Abstract
The relationship between metformin and prostate cancer (PCa) remains controversial. To clarify this association, the PubMed, Embase and Cochrane library databases were systematically searched from their inception dates to May 23, 2018, using the keywords “metformin” and “prostate cancer” to identify the related studies. The results included incidence, overall survival (OS), PCa-specific survival (CSS) and recurrence-free survival (RFS), which were measured as hazard ratios (HR) with a 95% confidence interval (95% CI) using Review Manager 5.3 software. A total of 30 cohort studies, including 1,660,795 patients were included in this study. Our study revealed that metformin treatment improves OS, CSS and RFS in PCa (HR = 0.72, 95% CI: 0.59–0.88, P = 0.001; HR = 0.78, 95% CI: 0.64–0.94, P = 0.009; and HR = 0.60, 95% CI: 0.42–0.87 P = 0.006, respectively) compared with non-metformin treatment. However, metformin usage did not reduce the incidence of PCa (HR = 0.86, 95% CI: 0.55–1.34, P = 0.51). In conclusion, compared with non-metformin treatment, metformin therapy can significantly improve OS, CSS and RFS in PCa patients. No association was noted between metformin therapy and PCa incidence. This study indicates a useful direction for the clinical treatment of PCa.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the second leading cause of malignancy deaths among men in the United States. Approximately 164,690 American males were diagnosed with PCa in 2017, and 29,430 will die of this disease1. Given the wide used of earlier detection modalities and advances in treatment, the incidence and mortality of PCa exhibit a sharp reductions1,2. However, Boorjian et al.3 reported that up to 40% of PCa patients faced challenges of cancer recurrence or progression during long-term follow-up.
Metformin, an oral biguanide mainly used to treat type 2 diabetes, has demonstrated anti-neoplastic effects in several types of solid tumours and hormone-sensitive tumours, such as colon cancer, pancreatic cancer and breast cancer4,5,6. Metformin inhibits cancer proliferation by activating the AMPK pathway and suppressing the expression of genes involved in mitosis7,8. Given that hyperinsulinaemia is associated with an increased risk of colorectal and breast cancer, a poor prognosis is often noted9. As an insulin sensitizer, metformin exhibits indirect antitumour effects by reducing insulin levels through the inhibition of hepatic gluconeogenesis. However, the effects of metformin use in prostate cancer, an analogous hormonally sensitive cancer in men, remain controversial. Several studies10,11 demonstrated that metformin reduces the risk of prostate cancer incidence and improve PCa outcomes. In contrast, other studied reported negative outcomes.
Given the association between metformin and cancer incidence, the prognosis of prostate cacncer remain unclear. In this study, we evaluated the incidence and prognostic value of metformin in prostate cancer.
Result
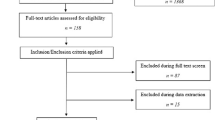
In total, 1004 publications were identified for eligibility through a literature search. After removing the duplicate studies and reviewing titles and abstracts, 30 studies and 1,660,795 individuals were included in our meta-analysis (Fig. 1).
Study characteristics
The baseline characteristics of all included studies are presented Table 1. Studies were published between 2012 and 2017. There were 12, 14, 7 and 8 publications associated with incidence, OS, CSS, and RFS, respectively. 19 studies were performed in the United States, 8 in Europe, 2 in Asia and 1 in Australia. Four studies were conducted in patients with prostatectomy, 4 with radiotherapy, 1 with ADT, and 1 with docetaxel. Eight studies included a mixture of these PCa treatments. Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of included studies, which ranged from 6 to 9 (Table 2).
Metformin therapy and PCa overall survival
Figure 2 indicated that incidence of PCa was assessed in 14 studies. The HR for PCa patients taking metformin compared with those not taking metformin was 0.72 [95% CI: 0.59∼0.88], P = 0.001. Interstudy heterogeneity was noted (I2 = 89%, P < 0.00001). Metformin therapy improved the OS of PCa patients who accepted radiotherapy (n = 3, HR = 0.44, [95% CI: 0.35∼0.55], P < 0.00001). The subgroup studies consist of study region, study design, sample sizes, diabetic only, study setting and cumulative duration (Table 3).
