Abstract
Falls in late postmenopausal women with osteopenia usually cause fractures with severe consequences. This 36-month randomized, double-blind and placebo-controlled trial with a 10-year observational follow-up study aimed to investigate the long-term effect of herbal formula Bushen Yijing Fang (BSYJF) on fall risk in the late postmenopausal women with osteopenia. 140 late postmenopausal women (Femoral neck T-score, −2.5~−2 SD) were recruited and randomized to orally receive calcium carbonate 300 mg daily with either BSYJF or placebo for 36 months. The effect was further investigated for another 10-year follow-up. During the 36-month administration, there were 12 falls in BSYJF group and 28 falls in placebo group, respectively, indicating 64% lower risk of falls (RR 0.36 [95% CI, 0.18 to 0.71]; P = 0.004) in BSYJF group. During the 10-year follow-up, 36% lower fall risk (RR 0.64 [95% CI, 0.46 to 0.89]; P = 0.009) was observed in BSYJF group. No significant difference was found in safety profile between two groups. Thirty-six-month administration of BSYJF reduced fall risk with an increase in bone mass, and its latent effect on fall risk was continually observed in the 10-year follow-up in late postmenopausal women with osteopenia. This clinical trial was registered at Chinese clinical trial registry (ChiCTR-IOR-16008942).
Similar content being viewed by others
Introduction
Falls commonly happen in late postmenopausal women1. Fall-related fractures in late postmenopausal women, e.g. hip fracture, usually cause disability and even mortality2,3, which could be induced by a combination of bone-dependent factors and falls3,4,5. Bone mineral density (BMD) is commonly used to diagnose osteoporosis (T score ≤ −2.5) and to predict individual fracture risk6. However, many fractures occur in postmenopausal women who have non-osteoporotic BMD values (T score > −2.5)7. Therefore, the bone-independent risk factor, such as the tendency to fall, needs to be taken into consideration to reduce fall-related fractures in late postmenopausal women with osteopenia. Both decreased muscle mass and impaired functional mobility (muscular and neurological) are two important risk contributors to falls8. Thus, the two contributors should be targeted to reduce fall risk for preventing fall-related fracture in late postmenopausal women with osteopenia.
To date, there is still no effective pharmacological agent to prevent falls in clinical. Many clinical trials have been performed to study the effects of Vitamin D on fracture and falls9,10. Although Vitamin D supplements show beneficial effects on muscle function5,11. However, a recently updated meta-analysis concludes that vitamin D supplements do not prevent fracture and falls12. Therefore, it is desirable to develop a therapeutic agent which has potential to reduce falls.
The Chinese herbal formula “Bushen Yijing Fang” (BSYJF), approved by China Food and Drug Administration (CFDA No. Z20090656) and commercially available as a patent drug, has been prescribed as “bone-invigorating” and “muscle-strengthening” drug by Practitioners to treat musculoskeletal disorders in China13. Our experimental data in aged ovariectomized (OVX) rats showed that BSYJF could attenuate the decrease of muscle fiber cross-sectional area (CSA), the twitch force of extensor digitorum longus (EDL) (Appendix Fig. 1) and elevate the mRNA of myosin heavy chain that responsible for performing sustained and tonic contractile activities14,15,16. Furthermore, BSYJF showed beneficial effect on neurological function in aging rats17. The evidence implied the potential of BSYJF to both maintain the muscle mass and improve the functional mobility muscularly and neurologically. Besides of the animal data, the short-term clinical observation also demonstrated that BSYJF could potentially increase bone mass in both osteoporotic rodents and postmenopausal women18,19,20,21,22.
In this study, we performed a 36-month randomized, double-blind and placebo-controlled trial in late postmenopausal women with osteopenia to investigate efficacy of BSYJF on fall risk and long-term safety in the 36-month administration. Further, the latent effect on fall risk was also evaluated in a 10-year observational follow-up after completing the 36-month trial. Moreover, we explored and validated the underlying mechanism of BSYJF by bioinformatics analysis.
Methods
Study design
This study was a 36-month randomized, double-blind and placebo-controlled trial with a 10-year observational follow-up. As the trial registration regulation wasn’t implemented in China when this trial commenced in 2000, this study was retrospectively registered in Chinese clinical trial registry (ChiCTR-IOR-16008942; Date of Registration: 30/07/2016). The original study design didn’t include a 10-year observational follow-up. To further investigate the latent effect of BSYJF on fall risk in late post-menopausal women, we conducted a 10-year observational follow-up after completing the 36-month trial. All experimental protocols were approved by the local ethics committee of Shanghai Academy of Traditional Chinese Medicine and all methods were performed in accordance with the relevant guidelines and regulations (Approval No. SZYGSLL-1999-002).
