supplementary Fig. S2a). We also observed disrupted synteny between slc25a14 and aifm1, which flank human, mouse and chicken rab33a, in zebrafish (Supplementary Fig. S2b). On the other hand, we identified rab33bb only in zebrafish (Supplementary Fig. S2d). These data indicate that zebrafish rab33ba shares synteny with mammalian rab33b but not with mammalian rab33a. Together, the present data suggest that zebrafish rab33a and rab33ba are orthologs of mammalian Rab33a and Rab33b, respectively.
Zebrafish rab33a and rab33ba are expressed in forebrain commissural neurons
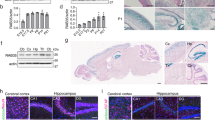
To examine the expression of rab33a and rab33ba in zebrafish embryos, we first performed RT-PCR using specific primers. RT-PCR analysis detected the expression of rab33a and rab33ba at 0, 24, 36 and 48 hours postfertilization (hpf) (Fig. 1a). Whole-mount in situ hybridization detected rab33a and rab33ba expression in the developing brain (Fig. 1b–e and Supplementary Fig. S3). At 24 hpf, rab33a was expressed in the forebrain, with high levels of the signal in the telencephalic dorsorostral cluster (DRC) (arrowheads, Fig. 1b′) and diencephalic ventrorostral cluster (VRC) (arrows, Fig. 1b′). These neuronal clusters are also referred as the nucleus of the tract of the anterior commissure and the nucleus of the tract of the postoptic commissure, respectively30. On the other hand, rab33ba was expressed widely in the forebrain including the DRC and VRC of 24 hpf embryos (arrowheads and arrows, Fig. 1c′). Neurons in the DRC and VRC start to extend axons at 20 hpf and form axonal tracts of the anterior commissure and postoptic commissure, respectively, by 36 hpf27,28,30. The expression of rab33a and rab33ba was also detected in the forebrain including the DRC and VRC of 36 hpf embryos (arrowheads and arrows, Fig. 1d′,e′), suggesting that these genes are expressed during axonal extension in the anterior and postoptic commissures. In addition, we detected the expression of rab33a and rab33ba in the hindbrain of 24 hpf and 36 hpf embryos (arrows, Fig. 1b–e).
Expression of zebrafish rab33a and rab33ba. (a) RT-PCR analysis of rab33a and rab33ba transcripts. Elongation factor 1a (EF1a) was used as a control. Developmental stages are denoted as hours post-fertilization (hpf). RT-PCR products produced in the presence (RT+) or absence (RT−) of reverse transcriptase were electrophoresed on 2% agarose gels. (b,c) Whole-mount in situ hybridization of rab33a (b) and rab33ba (c) at 24 hpf; (b′) and (c′) show the enlarged lateral (left) and ventral (right) views of the areas indicated by the rectangles. (d,e) Whole-mount in situ hybridization of rab33a (d) and rab33ba (e) at 36 hpf; (d′) and (e′) show the enlarged lateral (left) and ventral (right) views of the areas indicated by the rectangles. Arrowheads and arrows in (b′–e′) indicate the DRC and VRC, respectively. Arrows in (b–e) indicate the hindbrain. See the negative control data for whole-mount in situ hybridization (Supplementary Fig. S3). Scale bars: 100 μm.
Rab33a and Rab33ba mediate the formation of forebrain commissures
The expression of rab33a and rab33ba in the DRC and VRC suggest that Rab33a and Rab33ba may have a role in the formation of the anterior and postoptic commissures. To analyze the functions of rab33a and rab33ba, we generated rab33a and rab33ba mutants using the CRISPR/Cas9 system. To generate rab33a mutants, we injected rab33a gRNA and Cas9 mRNA into wild-type fertilized eggs. The injected fish were raised to adulthood and crossed with wild-type fish. The rab33a mutant allele contained a 10-bp deletion in the first exon, resulting in a frame shift and a premature stop codon after 13 aa (Fig. 2a and Supplementary Fig. S4a,b). To generate rab33ba mutants, we injected rab33ba gRNA and Cas9 mRNA into fertilized eggs from the rab33a heterozygous mutant fish. The injected fish were raised and crossed with wild-type fish. The rab33ba mutant allele harbored a 2-bp deletion (5-bp deletion and 3-bp insertion) in the first exon, resulting in a premature stop codon after 58 aa (Fig. 2a and Supplementary Fig. S5a,b). We identified homozygous and heterozygous fish for rab33a and rab33ba mutations by T7EI-based genotyping (Supplementary Figs S4c and S5c).