Metformin therapy and PCa-specific survival
Figure 3 indicates that CSS was assessed in 7 studies. The HRs for CSS in PCa patients taking metformin compared with those not taking metformin was 0.78 [95% CI: 0.64∼0.94], P = 0.009. Interstudy heterogeneity was noted (I2 = 67%, P = 0.006). Metformin therapy improved the CSS of PCa patients who accepted radiotherapy or mix treatment (n = 2 HR = 0.18, [95% CI: 0.07∼0.45], P = 0.0003; n = 3 HR = 0.78, [95% CI: 0.67∼0.91], P = 0.002 respectively). The subgroup studies consist of study region, study design, sample sizes, diabetic only and study setting (Table 4).
Metformin therapy and PCa recurrence free survival
Figure 4 indicates that RFS was assessed in 8 studies. The HRs for RFS in PCa patients taking metformin compared with those not taking metformin was 0.60, [95% CI: 0.42∼0.87] P = 0.006. Interstudy heterogeneity was noted (I2 = 63%, P = 0.009). In the subgroup of basic treatment, metformin therapy improved the RFS of PCa patients who accepted radiotherapy (n = 3 HR = 0.41, [95% CI: 0.29∼0.58], P < 0.00001). The subgroup studies consist of study region, sample sizes, diabetic only, study setting and study design (Table 5).
Metformin therapy and incidence of PCa
Figure 5 indicates that incidence of PCa was assessed in 12 studies. The HR for PCa patients taking metformin compared with those not taking metformin was 0.86 [95% CI: 0.55∼1.34], P = 0.51. Interstudy heterogeneity was noted (I2 = 98%, P < 0.00001). In our subgroup, 6 studies are classified according to their participants’ race, including African American, Hispanic/Latino, non-Hispanic white and Asian. Non-Hispanic whites with metformin therapy exhibit a reduced incidence of PCa (HR = 0.86, [95% CI: 0.76∼0.98], P = 0.02). No associations were found between metformin usage and African Americans, Hispanic/Latinos and Asians. The subgroup studies consist of study region, sample sizes, race, duration of metformin therapy, cumulative dose of metformin, Gleason of PCa, advanced PCa, diabetic only and cumulative duration. All subgroup analyses did not reveal any benefits for reducing the incidence of PCa (Table 6).
Assessment of heterogeneity
There was evidence of considerable heterogeneity in OS (I2 = 89%, P < 0.00001), CSS (I2 = 67%, P = 0.006), RFS (I2 = 63%, P = 0.009) and incidence of PCa (I2 = 98%, P < 0.00001). Subgroup analyses investigating potential sources of heterogeneity demonstrated that study region, study design, study setting, sample size and diabetic only were not significantly associated with the heterogeneity in this meta-analysis. We also conducted a sensitivity analysis in which one study was removed at a time and found that the Zaosky 2017 study was the source of heterogeneity in the meta-analysis for RFS. When the study by Zaosky 2017 was removed, the heterogeneity in RFS decreased (I2 = 57%, P = 0.03), and the results remained stable (HR = 0.66, 95% CI: 0.45–0.96). In the group of incidence, Tseng’s 2014 study was the source of statistical heterogeneity in the meta-analysis. When the study by Tseng 2014 was removed, the heterogeneity in incidence decreased (I2 = 67%, P = 0.0007), and a meta-analysis of incidence results remained stable (HR = 0.96, 95% CI: 0.84–1.10). When the abovementioned studies were removed, the meta-analysis of RFS and incidence demonstrated statistical robustness. No study markedly affected the heterogeneity in the group of OS and CSS. This sensitivity analysis confirms the robustness of our results.
Publication bias
Egger’s and Begg’s tests revealed the possibility of publication bias for OS (0.677), CSS (0.816), RFS (0.526) and incidence (0.284). No obvious publication bias was noted in our analysis.
Discussion
In the past few years, the controversial results of metformin in the incidence and prognosis of PCa have been increasing. Increasing experimental research reports that metformin exhibits its own advantages in PCa treatment in vitro. Comstock et al.12 reported that the cyclin D1 pathway was related to PCa cell cycle progression and androgen-dependent transcription. Metformin inhibits PCa cell proliferation by reducing cyclin D1 activity13. Metformin also reduces PCa cell viability and enhances apoptosis by downregulating androgen receptors in both androgen-dependent and androgen-independent prostate cancers14. Metformin activates the AMP-activated protein kinase (AMPK), which inhibits mTOR signalling. Given that mTOR is overexpressed in PCa, metformin reduces PCa growth15. In clinical research, the effect of metformin on PCa is uncertain. Mayer et al.16 reported that metformin used with docetaxel did not affect castration-resistant PCa-specific survival and overall survival. However, another study17 reported that ADT with metformin prolongs advanced PCa-specific survival and overall survival. To clarify the relationship between metformin and PCa, a total of 30 cohort studies encompassing 1,660,795 individuals were included in our present systematic review and meta-analysis.