Participants and setting
This trial was conducted in two sites in Shanghai (Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine & Institute of Orthopedics and Traumatology in Shanghai Academy of Traditional Chinese Medicine). All the participants were recruited via advertisement and provided written informed consent. Community-dwelling women (postmenopausal ≥10 years, aged 55~69 years) were invited via medical records and outreach materials. The women who had femoral neck T-score between −2.5~−2 SD for 12 months were included. The subjects were excluded if they (1) were diagnosed with neurological or musculoskeletal disorder, or chronic diseases, (2) took estrogen, calcitonin, fluoride, bisphosphonates, or adrenocortical hormone within one year, (3) took ≥ 4 prescription medications, (4) had postural hypotension: drop in systolic blood pressure ≥ 20 mmHg or to < 90 mmHg on standing, (5) had environmental hazards for falls or tripping, (6) had impairment in gait, (7) had impairment in transfer skills or balance, (8) had impairment in leg or arm muscle strength or range of motion (hip, ankle, knee, shoulder, hand, or elbow), (9) had alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels greater than 50% of upper normal limit, (10) had serum creatinine levels greater than 133 μmmol/l or 1.5 mg/dl (Appendix Table 1). All the included participants were provided a detailed written description about the study and an informed consent form before agreeing to participate and confirming eligibility. To protect the subjects who were randomized to placebo during the initial 36-month treatment phase, those who experienced a dramatic bone loss or fragility fracture were also excluded during 10-year observational follow-up.
Allocation, randomization and blinding
The participants were randomly assigned to either BSYJF or placebo group by using the randomization-number table with a 1:1 ratio. Briefly, randomization numbers were generated by an independent statistician from the department of statistics, Shanghai University of Traditional Chinese Medicine. This statistician was not involved in following data collection or analysis. The codes were concealed in sealed, opaque envelopes with date and signature labels. All the participants, investigators and coordinators were blinded to the treatment assignment. The blinding codes were given to a pharmacist to keep and could not be broken unless in emergency situations during the study. The treatment arrangements were made by the pharmacist in each center, who was blinded to the participants’ characteristics and not involved in the number generation and recruitment process.
Intervention and compliance
The participants were orally given calcium carbonate 300 mg daily with either BSYJF or placebo capsules (0.55 g/capsule, 3 capsules/time, 3 times/day) for 36 months continuously. The main ingredients in placebo were starch supplemented with food colorants and flavoring agents to mimic BSYJF capsules. Placebo capsules were identical to BSYJF capsules in size, weight, appearance, color and flavor.
The compliance was calculated at capsule collecting every three months (actual capsules consumption/required capsules consumption × 100%). The participants were considered evaluable if they were within 80% and 120% of compliance. All participants were arranged to join the Health Club and gathered once a month to facilitate the interaction between the participants and doctors.
Quality control for BSYJF capsules
The BSYJF is composed of 7 herbs, Herba Epimedii (Yinyanghuo [in Chinese], stem leaves of Epimedium brevicornum Maxim.), Radix Polygoni Multiflori (Heshouwu, root tuber of Polygonum multiflorum Thunb.), Herba Cistanches (Roucongrong, stem of Cistanche deserticola Y. C. Ma), Radix Astragali (Huangqi, root of Astragalus membranaceus), Rhizoma Drynariae (Gusuibu, rhizome of Drynaria fortune J. Sm.), Herba Dendrobii (Shihu, stem of Dendrobium nobile Lindl.) and Flos Chrysanthemi (Juhua, flower head of Chrysanthemum morifolium Ramat.), with a weight ratio in 10:6:6:10:6:6:6. The manufacturing processes of BSYJF capsules were strictly complied with Good Manufacturing Practice (GMP) standards and Chinese Pharmacopoeia. In addition, the stability and consistency of the components in different batches of BSYJF capsule used in this study were also confirmed by chromatographic fingerprint (Appendix Table 2). The BSYJF was recorded in National Drug Standard of CFDA (No. YBZ10052009) and available from Xia’men Chinese Medicine Factory Co., LTD (Trade name, Qi-Gu Capsule).
Endpoints
The primary endpoint was the number of falls at 36 months. Secondary endpoints were Timed Up and Go (TUG) test, lean mass of left thigh, femoral neck BMD, serum osteocalcin (OC), urine deoxypyridinoline (DPD), serum 25-OH-vitamin D (25(OH)D), 17 β-estradiol (E2) and endometrial thickness at 36 months. During the 36-month trial, the participants underwent the above assessment at baseline and 12, 24, 36 months.
Two independent orthopedic surgeons (Y.Z. and G.Z.) who were blinded to the characteristics of the participants performed the assessments.
Number of falls, fallers and fall-related hip fractures
A fall was defined as any event that led to an unplanned, unexpected contact with a supporting surface23. Falls resulting from pathologic fracture, major trauma or facial trauma, and/or unavoidable hazards such as transient ischemic attacks, chair collapsing or cerebrovascular accidents were excluded. A faller was defined as the participants sustained at least one fall or more. The participants were educated to record the information of new falls in the form immediately, including the date of fall and related-injury. The forms were collected at each clinical visit or monthly meeting in the health club for the 36-month trial and 10-year follow-up. The number of falls was accumulated at each time point24. The cumulative incidence of fallers was calculated as the total number of participants with first fall at each time point divided by the number of participants at risk25. The number of fall-related fractures was also recorded. A copy of radiograph confirming the fracture was obtained.