rab33a;rab33ba double mutants display a reduced cross-sectional area of the anterior commissure. (a) Schematic structures of Rab33a and Rab33ba proteins in wild-type and single mutant fish. Frameshift mutations in rab33a and rab33ba result in premature stop codons after aa positions 13 and 58, respectively. The gray boxes indicate amino acids added by the frameshift mutations, and the numbers in brackets indicate the numbers of these additional residues. The regions involved in GTP/GDP-binding and GTPase activity11,17 are indicated by the orange color. (b) A representative lateral view of a 36 hpf wild-type zebrafish brain immunolabeled with anti-acetylated tubulin antibody. See Movie 1. Abbreviations: AC, anterior commissure; POC, postoptic commissure. Scale bar: 100 μm. (c–i) Frontal views (upper panels) of the anterior commissures of wild-type control (c), rab33a−/− single mutant (d), rab33ba−/− single mutant (e) and rab33a−/−;rab33ba−/−double mutant (f) embryos at 36 hpf. In (g), rab33a and rab33ba mRNAs were injected into a rab33a−/−;rab33ba−/− double mutant embryo for rescue analysis. In (h,i) rab33a mRNA (h) and rab33ba mRNA (i) were injected into a rab33a−/−;rab33ba−/− double mutant embryo for rescue analysis. Scale bars: 50 µm. The lower panels show cross-sections at the dotted lines shown in the frontal views. Scale bars: 10 µm. (j) The cross-sectional area of the anterior commissure obtained from the data analyses in (c–i, lower panels). Wild-type control (n = 17), rab33a−/− single mutant (n = 18), rab33ba−/− single mutant (n = 18) and rab33a−/−;rab33ba−/− double mutant (n = 17) embryos, and rab33a−/−;rab33ba−/− double mutant embryos with rab33a and rab33ba mRNAs (n = 22), rab33a mRNA (n = 22) and rab33ba mRNA (n = 22) were analyzed at 36 hpf. Data are mean ± SEM; ***P < 0.01; ns, not significant (one-way ANOVA with Tukey’s post hoc test).
The DRC and VRC neurons start to develop axons at 24 hpf and form axonal tracts of the anterior and postoptic commissures by 36 hpf27,30,31. To visualize these axonal tracts, we performed whole-mount immunohistochemistry using anti-acetylated tubulin antibody27,29,30 (Fig. 2b). The anterior commissures in control and mutant embryos were analyzed at 36 hpf (Movie 1), and their cross-sectional areas were quantified at the midline (Fig. 2c–e). In control embryos, the cross-sectional area of the anterior commissure was 189.4 ± 10.1 μm2 (n = 17 embryos) (Fig. 2c,j), similar to that of the rab33a single mutant and the rab33ba single mutant embryos (Fig. 2d,e,j). We also generated a rab33a;rab33ba double mutant (Fig. 2f); the rab33a;rab33ba double mutation led to a significant reduction in the cross-sectional area of the anterior commissure (Fig. 2f,j). When rab33a and rab33ba mRNAs were injected into the double mutant embryos, the reduced cross-sectional area of the anterior commissure was rescued to a level similar to that of control embryos (Fig. 2g,j). Injection of rab33a mRNA or rab33ba mRNA into the double mutant also rescued the reduced cross-sectional area of the anterior commissure (Fig. 2h–j).