In our meta-analysis of PCa and metformin, we found that PCa patients who use metformin exhibited OS, CSS and RFS benefits compared with PCa patients who did not take metformin. This result is similar with previous meta-analysis, which reported that metformin was useful for OS and RFS18,19. However, Stopsack et al.18 included 4 studies and Xiao et al.19 included 6 studies. Thus, the meta-analysis was limited due to low event numbers in studies reporting CSS. However, unlike the previous meta-analysis, we found that metformin therapy was associated with CSS by including 7 studies. We first grouped the included studies based on basic treatment (prostatectomy, radiotherapy, ADT, etc.), metformin dose, and duration of metformin therapy and observed that patients who accepted both radical radiotherapy and metformin therapy had a significant improvement in OS, CSS and RFS in our meta-analysis. An in vitro study reported that metformin enhanced ionizing radiation activation of AMPK in PC-3 cells and reduced the surviving fraction of PC-320. These results demonstrated that metformin induced radiosensitizing effects. Interestingly, many studies reported21,22,23 that the prognosis of PCa patients who accepted prostatectomy was not associated with metformin use, dose or duration of use. These contradictory data between prostatectomy and radiotherapy were significant. Pre-operative ADT exhibited no survival benefit in men accepting prostatectomy24,25, but was benefical in radical radiotherapy26. Taira et al.21 hypothesized that prostatectomy without ADT may weaken the antineoplastic effect of metformin. According to our subgroup analyses, ADT with metformin improves PCa-specific survival and overall survival. However, ADT increases the incidence of metabolic syndrome, such as obesity, hyperinsulinaemia, insulin resistance and type-2 diabetes mellitus27. Given that metabolic syndrome is an important factor for biochemical failure after prostatectomy and radiotherapy, metformin exhibited therapeutic benefits for weight gain induced by medications and metabolic disturbances related to insulin resistance28,29,30. In benign prostate hyperplasia (BPH) xenograft models, metformin inhibits testosterone and attenuates prostate weight and pathological alterations31. These findings suggest that metformin not only reduced the side effects of ADT but also acted as chemotherapy for ADT through testosterone inhibition. Docetaxel is a first-line chemotherapy for treating castration-resistant prostate cancer (CRPC). Hyperglycaemia, which is a side effect of docetaxel, reduces the efficacy of docetaxel at inducing PCa cell apoptosis32. Biernacka et al. demonstrated that co-treatment with docetaxel and metformin led to additive effects to induce PCa cell apoptosis and alleviated the resistance induced by hyperglycaemia33. However, only two clinical studies examined the relationship between docetaxel and metformin16,34, and none of these studies revealed that metformin exhibited an additive effect with docetaxel. Further clinical studies are needed to discover whether metformin therapy could improve the prognosis of both PCa and CRPC.
Coinciding with the prognosis of PCa, the association between metformin therapy and PCa incidence is controversial. Bansal et al.10 demonstrated that diabetes reduced the diagnosis of PCa by 14% compared with those without diabetes. Some studies noted that compared with non-diabetic patients, diabetic patients exhibited reduced levels of testosterone, which decreased the incidence of low-grade PCa11,35,36,37. However, Azoulay et al.38 summarized data from the UK General Practice Research database and found that metformin intake increases the incidence of PCa. Various studies reported conflicting results with the association of metformin usage and PCa diagnosis39,40,41,42,43. To clarify this association, we included 12 studies and found no association between metformin usage and the incidence of PCa. A previous meta-analysis provided similar results between PCa risk and metformin exposure44. In contrast, two studies45,46 reported slight reductions (12% and 9%) in PCa risk and metformin use with substantial heterogeneity. (I2 = 74.7% and 51%). In their meta-analysis, all the included studies were published earlier than 2014. However, our study identified 12 studies and included 1,431,979 male subjects, a larger population group than previous studies45,46. Moreover, more than 92% studies were published in the past five years. Therefore, our results gained stronger statistical power.