TUG test
The TUG test was designed to evaluate the functional mobility of the participants. The TUG test, consisting of both walking speed and chair rise components, was easy to complete by older adults26. Briefly, the participants were instructed to rise from a standard arm chair, walk 3 meters, turn around, walk back to the chair at the normal pace and sit down23. This test had high sensitivity (87%) and specialty (87%) to assess individual functional mobility23. Higher scores represented a poorer degree of functional mobility. The change of TUG score was normalized by the baseline.
Lean mass of left thigh and femoral neck BMD
All the densitometry operators in the two sites were specifically trained to ensure the standard procedure. The lean mass of left thigh and the BMD of left femoral neck were derived from the total body scan by Dual-energy X-ray Absorptiometry (DXA) (DPX-L; Lunar, Madison, WI, USA)27. The same DXA apparatus was used for the same participant over the entire period. Monitoring of DXA scanner drift was achieved by the regular scanning of a calibration phantom. The precision of a measurement was 1.86% for femoral neck and 0.74% for lean mass of left thigh. The changes of the femoral neck BMD and lean mass of left thigh were normalized by their corresponding baseline, respectively.
OC and urine DPD
The OC and urine DPD were bone turnover markers for predicting bone formation and bone resorption, respectively. The OC was assessed by a commercial ELISA Kit (Quidel, San Diego, CA, USA). Intraassay and interassay coefficient of variations (CVs) were 5.2% and 7.5% for serum OC, respectively. Urine DPD was quantified by a commercial ELISA Kit (Ostex International, Seattle, WA, USA). Intraassay and interassay CVs were 5.9% and 6.5% for urine DPD, respectively.
Serum 25(OH)D and E2
The serum 25(OH)D and E2 were also important indices of mineral metabolism. 25(OH)D concentrations were measured by a commercial radioreceptor assay (Amersham, Bucks, UK). Normal values for this age group were 4.3–40.5 ng/ml (within assay variation was 4–6%). E2 was assessed by a commercial RIA kit (Jiu Ding, Tianjing, China). Intraassay and interassay CVs were 4.0% and 5.0%, respectively.
Endometrial thickness
Endometrial thickness was measured at the thickest portion of the endometrium by transvaginal ultrasound (Siemens Medical Solutions Systems, Issaquah, WA, USA) and both endometrial layers were included. The sensitivity of detecting endometrial abnormalities was 92% when an endometrial thickness of 5 mm was used as the upper limit of the normal value28.
Fasting blood and urine samples were collected at each time point. The serum was separated from the blood corpuscles by centrifugation and stored at −70 °C until the assessment. All urine samples were analyzed for their creatinine content, and excretion of DPD was corrected for creatinine excretion. The changes of the OC and DPD were normalized by their corresponding baseline, respectively.
For safety, biochemical examination (complete blood count, renal function, and liver function), blood pressure and electrocardiogram, and observation of abnormal symptoms/signs based on standardized patient report forms were performed at baseline and 12, 24, 36 months. During each clinical visit, abnormalities were evaluated and documented, and follow-up medical care was provided as needed. Potential adverse events were monitored for each subject.
The participants, who successfully entered the treatment allocation but failed to complete the whole period, were considered as dropout cases. The reasons for dropout were recorded, and the last data of these participants were also included in the data analysis. The subjects could voluntarily withdraw at any time during the trial. The trial could be terminated if serious adverse events, protocol deviation or loss to follow-up occurred and/or BSYJF was found to have no clinical value after long-term administration.
During the entire trial, the subjects were advised to follow the guideline for daily dietary intake and physical activities, such as intaking high-protein diet, avoiding alcohol abuse and smoking, and keeping active in daily physical activities. The questionnaire for daily dietary intake and physical activities were recorded at baseline and 12, 24, 36 months during 36-month clinical trial. Calcium and vitamin D supplement on their own were prohibited in 36-month clinical trial.
Ten-year follow-up
For the extension treatment-free follow-up phase, the participants were followed-up at the 3rd, 6th and 10th year to assess the latent effects of BSYJF on falls and fall-related hip fractures in late postmenopausal women. The number of falls, fallers and fall-related hip fractures were recorded and the serum 25(OH)D levels were measured at the 3rd, 6th and 10th year. The information of falls, fracture, daily dietary intake and physical activities were collected by phone call or health club gathering once a month.
Statistical analysis
Assuming the fall incidence among community-dwelling elderly women was 19% in placebo group29, we hypothesized that BSYJF would reduce 50% in fall risk annually30. A sample size of 110 (55 per group) was required for 90% power at one-sided 5% significance level. Assuming the dropout of 20%, 70 participants for each group were adopted.