We also analyzed the formation of the postoptic commissure in control and mutant embryos at 36 hpf (Fig. 3). In control embryos, the cross-sectional area of the postoptic commissure was 164.8 ± 12.4 μm2 (n = 11 embryos) (Fig. 3a,h), similar to that of the rab33a single mutants and the rab33ba single mutants (Fig. 3a–c,h). However, the cross-sectional area of the postoptic commissure was significantly reduced in embryos carrying the rab33a;rab33ba double mutation (Fig. 3d,h). Furthermore, injection of rab33a and rab33ba mRNAs, single rab33a mRNA or single rab33ba mRNA into the double mutant embryos rescued the reduced cross-sectional area of the postoptic commissure (Fig. 3e–h). The body length, head size and distance between eyes were similar among the wild-type, rab33a single mutant, rab33ba single mutant and rab33a;rab33ba double mutant fish (Supplementary Fig. S6), thus ruling out the possibility that developmental delays in the double mutant fish results in dysgenesis of the anterior and postoptic commissures. Together, these data indicate that Rab33a and Rab33ba mediate the formation of the anterior and postoptic commissures.
rab33a;rab33ba double mutants display a reduced cross-sectional area of the postoptic commissure. (a–g) Representative frontal views (upper panels) of the postoptic commissures of wild-type control (a), rab33a−/− single mutant (b), rab33ba−/− single mutant (c) and rab33a−/−;rab33ba−/− double mutant (d) embryos at 36 hpf. In (e), rab33a and rab33ba mRNAs were injected into a rab33a−/−;rab33ba−/− double mutant embryo for rescue analysis. In (f,g), rab33a mRNA (f) or rab33ba mRNA (g) were injected into a rab33a−/−;rab33ba−/− double mutant embryo for rescue analysis. Scale bars: 50 µm. The lower panels show the cross-sections at the dotted lines shown in the frontal views. Scale bars: 10 µm. (h) The cross-sectional area of the postoptic commissure obtained from the data analyses in (a–g; lower panels). Wild-type control (n = 11), rab33a−/− single mutant (n = 11), rab33ba−/− single mutant (n = 14) and rab33a−/−;rab33ba−/− double mutant (n = 14) embryos, and rab33a−/−;rab33ba−/− double mutant embryos with rab33a and rab33ba mRNAs (n = 13), rab33a mRNA (n = 14) and rab33ba mRNA (n = 10) were analyzed at 36 hpf. Data are mean ± SEM; ***P < 0.01; **P < 0.02; ns, not significant (one-way ANOVA with Tukey’s post hoc test).
Rab33a and Rab33ba mediate the outgrowth of anterior commissural axons
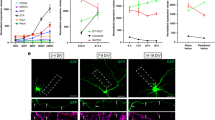
Dysgenesis of the anterior and postoptic commissures in the rab33a;rab33ba double mutant fish raises the possibility that these molecules promote outgrowth of the forebrain commissure axons cooperatively. Zhang et al.29 reported a method to trace the projection of individual axons in the anterior commissure using the Gal4/UAS system. To analyze the axonal projection from DRC neurons in the anterior commissure, we coinjected a plasmid carrying emx3:Gal4FF and a plasmid carrying UAS:tdTomato into fertilized eggs, as described previously29. Under the telencephalon-specific enhancer emx3, Gal4FF activates the expression of tdTomato in telencephalic neurons. Figure 4a shows a single tdTomato-labeled DRC neuron in a wild-type 36 hpf embryo. At this stage, most of the labeled axons in the anterior commissure projected to the contralateral DRC (Fig. 4b), The mean length of axons in the anterior commissure was 209.2 ± 13.1 (n = 9 cells) (Fig. 4e). On the other hand, the mean axon length in the rab33a;rab33ba double mutants was 114.5 ± 20.9 (n = 9 cells), which is significantly shorter than that observed in control embryos (Fig. 4c–e). These results indicate that Rab33a and Rab33ba are involved in the outgrowth of the anterior commissural axons.
The rab33a;rab33ba double mutation inhibits axonal extension in the anterior commissure. (a,c) Representative dorsal views (upper panels) of the anterior commissures immunolabeled with anti-acetylated tubulin antibody and individual DRC neurons expressing tdTomato in wild-type control (a) and rab33a−/−;rab33ba−/− double mutant (c) embryos at 36 hpf. The lower panels show only the tdTomato-labeled neurons in the upper panels. The tips of the axons are indicated by arrows. Scale bars: 50 µm. (b,d) The trajectories of individual axons of the tdTomato-labeled DRC neurons in wild-type control (b) and rab33a−/−;rab33ba−/− double mutant (e) embryos at 36 hpf. The cell body positions are normalized at (x = 0 μm, y = 0 μm). (e) The length of the axon of DRC neurons in the anterior commissure obtained from the data analyses in (b,d). Axons of wild-type control (n = 9) and rab33a−/−;rab33ba−/− double mutant (n = 9) neurons were analyzed at 36 hpf. Data are expressed as mean ± SEM; ***P < 0.01 (unpaired Student’s t test).