PCa occurrence and outcomes vary considerable between racial and ethnic groups. Siegel et al.1 reported that PCa incidence and mortality are generally highest among American Africans, whereas Asians exhibited the lowest PCa rates. However, compared with non-Asian patients with type 2 diabetes, Asian patients with type 2 diabetes exhibit a significantly increased risk of PCa10,47. One large population-based study reported that Hispanics undergoing metformin therapy exhibited an evident reduction in PCa incidence, whereas metformin usage is not associated with PCa incidence among African Americans and non-Hispanic whites48. Previous meta-analyses on this topic revealed no association between metformin and incidence of PCa in either Western-based or Asian-based populations49. However, Western and Asian populations were only classified based on geography, and this studies were limited by significant heterogeneity (I2 = 88%). Unlike the previous study, we classified all participants as African American, Latino/Hispanic, Non-Hispanic white and Asian. We found that metformin use is associated with a 14% reduction in the risk of PCa among non-Hispanic whites with the presence of heterogeneity (I2 = 49%). However, metformin therapy did not decrease the risk of PCa among American Africans (I2 = 26%). A high degree of heterogeneity was noted among Hispanics/Latinos and Asian (94% and 100%, respectively). This high heterogeneity is consistent with a previous study50. We found that this evidence heterogeneity is heavily influenced by the studies of Raval and Tseng, which were large studies with an extreme risk estimate. Given that there were only two studies in each subgroup, these studies also had a high level of precision and a high Newcastle-Ottawa score, we included this study. In other subgroup analyses, duration of metformin therapy, cumulative metformin dose, and study region exhibited no association with the incidence of PCa. Moreover, we found that metformin usage was not associated with the Gleason scores of PCa.
There was evidence of considerable heterogeneity in OS (I2 = 89%, P < 0.00001), CSS (I2 = 67%, P = 0.006), RFS (I2 = 63%, P = 0.009) and incidence of PCa (I2 = 98%, P < 0.00001). A sensitivity analysis found that Zaosky 2017 and Tseng 2014 were the sources of heterogeneity in the meta-analysis for RFS and PCa risk. The study by Zaorsky 2017 failed to report the exact start and stop times of metformin. Information on the timing and amount of metformin use was unclear, which might cause a time bias and lead to heterogeneity. In Tseng 2014, no information was available on lifestyle variables, such as smoking status, alcohol consumption, or diet, that potentially influenced the risk of PCa. Although 4 studies39,41,43,49 also fail to record the lifestyle in the group of incidence. Differing from these studies which lack information on lifestyle factors, Tseng only focuses on the local Asian administrative databases. We suppose that the lack of information on lifestyle factors in Asian may be the potential reason for heterogeneity in Tseng et al. Moreover, there are only two studies which focus on the Asian in the group of incidence. We use subgroup analysis and found that these two Asian studies also have a high degree of heterogeneity (I2 = 100%). The participants in Tseng et al. were from the National Health Insurance reimbursement database, which is a local Asian database in Taiwan.While in Chen et al.49, the participants were Asian Canadian and come from British Columbia Cancer Agency in Canada. This difference of such criteria included may also lead to the heterogeneity in Tseng 2014. The Newcastle-Ottawa score revealed that these two studies were not more biased compared with other studies and had large sample sizes. Therefore, it is inappropriate to exclude these two studies from our meta-analysis.
There were various strengths of our meta-analysis. First, we comprehensively searched relevant studies using Embase, PubMed and Cochrane without publication date or publication type limits by extracting the maximal number of dates in suitable studies. Second, a total of 30 cohort studies including 1,660,795 individuals were included in our studies, which allowed us to quantitatively assess the relationship between metformin intake and PCa. Third, various subgroup analyses, such as PCa treatment, race, duration of metformin therapy, cumulative metformin dose and PCa Gleason score, could provide precise evidence for metformin use in clinical practice. Fourth, we only included the patients with metformin monotherapy and reduced the anticancer bias of other medications.
There were some limitations of our meta-analysis. First, two studies51,52 did not report the number of metformin users and non-metformin users, and 1 study53 did not separate type 1 and type 2 diabetes, which may affect the accuracy of the final result. Second, the accuracy of the summary estimates is influenced by different survival analysis methods. Although a multivariate Cox proportional hazards model was used in most of the studies, 2 studies54,55 did not report their statistical models. Third, because our meta-analysis exclusively focused on studies written in English, a language bias might exist. Fourth, most of our included studies were retrospective studies, which affected the quality of evidence for our meta-analysis.