An intention-to-treat approach was used for all the analyses. The number of falls was presented as risk ratio (RR), determined by the Negative binomial with log link in generalized estimating equations (GEEs) with an unstructured covariance matrix. The rate of fallers at 36 months was presented as hazard ratio (HR), determined by a Cox regression analysis. The rate of fallers at 10-year follow-up, as well as the number of fall-related hip fractures, were presented as odds ratio (OR), determined by the Binary logistic regression with Hosmer-Lemeshow method. The Kaplan–Meier analysis was also performed to demonstrate differences in the time to the first fall between two groups. Censoring time was defined as the time from randomization until the last follow-up. A sensitivity analysis was performed by Negative binomial with log link in GEEs.
The continuous outcomes at different time points were analyzed by Linear model in GEEs. The differences between groups were calculated by analysis of covariance model (ANCOVA) or Wilcoxon rank-sum test. The difference within-group was analyzed by t-test. A sensitivity analysis was performed by Linear model in GEEs.
The age, time since menopause, body mass index (BMI) and history of fall at baseline were used as covariates. The analysis for adverse events was performed by chi-square test. All variables were presented as mean (SD) or median (IQR), and provided with 95% confidence interval (CI) or percentage. All tests were used two-sided and set at the 5% level using IBM SPSS 20.0.
Results
Baseline characteristics
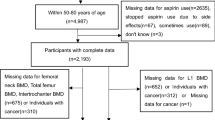
From 2000 to 2001, 616 subjects were recruited for screening and 140 participants were eligible (Fig. 1). The participants were randomly assigned to two groups. The number of subject recruited in each season in two groups was balanced. The demographic and key characteristics at baseline were well-balanced between two groups (Table 1).
Dropout and compliance
For the 36-month trial, twenty-seven participants withdrew from the trial: 13/70 in BSYJF and 14/70 in placebo. The reasons were 1) lack of family support (6 [46%] in BSYJF and 5 [36%] in placebo); 2) family relocation (4 [31%] in BSYJF and 4 [28%] in placebo); 3) Unwilling for long-term medications (3 [23%] in BSYJF and 5 [36%] in placebo) (Fig. 1). The compliance of BSYJF/placebo capsules administration was 91.61% (SD 0.51%) and 91.55% (SD 0.54%), respectively (Appendix Table 3). For the 10-year observational follow-up, 15 participants were lost to follow-up: 7/57 in BSYJF and 8/56 in placebo The reason was family relocation (Fig. 1).
Endpoints for 36-month trial
For the number of falls, there were 12 falls in BSYJF and 28 falls in placebo at 36 months, respectively. The fall risk was 64% lower in BSYJF than that in placebo (RR 0.36 [95% CI, 0.18 to 0.71]; P = 0.004). For the number of fallers, there were 8 fallers in BSYJF and 17 fallers in placebo at 36 months, respectively. The rate of fallers in BSYJF was 60% lower compared with that in placebo (HR 0.40 [95% CI, 0.17 to 0.95]; P = 0.038). For the number of fall-related hip fractures, there was 1 case in each group at 36 months, respectively (Fig. 2 and Table 2).
The change patterns of fall-related (upper panel) and bone-dependent (lower panel) fracture risk factors in late postmenopausal women during 36-month clinical trial. The fall-related fracture risk factors include the number of falls (upper left), the first fall in the time-to-event analysis (upper middle-left), lean mass (upper middle-right) and Timed Up and Go (TUG) score (upper right). The bone-dependent fracture risk factors include femoral neck BMD (lower left), serum osteocalcin levels (lower middle) and urine deoxypyridinoline levels (lower right). The Kaplan–Meier analysis was performed to demonstrate differences in the time to the first fall between two groups. Negative binomial model with log link was used to analyze the number of falls. The Linear model in generalized estimating equations (GEEs) analysis was used to analyze the change patterns over time between two groups. Error bars indicate pointwise 95% confidence intervals for the value of lean mass of left tight, and interquartile ranges for the values of formal neck BMD, TUG, and the levels of osteocalcin and deoxypyridinoline. Note: *P < 0.05 for within-group comparisons in BSYJF group or placebo group in percentage change from baseline. #P < 0.05 for between-group difference at 36 months. BMD = bone mineral density, BSYJF = Bushen Yijing Fang; TUG = Timed Up and Go.
There were significant differences between the BSYJF and placebo in the TUG score, lean mass of left thigh, femoral neck BMD and biochemical markers (OC and urine DPD) at 36 months, respectively (P ≤ 0.001 for interaction between time and group). Within each group, compared to the baseline, the TUG score in placebo significantly increased at 36 months (4.22%, P < 0.001), whereas BSYJF maintained TUG score (−2.26%, P = 0.071). The lean mass in placebo significantly decreased at 36 months (−5.71%, P < 0.001), whereas BSYJF maintained the lean mass (2.05%, P = 0.24). The femoral neck BMD in BSYJF was increased from baseline (3.86%, P < 0.001), whereas that in placebo was significantly decreased (−4.05%, P < 0.001). The OC levels did not change in placebo at 36 months (3.06%, P = 0.36), whereas BSYJF significantly increased OC at 36 months (11.28%, P = 0.001). The urine DPD levels increased in placebo at 36 months (8.38%, P = 0.001), whereas BSYJF slightly decreased DPD level at 36 months (−1.86%, P = 0.65) (Fig. 2, Table 2 and Appendix Table 4). There was no significant difference in the change patterns of 25(OH)D (P = 0.137), serum E2 levels (P = 0.53) and endometrial thickness (P = 0.27) between two groups during 36-month intervention, respectively (Table 2 and Appendix Table 5).