Discussion
In this study, we showed that rab33a and rab33ba are expressed in the telencephalic DRC and diencephalic VRC, from which neurons project axons to the anterior and postoptic commissures, respectively. The rab33a;rab33ba double mutant fish displayed dysgenesis of the anterior and postoptic commissures. Furthermore, the rab33a;rab33ba double mutation inhibited axonal extension in the anterior commissure. These results suggest that Rab33a and Rab33ba promote the outgrowth of forebrain commissural axons and the formation of forebrain commissures in the developing zebrafish brain. To our knowledge, this is the first report that describes the role of Rab33a in mediating axon outgrowth in vivo. In addition, our data provide the first evidence that zebrafish Rab33ba, an ortholog of mammalian Rab33b, is involved in axon outgrowth.
Recent studies reported mutations of RAB33B in human patients with Smith–McCort dysplasia23,24,25. Smith-McCort dysplasia is a skeletal dysplasia characterized by a short neck and short trunk dwarfism with a barrel-shaped chest and rhizomelic limb shortening. In the present study, we did not observe these severe phenotypes in the body of the rab33a;rab33ba double mutant fish. This appears to be consistent with the in situ hybridization data, which showed that the bodies of zebrafish embryos lacked clear signals for rab33a and rab33ba. As rab33a and rab33ba are expressed in the hindbrain and other forebrain regions, we do not rule out the possibility that they also mediate axon outgrowth of the neurons located in these regions. Further analyses in the hindbrain and other forebrain regions with suitable markers will be required to detect additional defects.
Concerning the molecular mechanism for Rab33a- and Rab33ba-mediated axon outgrowth, rab33a single mutant and rab33ba single mutant fish did not show remarkable defects although the rab33a;rab33ba double mutant fish displayed dysgenesis of the forebrain commissures. In addition, injection of single rab33a mRNA or single rab33ba mRNA into the double mutant embryos rescued the dysgenesis of the forebrain commissures in the rab33a;rab33ba double mutant. Possible functional redundancy between Rab33a and Rab33ba suggests that these molecules may promote axonal extension thorough a similar mechanism. Our previous study reported that Rab33a in cultured rat hippocampal neurons promotes axonal extension by mediating anterograde axonal transport of post-Golgi vesicles and their concomitant exocytosis at the growth cone14. Thus, it is likely that zebrafish Rab33a promotes extension of the forebrain commissural axons by mediating axonal transport of post-Golgi vesicles. In addition, since mammalian Rab33b is also localized in the Golgi apparatus20,21,22, zebrafish Rab33ba may promote axon outgrowth in a similar manner. At present, the effectors of Rab33a that mediate the axonal transport of the post-Golgi vesicles are unknown. The detailed molecular mechanisms how Rab33a and Rab33ba promote axon outgrowth remain for future analyses.
Methods
Zebrafish husbandry
All relevant aspects of the experimental procedures were approved by the Institutional Animal Care and Use Committee of Nara Institute of Science and Technology (reference No. 1321 and 1811) and were performed in accordance with relevant guidelines and regulations. Embryos were obtained from wild-type and mutant fish and were raised at 28.5 °C as described previously32.
RT-PCR and cDNA cloning
Total RNA was purified from zebrafish embryos using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. The RNA sample was used to synthesize cDNA using MLV RT (H-) Point Mutant (Promega) with primer AP (Supplementary Table S1) for reverse transcription. Specific cDNAs were PCR-amplified using the following primers: rab33a-h and rab33a-t for rab33a, and rab33ba-h and rab33ba-t for rab33ba. We used EF1a-f and EF1a-r primers as a positive control. The primers used are listed in Supplementary Table S1. The cDNAs were cloned into pGEM-T and sequenced using an ABI PRISM 3130 (Applied Biosystems).