In conclusion, our meta-analysis suggested that metformin therapy exhibits advantages in improving the prognosis of PCa, but no association was noted between metformin usage and PCa incidence. Moreover, PCa patients with metformin therapy accepting radical radiotherapy exhibited more dramatic effects on OS, CSS and RFS. These studies demonstrated a useful direction for the clinical treatment of PCa. Further randomized controlled trials are needed to confirm the association of PCa and metformin usage.
Materials and Methods
Study selection
Two authors (He & Hu) performed an electronic search of the PubMed, Embase, and Cochrane databases for relevant English studies (the last search update was May 20, 2018). The search strategies included ‘metformin’, ‘biguanide’, ‘Dimethylbiguanidine’, ‘Prostate cancer’ and ‘Prostate Neoplasms’. All the included studies met the following criteria: 1) Study designs must be prospective or retrospective cohort study. Studies must compare metformin users and non-metformin users. 2) Studies must analyse the PCa incidence, overall survival (OS), PCa-specific mortality (CSS) or recurrence-free survival (RFS). We excluded the following types of studies: reviews, case-control studies, studies of interventions other than metformin, articles assessing outcomes following metformin use in animal models, metformin use in other populations, studies including metastatic PCa patients at diagnosis and in vitro studies. Language selection focused on articles written in English. Hazard Ratio (HR) was used as the measure across studies. Given that the PCa incidence was relatively low, odds ratio (OR) were used as an estimate of HR. The prognostic outcomes estimate HRs/RRs with 95% CIs. RFS was defined as the time from the date of PCa patients accepting prostatectomy, radiation therapy or androgen deprivation therapy to the date of biochemical recurrence. After removing duplicate publications, two authors (He & Hu) independently assessed the primary literature by assessing titles and abstracts and then identified the final relevant studies based on eligibility.
Data extraction and Quality assessment
Two authors (He & Ye) extracted data and information from final studies, such as the first author, year of publication, study region, sample size, study design, follow-up period, type of treatment, and survival endpoints. Two authors (He & Ye) assessed the final studies, scored them using the NOS56 and reached a consensus value for each study independently.
Statistical analysis
Review Manager 5.3 (RevMan 5.3) was employed to conduct all statistical analyses. PCa incidence and survival estimates were abstracted from the final studies and pooled using a random-effects model. Standard Cochran’s Q test and I2 statistics were used to identify heterogeneity between the included studies. A value of I2 statistics >50% and p-value <0.1 indicated significant heterogeneity. When heterogeneity was significant, we explored the potential influential variables between included studies and pooled the results into subgroup analyses. Publication bias was detected with the Begg and Egger’s regression intercept test by using STATA 13. (Stata Corp LP, college Station, TX).
Ethics approval
Ethical approval was not sought as the study was based entirely on previously published data.
Data Availability
The study was based entirely on previously published data.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. 68, 7–30, https://doi.org/10.3322/caac.21442 (2018).
Etzioni, R. et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 19, 175–181, https://doi.org/10.1007/s10552-007-9083-8 (2008).
Boorjian, S. A. et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol 61, 664–675, https://doi.org/10.1016/j.eururo.2011.11.053 (2012).
Duan, W. et al. Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett 385, 225–233, https://doi.org/10.1016/j.canlet.2016.10.019 (2017).
Wahdan-Alaswad, R. et al. Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle 15, 1046–1059, https://doi.org/10.1080/15384101.2016.1152432 (2016).
DeCensi, A. et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prevention Research 3, 1451–1461, https://doi.org/10.1158/1940-6207.CAPR-10-0157 (2010).
Mayer, M. J., Klotz, L. H. & Venkateswaran, V. Metformin and prostate cancer stem cells: A novel therapeutic target. Prostate Cancer and Prostatic Diseases 18, 303–309, https://doi.org/10.1038/pcan.2015.35 (2015).
Vazquez-Martin, A., Oliveras-Ferraros, C., Lopez-Bonet, E. & Menendez, J. A. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle 8, 3679–3683, https://doi.org/10.4161/cc.8.22.9905 (2009).
Dowling, R. J. O., Niraula, S., Stambolic, V. & Goodwin, P. J. Metformin in cancer: Translational challenges. Journal of Molecular Endocrinology 48, R31–E43, https://doi.org/10.1530/JME-12-0007 (2012).