Endpoints for 10-year extension follow-up
At 10-year extension follow-up phase, there were 81 falls in BSYJF and 123 falls in placebo, respectively. The fall risk was 36% lower in BSYJF than that in placebo (RR 0.64 [95% CI, 0.46 to 0.89]; P = 0.009). For the number of fallers, there were 31 fallers in BSYJF and 33 fallers in placebo at 10-year follow-up, respectively. The rate of fallers in BSYJF was 25% lower compared with that in placebo (OR 0.75 [95% CI, 0.32 to 1.79]; P = 0.52). For the number of fall-related hip fractures, there were 1 case in BSYJF and 3 cases in placebo, respectively. There was 77% reduction in hip fracture rate in BSYJF during the 10-year extension follow-up (OR 0.23 [95% CI, 0.02 to 2.98]; P = 0.26) (Table 3).
There was no significant difference in the change patterns of 25(OH)D between two groups during 10-year follow-up (P = 0.06) (Table 3 and Appendix Table 6). The serum 25(OH)D levels in both groups slightly decreased during the whole study.
Daily dietary intake and physical activities
Both the time of daily physical activity and the calcium/vitamin D intake between two groups was similar during 36-month clinical trial and 10-year extension follow-up.
Adverse events
Related adverse events were summarized in Table 4. In those tolerable adverse events, 9 participants were reported in BSYJF group and 8 in placebo group (13% vs. 11%; RR, 1.13 [95% CI, 0.46 to 2.75]; P = 0.80). Gastrointestinal complaints, such as stomach discomfort, constipation, and nausea/vomiting, were the most common complaints (5 in BSYJF and 4 in placebo).
Minimally elevated liver enzyme levels were found in 2 women in BSYJF and 1 woman in placebo. The elevated liver enzyme levels returned to the normal range by the next test in all the 3 participants.
Sensitivity analysis
Sensitivity analysis showed no change in the number of falls at 36 months (RR 0.41[95% CI, 0.17 to 0.99]; P = 0.048) and other outcomes (Appendix Table 7).
Discussion
The study is the first randomized, double-blind and placebo-controlled trial with a 10-year observational follow-up to investigate the long-term effect of Chinese herb formula on fall risk in the late postmenopausal women with osteopenia.
With the increasing age, fragility fractures in the postmenopausal women is an ongoing concern for orthopaedic surgeons. The most common sites for fragility fracture are the vertebrae, hip (proximal femur) and wrist (distal forearm). Unlike fractures at other two sites, vertebral fractures are the most common fragility fractures caused by osteoporosis, only a minority of vertebral fractures result from a fall31. Conversely, most fall-related fractures are nonvertebral ones, and the site and type of fracture are dependent on the direction of the fall. Falling sideways or straight down is more likely to result in a hip fracture32,33, while falling backwards or obliquely forward is more likely to cause a distal forearm fracture34. However, distal forearm fractures do not tend to occur in frail individuals, since the elderly tend to move slowly and are unable to put out their hand to break a fall, in turn, decrease their risk for distal forearm fracture35,36. Therefore, hip fracture is the most representative as the fall-related fragility fractures. In the current trial, totally 7 hip fractures were reported.
BSYJF reduced fall risk, evidenced by the lowered fall number in BSYJF group during 36-month trial. Surprisingly, its latent effect on fall risk was still observed in 10-year follow-up in late postmenopausal women with osteopenia. Muscle mass and functional mobility were recognized as significant predictors for falls37. The lowered number of falls could be partially explained by the maintained thigh lean mass and TUG score in those women treated with BSYJF. The beneficial effect of BSYJF on muscle was similar with our animal data. BSYJF also modified bone-dependent risk factors, evidenced by the increased femoral neck BMD in BSYJF group at 36 months. It might be explained by the promoted bone formation (promoted increase in serum OC level) and attenuated bone resorption (prevented increase in urine DPD level) in the late postmenopausal women treated with BSYJF.
It has been reported that other risk factors (e.g., impairment of gait or balance, neurological disorders and environment hazards) also contribute to falls38. In this clinical study, these potential risk factors were excluded at baseline before recruitment. There is seasonal difference in falls and fractures among the elderly, more falls and fractures with more severe outcomes occur in winter39. In our study, the number of subject recruited in each season in two groups was balanced, which the influence of season factor could be considered minimized. Recently, daily physical activities and daily dietary intake, especially calcium and vitamin D intake, have been demonstrated to influence fall risk40. During the 36-month clinical trial and 10-year follow-up, the daily dietary intake and physical activities between two groups were similar. Serum 25(OH)D also showed similar level between two groups. After excluding the above potential risk factors, the difference in fall risk between the two groups could be associated with BSYJF.