DNA electrophoresis
The DNA electrophoresis in Fig. 1a and Supplementary Figs S4c, S5c and S8c–g was performed using agarose gels. The images in Fig. 1a and Supplementary Fig. S8c–g were cropped from full-length gel images in Supplementary Figs S7 and S9, respectively.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described previously33. The following plasmids were constructed for synthesizing in situ probes. For constructing pCRII-rab33a, rab33a was PCR-amplified using the primers rab33a-h and rab33a-t and cloned into pCRII-TOPO (Invitrogen). For constructing pCRII-rab33ba, rab33ba in pGEM-T was subcloned into pCRII-TOPO. Plasmids pCRII-rab33a and pCRII-rab33ba were digested with NotI, and antisense probes of rab33a and rab33ba were synthesized using SP6 RNA polymerase (Roche). Plasmids pCRII-rab33a and pCRII-rab33ba were digested with BamHI, and sense probes of rab33a and rab33ba were synthesized using T7 RNA polymerase (Roche). All in situ probes were synthesized from cDNAs using the DIG RNA labeling kit (Roche), according to the manufacturer’s instructions. Images were acquired using a Leica MZFL III and processed using Adobe Photoshop Elements 12 and Fiji34.
Generation of zebrafish rab33a and rab33ba mutants
Zebrafish mutants of rab33a and rab33ba were generated using the CRISPR/Cas9 system35. Vectors for customized guide RNAs (gRNAs) were constructed as described previously35. Plasmid pT7-rab33a was constructed by cloning the two annealed oligonucleotides rab33a-f-ex1 and rab33a-r-ex1. pT7-rab33ba was constructed by cloning the two annealed oligonucleotides rab33ba-f-ex1 and rab33ba-r-ex1. To generate rab33a mutants, the gRNAs and Cas9 mRNA were synthesized and injected into wild-type fertilized eggs as described previously35. The injected embryos were raised and crossed with wild-type zebrafish. To generate rab33ba mutants, we injected rab33ba gRNA and Cas9 mRNA into fertilized eggs from the rab33a heterozygous mutant fish. The injected fish were raised and crossed with wild-type fish. To identify rab33a and rab33ba mutations, a T7EI assay was performed as described previously35. We used rab33a-ex1-5′ and rab33a-ex1–3′ primers for rab33a, and rab33ba-ex1–5′ and rab33ba-ex1–3′ primers for rab33ba (Supplementary Table S1). The PCR products were sequenced using an ABI PRISM3130 (Applied Biosystems). We confirmed the mutations of rab33a and rab33ba by genotyping analyses (Supplementary Figs S4 and S5). In addition, RT-PCR analyses confirmed the absence of the wild-type rab33a transcript in rab33a single mutant or rab33a;rab33ba double mutant embryos (Supplementary Fig. S8d). Mutant rab33ba transcript expression was detected in rab33ba single mutant and rab33a;rab33ba double mutant embryos, but not in wild-type or rab33a single mutant embryos (Supplementary Fig. S8f). We used rab33a-WT-5′ and rab33a-t primers to detect wild-type rab33a transcript, and rab33ba-MU-5′ and rab33ba-t primers to detect the mutant rab33ba transcript (Supplementary Table S1).
Genotyping
T7EI-mediated genotyping was performed using the primers rab33a-ex1–5′ and rab33a-ex1–3′ to identify rab33a mutations, and rab33ba-ex1–5′ and rab33ba-ex1–3 to identify rab33ba mutations. The genotyping was performed using two different T7EI assays. The first T7EI assay was performed as described previously35. In the second T7EI assay, PCR products obtained from samples were mixed with those from the wild type before denaturation at 94 °C for 5 min, annealing at room temperature and digestion of the annealed products with T7EI. The first T7EI assay distinguished heterozygous fish from wild-type and homozygous fish. The second T7EI assay distinguished between homozygous and wild-type fish.
Microinjection
Microinjection was performed as described previously35. Fertilized eggs were injected with gRNAs (50 pg/embryo) and Cas9 mRNA (300 pg/embryos). In rescue experiments, fertilized eggs were injected with rab33a mRNA (25 pg/embryo) and rab33ba mRNA (25 pg/embryo).