Bansal, D., Bhansali, A., Kapil, G., Undela, K. & Tiwari, P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis 16(151-158), s151, https://doi.org/10.1038/pcan.2012.40 (2013).
Gong, Z. et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15, 1977–1983, https://doi.org/10.1158/1055-9965.epi-06-0477 (2006).
Comstock, C. E., Revelo, M. P., Buncher, C. R. & Knudsen, K. E. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br J Cancer 96, 970–979, https://doi.org/10.1038/sj.bjc.6603615 (2007).
Ben Sahra, I. et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27, 3576–3586, https://doi.org/10.1038/sj.onc.1211024 (2008).
Wang, Y. et al. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 75, 1187–1196, https://doi.org/10.1002/pros.23000 (2015).
Dowling, R. J., Zakikhani, M., Fantus, I. G., Pollak, M. & Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 67, 10804–10812, https://doi.org/10.1158/0008-5472.can-07-2310 (2007).
Mayer, M. J., Klotz, L. H. & Venkateswaran, V. The Effect of Metformin Use during Docetaxel Chemotherapy on Prostate Cancer Specific and Overall Survival of Diabetic Patients with Castration Resistant Prostate Cancer. Journal of Urology 197, 1068–1075, https://doi.org/10.1016/j.juro.2016.10.069 (2017).
Richards, K. et al. Metformin use is associated with improved survival in veterans with advanced prostate cancer on androgen deprivation therapy. Journal of Urology 197, e715–e716 (2017).
Stopsack, K. H., Ziehr, D. R., Rider, J. R. & Giovannucci, E. L. Metformin and prostate cancer mortality: a meta-analysis. Cancer Causes Control 27, 105–113, https://doi.org/10.1007/s10552-015-0687-0 (2016).
Xiao, Y. et al. The impact of metformin use on survival in prostate cancer: a systematic review and meta-analysis. Oncotarget 8, 100449–100458 (2017).
Sanli, T. et al. Ionizing radiation activates AMP-activated kinase (AMPK): A target for radiosensitization of human cancer cells. International Journal of Radiation Oncology Biology Physics 78, 221–229, https://doi.org/10.1016/j.ijrobp.2010.03.005 (2010).
Taira, A. V. et al. Metformin is not associated with improved biochemical free survival or cause-specific survival in men with prostate cancer treated with permanent interstitial brachytherapy. Journal of Contemporary Brachytherapy 6, 254–261, https://doi.org/10.5114/jcb.2014.45757 (2014).
Kaushik, D. et al. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urologic Oncology: Seminars and Original Investigations 32, 43.e41–43.e47, https://doi.org/10.1016/j.urolonc.2013.05.005 (2014).
Allott, E. H. et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis 16, 391–397, https://doi.org/10.1038/pcan.2013.48 (2013).
Aus, G. et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int 90, 561–566 (2002).
Soloway, M. S. et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 167, 112–116 (2002).
Roach, M. 3rd et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 26, 585–591, https://doi.org/10.1200/jco.2007.13.9881 (2008).
Crawley, D. et al. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int J Cancer 139, 2698–2704, https://doi.org/10.1002/ijc.30403 (2016).
Diamanti-Kandarakis, E., Economou, F., Palimeri, S. & Christakou, C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci 1205, 192–198, https://doi.org/10.1111/j.1749-6632.2010.05679.x (2010).
Wu, R. R. et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. Jama 299, 185–193, https://doi.org/10.1001/jama.2007.56-b (2008).
Bianchi, C., Penno, G., Romero, F., Del Prato, S. & Miccoli, R. Treating the metabolic syndrome. Expert Rev Cardiovasc Ther 5, 491–506, https://doi.org/10.1586/14779072.5.3.491 (2007).
Mosli, H. H. et al. Metformin Attenuates Testosterone-Induced Prostatic Hyperplasia in Rats: A Pharmacological Perspective. Sci Rep 5, 15639, https://doi.org/10.1038/srep15639 (2015).
Biernacka, K. M. et al. Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat Cancer 20, 741–751, https://doi.org/10.1530/erc-13-0077 (2013).
Biernacka, K. M. et al. Hyperglycaemia-induced resistance to Docetaxel is negated by metformin: A role for IGFBP-2. Endocrine-Related Cancer 24, 17–30, https://doi.org/10.1530/ERC-16-0095 (2017).
Niraula, S. et al. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J 7, E74–81, https://doi.org/10.5489/cuaj.267 (2013).