According to the bioinformatics analysis, seven herbs in BSYJF were classified into two categories based on their target genes in fall-related network, herbs target ≥ 3 genes (HT3G) or herbs target ≤ 2 genes (HT2G) (Supplement). HT3G was demonstrated to be the active component within BSYJF in regulating muscle mass and muscle strength, evidenced by the unimproved muscle property in OVX rats after HT2G treatment. However, HT2G within BSYJF was not expendable due to its small target gene number. Bone became fragile after treatment of BSYJF without HT2G. These evidence implied that the seven herbs within BSYJF should be used as a whole to modify fall-related fracture risk factors.
To date, the guidance for fracture prevention in postmenopausal osteopenic women mainly focuses on the bone-dependent risk factors, the recommended therapy is calcium plus vitamin D supplements41. Different interventions have shown some beneficial effect in clinical trials to prevent falls among the elderly, including exercises (e.g., Tai Chi42), environment modification43, medication9 or the combination interventions44. Although vitamin D supplements exert a small positive impact on lower limb muscle strength (standardized mean difference [SMD] of 0.19 kg force; 95% CI, 0.05 to 0.34; 19 trials), a significant heterogeneity in the meta-analysis is reported45 and no effect is found in either muscle mass (SMD of 0.058 kg; 6 trials)45 or TUG (SMD of 0.3 s; 95% CI, 0.1 s to 0.5 s; 5 trials)46. In addition, the updated meta-analyses show that vitamin D supplements have no effects on falls (RR 0.98 [95% CI, 0.94 to 1.02]; 23 trials with more than 30 000 participants)47. In current study, BSYJF treatment did not affect the serum 25(OH)D levels during the whole study, implying BSYJF exerts beneficial effects independent of vitamin D. Therefore, BSYJF may have a greater advantage than vitamin D supplement on modifying fall-related fracture risk factors in late postmenopausal women with osteopenia.
BSYJF was not associated with a detectable hyperplasia and estrogen-like effect on the uterus in this study, which was evidenced by no significant change in endometrial thickness and serum E2 in BSYJF-treated women. The findings implied the tissue selectivity of the BSYJF containing phytoestrogenic compounds. The results were consistent with one of our previous published clinical trial of epimedium, one of the major herbs in BSYJF48.
No severe adverse events occurred during treatment period. The number of gastrointestinal complaints in BSYJF was close to that in placebo, which was consistent with the findings in previous trial49 and another study of Chinese herbal formula (Xianlinggubao, XLGB)50. No significant difference in the complaints with minimally elevated liver enzyme levels between two groups was observed, although a meta-analysis suggested that phytoestrogens were with moderately increased risk of adverse gastrointestinal effects compared with placebo51. Consistently, no prominent adverse events of BSYJF were reported by CFDA (http://eng.cfda.gov.cn/; No. Z20090656).
Limitations of this research were noted as follows: (1) Given the fact that fall risk was multifactorial40, some fall related factors, such as lifestyles and cognitive abilities, were not taken into consideration in this study. (2) Lack of information concerning participants’ physical activity, co-morbidities and medications during the 10-year extension follow-up phase limited the cause–effect relationship between BSYJF and outcomes.
In conclusion, 36-month administration of BSYJF reduced fall risk with an increase in femoral neck bone mass, and its latent effect on fall risk was continually observed in 10-year follow-up in late postmenopausal women with osteopenia.
Data Availability
All data generated or analysed during this study are included in this published article and its Supplementary Information.
References
Geusens, P., Milisen, K., Dejaeger, E. & Boonen, S. Falls and fractures in postmenopausal women: a review. The journal of the British Menopause Society 9, 101–106 (2003).
Nelson, H. D., Helfand, M., Woolf, S. H. & Allan, J. D. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Annals of internal medicine 137, 529–541 (2002).
Stone, K. L. et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 18, 1947–1954, https://doi.org/10.1359/jbmr.2003.18.11.1947 (2003).
Schuit, S. C. et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 34, 195–202 (2004).
Gallagher, J. C. The effects of calcitriol on falls and fractures and physical performance tests. The Journal of steroid biochemistry and molecular biology 89–90, 497–501, https://doi.org/10.1016/j.jsbmb.2004.03.059 (2004).
Marshall, D., Johnell, O. & Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312, 1254–1259 (1996).
Cranney, A., Jamal, S. A., Tsang, J. F., Josse, R. G. & Leslie, W. D. Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177, 575–580, https://doi.org/10.1503/cmaj.070234 (2007).
Rutherford, O. M. Is there a role for exercise in the prevention of osteoporotic fractures? British journal of sports medicine 33, 378–386 (1999).
Bolland, M. J., Grey, A., Gamble, G. D. & Reid, I. R. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2, 573–580, https://doi.org/10.1016/S2213-8587(14)70068-3 (2014).
Zhao, J. G., Zeng, X. T., Wang, J. & Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 318, 2466–2482, https://doi.org/10.1001/jama.2017.19344 (2017).
Grados, F. et al. Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint, bone, spine: revue du rhumatisme 70, 203–208 (2003).