Whole-mount immunohistochemistry
Whole-mount immunohistochemistry was performed as described previously36, with slight modifications. Embryos were fixed in 4% formaldehyde (PFA) overnight at 4 °C and washed and blocked with blocking buffer (0.5% Triton-X, 4% normal goat serum, and 0.1% BSA in phosphate buffer) for 2 h at room temperature. The embryos were incubated with anti-acetylated tubulin antibody (Sigma) (1:1000) to label axonal tracts, followed by incubation with Alexa Fluor 488-conjugated anti-mouse IgG (Molecular Probes) (1:500) overnight at 4 °C.
Measurement of head size and distance between eyes
The head size and distance between eyes of the control and mutant fish were measured as described previously37.
Microscopy
Embryos were embedded in 1% low melting point agarose (Invitrogen). To take confocal images of the postoptic commissure, we removed the yolks from embryos with forceps. Images were captured with a Zeiss LSM710 and the cross-sections of the midline of the forebrain commissures were constructed using Imaris 8.0 and Fiji34. The cross-sectional areas were measured using Fiji.
Genetic single-cell labeling
A mixture of two plasmids emx3:Gal4FF and UAS:tdTomato (10 ng/μl each) was injected into one-cell-stage embryos, as described previously29. The injected embryos expressing tdTomato were screened by a fluorescent dissection microscopy at 24 hpf and fixed at 36 hpf. The embryos were immunostained with anti-acetylated tubulin antibody and observed by a Zeiss LSM710. Axon length was measured using Fiji.
Statistical analysis
Results are expressed as mean ± standard error (SEM). Statistical analyses were performed with GraphPad Prism 7. Statistical significance was determined by the unpaired Student’s t test. For multiple comparisons, we used one-way ANOVA with Tukey’s post hoc test.
References
Pfenninger, K. H. Plasma membrane expansion: a neuron’s Herculean task. Nat. Rev. Neurosci. 10, 251–261 (2009).
Bray, D. Surface movements during the growth of single explanted neurons. Proc. Natl. Acad. Sci. USA 65, 905–910 (1970).
Lockerbie, R. O., Miller, V. E. & Pfenninger, K. H. Regulated plasmalemmal expansion in nerve growth cones. J. Cell. Biol. 112, 1215–1227 (1991).
Craig, A. M., Wyborski, R. J. & Banker, G. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature 375, 592–594 (1995).
Futerman, A. H. & Banker, G. A. The economics of neurite outgrowth–the addition of new membrane to growing axons. Trends. Neurosci. 19, 144–149 (1996).
Pfeffer, S. R. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491 (2001).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 (2001).
Fukuda, M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 65, 2801–2813 (2008).
Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009).
Zhen, Y. & Stenmark, H. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 128, 3171–3176 (2015).
Koda, T. & Kakinuma, M. Molecular cloning of a cDNA encoding a novel small GTP-binding protein. FEBS Lett. 328, 21–24 (1993).
Cheng, E. et al. Rab33A: characterization, expression, and suppression by epigenetic modification. J. Invest. Dermatol. 126, 2257–2271 (2006).
Lee, M. S. et al. Selection of neural differentiation-specific genes by comparing profiles of random differentiation. Stem Cells 24, 1946–1955 (2006).
Nakazawa, H. et al. Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J. Neurosci. 32, 12712–12725 (2012).
Imai, A., Tsujimura, M., Yoshie, S. & Fukuda, M. The small GTPase Rab33A participates in regulation of amylase release from parotid acinar cells. Biochem. Biophys. Res. Commun. 461, 469–474 (2015).
Ishibashi, K., Uemura, T., Waguri, S. & Fukuda, M. Atg16L1, an essential factor for canonical autophagy, participates in hormone secretion from PC12 cells independently of autophagic activity. Mol. Biol. Cell 23, 3193–3202 (2012).
Zografou, S. et al. A complete Rab screening reveals novel insights in Weibel-Palade body exocytosis. J. Cell Sci. 125, 4780–4790 (2012).
Fukuda, M., Kobayashi, H., Ishibashi, K. & Ohbayashi, N. Genome-wide investigation of the Rab binding activity of RUN domains: development of a novel tool that specifically traps GTP-Rab35. Cell Struct. Funct. 36, 155–170 (2011).
Mori, T., Wada, T., Suzuki, T., Kubota, Y. & Inagaki, N. Singar1, a novel RUN domain-containing protein, suppresses formation of surplus axons for neuronal polarity. J. Biol. Chem. 282, 19884–19893 (2007).