Kasper, J. S., Liu, Y. & Giovannucci, E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer 124, 1398–1403, https://doi.org/10.1002/ijc.24044 (2009).
Bonovas, S., Filioussi, K. & Tsantes, A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47, 1071–1078, https://doi.org/10.1007/s00125-004-1415-6 (2004).
Leitzmann, M. F. et al. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control 19, 1267–1276, https://doi.org/10.1007/s10552-008-9198-6 (2008).
Azoulay, L., Dell’Aniello, S., Gagnon, B., Pollak, M. & Suissa, S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 20, 337–344, https://doi.org/10.1158/1055-9965.epi-10-0940 (2011).
Häggström, C. et al. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. International Journal of Cancer 140, 611–617, https://doi.org/10.1002/ijc.30480 (2017).
Raval, A. D., Mattes, M. D., Madhavan, S. & Pan, X. Association between Metformin Use and Cancer Stage at Diagnosis among Elderly Medicare Beneficiaries with Preexisting Type 2 Diabetes Mellitus and Incident. Prostate Cancer. 2016, 2656814, https://doi.org/10.1155/2016/2656814 (2016).
Nordström, T., Clements, M., Karlsson, R., Adolfsson, J. & Grönberg, H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. European Journal of Cancer 51, 725–733, https://doi.org/10.1016/j.ejca.2015.02.003 (2015).
Feng, T. et al. Metformin use and risk of prostate cancer: results from the REDUCE study. Cancer Prev Res (Phila) 8, 1055–1060, https://doi.org/10.1158/1940-6207.capr-15-0141 (2015).
Haring, A. et al. Antidiabetic drug use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scandinavian Journal of Urology 51, 5–12, https://doi.org/10.1080/21681805.2016.1271353 (2017).
Zhang, P., Li, H., Tan, X., Chen, L. & Wang, S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 37, 207–218, https://doi.org/10.1016/j.canep.2012.12.009 (2013).
Deng, D. et al. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: evidence from a meta-analysis. Diabetes/metabolism research and reviews 31, 595–602, https://doi.org/10.1002/dmrr.2645 (2015).
Yu, H. et al. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One 9, e116327, https://doi.org/10.1371/journal.pone.0116327 (2014).
Jian Gang, P., Mo, L., Lu, Y., Runqi, L. & Xing, Z. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocrine research 40, 54–61, https://doi.org/10.3109/07435800.2014.934961 (2015).
Wang, C. P. et al. Metformin for reducing racial/ethnic difference in prostate cancer incidence for men with type II diabetes. Cancer Prevention Research 9, 779–787, https://doi.org/10.1158/1940-6207.CAPR-15-0425 (2016).
Chen, C. B., Eurich, D. T., Majumdar, S. R. & Johnson, J. A. Metformin and the risk of prostate cancer across racial/ethnic groups: A population-based cohort study. Prostate Cancer and Prostatic Diseases 20, 122–126, https://doi.org/10.1038/pcan.2016.65 (2017).
Chen, C. B., Eskin, M., Eurich, D. T., Majumdar, S. R. & Johnson, J. A. Metformin, Asian ethnicity and risk of prostate cancer in type 2 diabetes: a systematic review and meta-analysis. Bmc Cancer 18, 65 (2018).
Habel, L. A. et al. Duration of metformin use and risk of prostate cancer in men with diabetes. Pharmacoepidemiology and Drug Safety 23, 170, https://doi.org/10.1002/pds.3701 (2014).
Lu-Yao, G. L. et al. Combination statin/metformin and prostate cancer specific mortality: A population-based study. Journal of Clinical Oncology 33 (2015).
But, A., Wang, H., Männistö, S., Pukkala, E. & Haukka, J. Assessing the Effect of Treatment Duration on the Association between Anti-Diabetic Medication and Cancer Risk. PLoS One 9, https://doi.org/10.1371/journal.pone.0113162 (2014).
Bensimon, L., Yin, H., Suissa, S., Pollak, M. N. & Azoulay, L. The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol Biomarkers Prev 23, 2111–2118, https://doi.org/10.1158/1055-9965.epi-14-0056 (2014).
Spratt, D. E. et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 63, 709–716, https://doi.org/10.1016/j.eururo.2012.12.004 (2013).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605, https://doi.org/10.1007/s10654-010-9491-z (2010).