Bolland, M. J., Grey, A. & Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol 6, 847–858, https://doi.org/10.1016/S2213-8587(18)30265-1 (2018).
Chen, Y. Y., Hsue, Y. T., Chang, H. H. & Gee, M. J. The association between postmenopausal osteoporosis and kidney-vacuity syndrome in traditional Chinese medicine. The American journal of Chinese medicine 27, 25–35, https://doi.org/10.1142/S0192415X99000057 (1999).
Zierath, J. R. & Hawley, J. A. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS biology 2, e348, https://doi.org/10.1371/journal.pbio.0020348 (2004).
Dong, P. et al. Effect of Bushen Yijing Fang on the expression of myosin heavy chain (MHC-I) gene mRNA of quadriceps femoris in ovariectomized rats. China J Orthop Traumatol. 9, 1–9, https://doi.org/10.3969/j.issn.1005-0205.2001.04.001 (2001).
Zhang, G. et al. Mechanical characteristics of the femoral neck bone quality and the gene expression related to the attached muscle strength in postmenopausal osteoporotic rat model induced by ovariectomy. Chin J Geriatr. 21, 210–213 (2002).
Zhang, G., Ma, J. & Zhang, Q. Experimental study on effect of bushen yijing recipe in delaying senility of bone and brain of aged male rats. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine 20, 43–45 (2000).
Song, X., Song, Z. & Shen, Z. Clinical observation on prevention and treatment of postmenopausal osteoporosis with Chinese kidney-nourishing herb. Pract J Intergrating Chin Modern Med 10, 892%\ 2016-2010-2024 2002:2007:2000 (1997).
Song, X., Cheng, B. & Shen, P. Clinical reaserch on prevention and treatment of postmenopausal osteoporosis with Chinese kidney-nourishing herb. Shanghai J Tradit Chin Med 34, 28–29 (2000).
Shen, P., Chen, D. & Zhang, G. Study on efficacy of Chinese Kidney-Tonifying Recipe in male rats with osteoporosis induced by dexamethasone and its mechanism. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine 18, 290–292 (1998).
Song, X., Shi, Y., Shen, P., Wang, H. & Chen, B. Experimental study of Chinese kidney-nourishing herb on postmenopausal osteoporosis. J Shanghai Railway Med Colloge. 9, 141–145 (1995).
Zhang, G. et al. Evaluation of Bushen Yijing Fang in delaying the aging of male rats and its mechanism. J Tradit Chin Orthop Traumatol 11(3–5), +62 (1999).
Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society 39, 142–148 (1991).
Jensen, J., Lundin-Olsson, L., Nyberg, L. & Gustafson, Y. Fall and injury prevention in older people living in residential care facilities. A cluster randomized trial. Annals of internal medicine 136, 733–741 (2002).
Cosman, F. et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. The New England journal of medicine 375, 1532–1543, https://doi.org/10.1056/NEJMoa1607948 (2016).
Yim-Chiplis, P. K. & Talbot, L. A. Defining and measuring balance in adults. Biological research for nursing 1, 321–331, https://doi.org/10.1177/109980040000100408 (2000).
Alfredson, H., Nordstrom, P. & Lorentzon, R. Aerobic workout and bone mass in females. Scandinavian journal of medicine & science in sports 7, 336–341 (1997).
Smith-Bindman, R. et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 280, 1510–1517 (1998).
Aoyagi, K. et al. Falls among community-dwelling elderly in Japan. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 13, 1468–1474, https://doi.org/10.1359/jbmr.1998.13.9.1468 (1998).
Kannus, P. Preventing osteoporosis, falls, and fractures among elderly people. Promotion of lifelong physical activity is essential. BMJ 318, 205–206 (1999).
Cooper, C., Atkinson, E. J., O’Fallon, W. M. & Melton, L. J. 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7, 221–227, https://doi.org/10.1002/jbmr.5650070214 (1992).
Greenspan, S. L. et al. Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med 104, 539–545 (1998).
Nankaku, M., Kanzaki, H., Tsuboyama, T. & Nakamura, T. Evaluation of hip fracture risk in relation to fall direction. Osteoporos Int 16, 1315–1320, https://doi.org/10.1007/s00198-005-1843-2 (2005).
Palvanen, M. et al. The injury mechanisms of osteoporotic upper extremity fractures among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporos Int 11, 822–831 (2000).
Kelsey, J. L. et al. Reducing the risk for distal forearm fracture: preserve bone mass, slow down, and don’t fall! Osteoporos Int 16, 681–690, https://doi.org/10.1007/s00198-004-1745-8 (2005).
Graafmans, W. C., Ooms, M. E., Bezemer, P. D., Bouter, L. M. & Lips, P. Different risk profiles for hip fractures and distal forearm fractures: a prospective study. Osteoporos Int 6, 427–431 (1996).
Frank-Wilson, A. W. et al. Lower leg muscle density is independently associated with fall status in community-dwelling older adults. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27, 2231–2240, https://doi.org/10.1007/s00198-016-3514-x (2016).