Zheng, J. Y. et al. A novel Rab GTPase, Rab33B, is ubiquitously expressed and localized to the medial Golgi cisternae. J. Cell Sci. 111, 1061–1069 (1998).
Valsdottir, R. et al. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 508, 201–209 (2001).
Itoh, T. et al. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol. Biol. Cell 19, 2916–2925 (2008).
Alshammari, M. J., Al-Otaibi, L. & Alkuraya, F. S. Mutation in RAB33B, which encodes a regulator of retrograde Golgi transport, defines a second Dyggve–Melchior–Clausen locus. J. Med. Genet. 49, 455–461 (2012).
Dupuis, N. et al. A novel RAB33B mutation in Smith-McCort dysplasia. Hum. Mutat. 34, 283–286 (2013).
Salian, S. et al. Additional three patients with Smith-McCort dysplasia due to novel RAB33B mutations. Am. J. Med. Genet. A 173, 588–595 (2017).
Hall, T. E., Martel, N., Lo, H. P., Xiong, Z. & Parton, R. G. A plasmid library of full-length zebrafish rab proteins for in vivo cell biology. Cell. Logist. 7, e1301151 (2017).
Chitnis, A. B. & Kuwada, J. Y. Axonogenesis in the brain of zebrafish embryos. J. Neurosci. 10, 1892–1905 (1990).
Ross, L. S., Parrett, T. & Easter, S. S. Jr. Axonogenesis and morphogenesis in the embryonic zebrafish brain. J. Neurosci. 12, 467–482 (1992).
Zhang, C., Gao, J., Zhang, H., Sun, L. & Peng, G. Robo2-Slit and Dcc-Netrin1 coordinate neuron axonal pathfinding within the embryonic axon tracts. J. Neurosci. 32, 12589–12602 (2012).
Barresi, M. J., Hutson, L. D., Chien, C. B. & Karlstrom, R. O. Hedgehog regulated Slit expression determines commissure and glial cell position in the zebrafish forebrain. Development 132, 3643–3656 (2005).
Wilson, S. W., Ross, L. S., Parrett, T. & Easter, S. S. Jr. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development 108, 121–145 (1990).
Westerfield, M. The zebrafish book. University of Oregon Press (2007).
Schulte-Merker, S. Looking at embryos. In zebrafish: a practical approach. Oxford Univ Press, 39–58 (2002).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Jao, L. E., Wente, S. R. & Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 110, 13904–13909 (2013).
Karlstrom, R. O. et al. Zebrafish mutations affecting retinotectal axon pathfinding. Development 123, 427–438 (1996).
Loviglio, M. N. et al. The immune signaling adaptor LAT contributes to the neuroanatomical phenotype of 16p11.2 BP2-BP3 CNVs. Am. J. Hum. Genet. 101, 564–577 (2017).
Acknowledgements
We thank Seiya Morishita, Kosuke Naka, Minato Uozumi, Emiko Watase, Maiko Yokouchi, Hiroko Shigesato, Mieko Ueda and Kouki Abe for maintaining the zebrafish, Dr. Gang Peng for providing the emx3:Gal4FF and UAS:tdTomato plasmids, Dr. Takaaki Matsui, Dr. Yasumasa Bessho and Dr. Yasumasa Ishida for helpful discussions. This research was supported in part by a JSPS Grant-in-Aid for Scientific Research on Innovative Areas (JP25102010), JSPS KAKENHI (JP23370088, JP26290007 and JP 26840081), AMED under Grant Number JP18gm0810011, the Foundation for Nara Institute of Science and Technology and the Osaka Medical Research Foundation for Incurable Diseases.
Author information
Authors and Affiliations
Contributions
H.L.-G., A.U. and N.I. conceived and designed the experiments. H.L.-G. and A.U. conducted the experiments and analyzed data. H.L.-G., A.U. and N.I. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, L., Urasaki, A. & Inagaki, N. Rab33a and Rab33ba mediate the outgrowth of forebrain commissural axons in the zebrafish brain. Sci Rep 9, 1799 (2019). https://doi.org/10.1038/s41598-018-38468-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38468-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.