Zaorsky, N. G. et al. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin Genitourin Cancer 15, 326–335.e323, https://doi.org/10.1016/j.clgc.2016.08.020 (2017).
Jarrard, D. F. et al. Impact of metformin on prostate cancer (PC) outcomes in the E3805 CHAARTED trial. Journal of Clinical Oncology 35 (2017).
Chong, R. W., Vasudevan, V., Zuber, J. & Solomon, S. S. Metformin Has a Positive Therapeutic Effect on Prostate Cancer in Patients With Type 2 Diabetes Mellitus. Am J Med Sci 351, 416–419, https://doi.org/10.1016/j.amjms.2016.01.013 (2016).
Joentausta, R. M., Kujala, P. M., Visakorpi, T., Tammela, T. L. J. & Murtola, T. J. Tumor features and survival after radical prostatectomy among antidiabetic drug users. Prostate Cancer and Prostatic Diseases 19, 367–373, https://doi.org/10.1038/pcan.2016.32 (2016).
Xu, H. et al. Validating drug repurposing signals using electronic health records: a case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc 22, 179–191, https://doi.org/10.1136/amiajnl-2014-002649 (2015).
Randazzo, M. et al. Influence of metformin use on PSA values, free-to-total PSA, prostate cancer incidence and grade and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol 33, 1189–1196, https://doi.org/10.1007/s00345-014-1426-y (2015).
Lee, H., Kuk, H., Byun, S. S., Lee, S. E. & Hong, S. K. Preoperative glycemic control status as a significant predictor of biochemical recurrence in prostate cancer patients after radical prostatectomy. PLoS One 10, e0124761, https://doi.org/10.1371/journal.pone.0124761 (2015).
Reznicek, D., Klyushnenkova, E. & Alexander, R. Metformin use predicts an overall survival advantage in diabetic veterans with prostate cancer. Journal of Urology 193, e146–e147 (2015).
Danzig, M. R. et al. Synergism between metformin and statins in modifying the risk of biochemical recurrence following radical prostatectomy in men with diabetes. Prostate Cancer and Prostatic Diseases 18, 63–68, https://doi.org/10.1038/pcan.2014.47 (2015).
Rieken, M. et al. Association of diabetes mellitus and metformin use with biochemical recurrence in patients treated with radical prostatectomy for prostate cancer. World J Urol 32, 999–1005, https://doi.org/10.1007/s00345-013-1171-7 (2014).
Onitilo, A. A. et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur J Cancer Prev 23, 134–140, https://doi.org/10.1097/CEJ.0b013e3283656394 (2014).
Tseng, C. H. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer 50, 2831–2837, https://doi.org/10.1016/j.ejca.2014.08.007 (2014).
Zannella, V. E. et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clinical Cancer Research 19, 6741–6750, https://doi.org/10.1158/1078-0432.CCR-13-1787 (2013).
Margel, D. et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 31, 3069–3075, https://doi.org/10.1200/jco.2012.46.7043 (2013).
Magliano, D. J., Davis, W. A., Shaw, J. E., Bruce, D. G. & Davis, T. M. E. Incidence and predictors of all-cause and site-specific cancer in type 2 diabetes: The Fremantle Diabetes Study. European Journal of Endocrinology 167, 589–599, https://doi.org/10.1530/EJE-12-0053 (2012).
Author information
Authors and Affiliations
Contributions
K.H. and H.H.: performed study searches, acquisited the data. K.H. and S.Y.: analyzed and interpretated the data. K.H. and H.W. wrote the manuscript and made revisions. L.Y. and R.C. designed the study, critical review and made the decision to submit.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, K., Hu, H., Ye, S. et al. The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Sci Rep 9, 2218 (2019). https://doi.org/10.1038/s41598-018-38285-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38285-w
This article is cited by
-
Understanding Cardiovascular Risk in Prostate Cancer: Role of Disparities, Diabetes, and Aging
Current Treatment Options in Cardiovascular Medicine (2024)
-
Novel sequential therapy with metformin enhances the effects of cisplatin in testicular germ cell tumours via YAP1 signalling
Cancer Cell International (2022)
-
Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF)
Scientific Reports (2022)
-
Family history, obesity, urological factors and diabetic medications and their associations with risk of prostate cancer diagnosis in a large prospective study
British Journal of Cancer (2022)
-
Metabolic syndrome and its pharmacologic treatment are associated with the time to castration-resistant prostate cancer
Prostate Cancer and Prostatic Diseases (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.