Tinetti, M. E. et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. The New England journal of medicine 331, 821–827, https://doi.org/10.1056/NEJM199409293311301 (1994).
Caberlon, I. C. & Bos, A. J. Seasonal differences in falls and fractures among the elderly in the southern Brazilian state of Rio Grande do Sul. Ciencia & saude coletiva 20, 3743–3752, https://doi.org/10.1590/1413-812320152012.20602014 (2015).
Uusi-Rasi, K. et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA internal medicine 175, 703–711, https://doi.org/10.1001/jamainternmed.2015.0225 (2015).
Weaver, C. M. et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27, 367–376, https://doi.org/10.1007/s00198-015-3386-5 (2016).
Hwang, H. F. et al. Effects of Home-Based Tai Chi and Lower Extremity Training and Self-Practice on Falls and Functional Outcomes in Older Fallers from the Emergency Department-A Randomized Controlled Trial. Journal of the American Geriatrics Society 64, 518–525, https://doi.org/10.1111/jgs.13952 (2016).
Lin, M. R., Wolf, S. L., Hwang, H. F., Gong, S. Y. & Chen, C. Y. A randomized, controlled trial of fall prevention programs and quality of life in older fallers. Journal of the American Geriatrics Society 55, 499–506, https://doi.org/10.1111/j.1532-5415.2007.01146.x (2007).
Assantachai, P., Chatthanawaree, W., Thamlikitkul, V., Praditsuwan, R. & Pisalsarakij, D. Strategy to prevent falls in the Thai elderly: a controlled study integrated health research program for the Thai elderly. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 85, 215–222 (2002).
Beaudart, C. et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. The Journal of clinical endocrinology and metabolism 99, 4336–4345, https://doi.org/10.1210/jc.2014-1742 (2014).
Rosendahl-Riise, H., Spielau, U., Ranhoff, A. H., Gudbrandsen, O. A. & Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association, https://doi.org/10.1111/jhn.12394 (2016).
Bolland, M. J., Grey, A. & Reid, I. R. Vitamin D supplements do not prevent falls. BMJ 353, i3005, https://doi.org/10.1136/bmj.i3005 (2016).
Zhang, G., Qin, L. & Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 22, 1072–1079, https://doi.org/10.1359/jbmr.070405 (2007).
Shi, Y. et al. Clinical research of Migu capsule in the treatment of postmenopausal osteoporosis for two years. Bull Med Res. 33, 53–54, https://doi.org/10.3969/j.issn.1673-548X.2004.04.028 (2004).
Zhu, H. M. et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 23, 1317–1327, https://doi.org/10.1007/s00198-011-1577-2 (2012).
Tempfer, C. B. et al. Side effects of phytoestrogens: a meta-analysis of randomized trials. The American journal of medicine 122, 939–946 e939, https://doi.org/10.1016/j.amjmed.2009.04.018 (2009).
Acknowledgements
The study was funded by the Ministry of Science & Technology of China (Project No. 96-906-09-05), Shanghai Science & Technology Commission (Project No. 004319226), National Natural Science Foundation of China (Project No. 81201406), General Research Fund (GRF) of Research Grant Committee (RGC) (Project No. 14112915, HKBU_261113, HKBU_478312). The authors thank Prof. Meiyu Shi and Prof. Hua Lu for his insightful suggestions and comments on the data and statistical guidance; they also thank nurses for their help and the participants for their cooperation.
Author information
Authors and Affiliations
Contributions
Y.Z., G.Z. and Y.S. designed the research; Y.Z., G.Z., X. Wang, J.Z., W.S., Y.Z., H.Z., Y.X. and Y.S. performed the clinical trial; Y.Z. collected the data; A.L., B.-T.Z., C.Z., Z.L., Z.B. and L.Z. interpreted the data; X. Wang, Z.-K.Z., B.G., L.D., and B.H. drafted the manuscript; Z.-K.Z., J. Li, Z.Z., C.L., J. Liu and B.T.Z. performed the animal study; L.D., X. Wu and L.W. were in charge of the quality control for BSYJF capsules; B.H., D.G. and A.L. performed the bioinformatics analysis; H.Q. performed the first bio-statistical analysis; Bioinformedicine Service, San Diego, CA, USA. double-checked the bio-statistical analysis; Y.S., B.-T.Z., G.Z. and A.L. obtained the funds for the research.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, Y., Wang, X., Zhang, ZK. et al. Bushen Yijing Fang Reduces Fall Risk in Late Postmenopausal Women with Osteopenia: A Randomized Double-blind and Placebo-controlled Trial. Sci Rep 9, 2089 (2019). https://doi.org/10.1038/s41598-018-38335-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38335-3
This article is cited by
-
Determination of the Combined Effects of Asian Herbal Medicine with Calcium and/or Vitamin D Supplements on Bone Mineral Density in Primary Osteoporosis: A Systematic Review and Meta-Analysis
Osteoporosis International (2024)
-
To investigate the mechanism of Yiwei Decoction in the treatment of premature ovarian insufficiency-related osteoporosis using transcriptomics, network pharmacology and molecular docking techniques
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.