Abstract
Cognitive impairment is a core feature of schizophrenia (SCH). In addition to the toxic effect of Bilirubin (BIL), it has antioxidant properties that were associated with the psychopathology and cognitive impairment of psychiatric disorders. The aim of this study was to examine the correlation of serum total BIL (TBIL) concentration with cognitive impairment in SCH patients. We recruited 34 SCH patients and 119 healthy controls (HCs) in this case-control design. Cognition was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Serum TBIL concentration was measured using the immunoturbidimetric method. Serum TBIL concentration was significantly decreased in SCH patients compared to HCs after adjusting for age, gender, and education. Serum TBIL concentration in SCH patients was also positively correlated with the RBANS immediate memory score. Further stepwise multiple regression analysis confirmed the positive association between serum TBIL concentration and immediate memory score in SCH patients. Our findings supported that the decline in serum TBIL concentration was associated with the immediate memory impairment and psychopathology of SCH.
Similar content being viewed by others
Introduction
Schizophrenia (SCH) is a severe psychiatric disorder that affects around 0.7% of the population worldwide1. Although SCH primarily involves a series of psychiatric symptoms, cognitive impairment is regarded as a core feature of SCH that precedes the onset of psychotic symptoms, and persists throughout the illness course2,3,4,5,6. SCH-associated cognitive impairment emerges in almost all cognitive domains, and may influence treatment outcomes, rehabilitation, quality of life, and even employment7,8,9,10,11,12,13,14. Collectively, these findings support the notion that cognitive impairment should be considered a potential treatment target for SCH and an important focus of future studies. However, the underlying pathophysiology of cognitive impairment in SCH patients remains unclear and requires further investigation.
Bilirubin (BIL) is a powerful antioxidant that is believed to stem from heme15,16. It has been reported that BIL demonstrates a strong protective function in the body when the mechanism of defense against oxidative stress is challenged17. Interestingly, both increased and decreased total BIL (TBIL) concentration (or a diversity of BIL-related effects) associated with SCH patients has been reported in previous studies. For example, several studies have shown that plasma TBIL concentration in SCH patients is significantly decreased compared to healthy controls (HCs)18,19,20,21,22. Similarly, plasma TBIL concentration in first-episode SCH patients is found to be significantly lower than that in HCs22. Further study has indicated that both male and female SCH patients have an approximately two-fold lower BIL concentration compared to HCs23. Moreover, the urinary concentration of biopyrrins generated from BIL in SCH patients is significantly higher than that in HCs24,25. These studies support the hypothesis that the antioxidant system defects exist in SCH patients26,27. However, two recent studies have indicated that serum BIL concentration in SCH patients is significantly elevated compared to HCs28,29. Also, previous studies have shown that there is a significant association between moderate hyperbilirubinemia and SCH24,30,31,32,33,34. Therefore, further studies should be conducted to resolve the inconsistency among these findings.
Previous studies have shown that abnormal BIL concentration may influence cognitive function. For example, serum BIL concentration is reported to be positively correlated with the Mini-Mental State Examination scores and Montreal Cognitive Assessment (including attention, delayed recall and abstract) in patients with mild cognitive impairment (MCI)35. Another study finds a positive correlation between serum BIL concentration and cognitive function originated from a great number of cognitive domains in patients with subcortical ischemic vascular disease (SIVD)36. Abnormal concentration of BIL in the brain can cause microglia and astrocyte activation, impaired myelination, and neuronal cell death37. Moreover, serum TBIL concentration in Korean adults is closely associated with the leukoaraiosis, which is relevant to cognitive impairment38,39,40,41. These findings indicate that serum TBIL concentration may influence cognitive function in SCH patients. However, to our knowledge, no study has investigated the relationship between serum TBIL concentration and cognitive impairment in SCH patients. Therefore, the objective of this study was to examine whether serum TBIL concentration is significantly associated with cognitive impairment in SCH patients in a Chinese population.
Results
Sociodemographic and clinical characteristics
Table 1 showed the sociodemographic and clinical characteristics of all subjects. SCH patients and HCs did not differ in age, gender, and education. There were significant differences in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score and subscores of immediate memory, attention, language, and delayed memory between the two groups. These between-group differences remained significant after adjusting for age, gender and education. These significant cognitive differences also passed the Bonferroni correction. Further Pearson’s correlation analysis found that serum TBIL concentration was not related to the Positive and Negative Syndrome Scale (PANSS) total score, PANSS negative symptom, PANSS positive symptom, and PANSS general psychopathology in SCH patients.
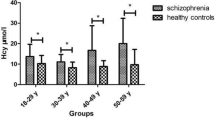
Serum TBIL concentration between SCH patients and HCs
Figure 1 showed that serum TBIL concentration in SCH patients was significantly decreased compared to HCs. This between-group difference remained significant after adjusting for age, gender and education.
Associations of serum TBIL concentration with the RBANS scores
The Pearson’s correlation analysis found a linear correlation of serum TBIL concentration with the immediate memory score in SCH patients, but not in HCs, as shown in Fig. 2. There were no significant correlations between serum TBIL concentration and other cognitive scores in SCH patients and HCs, respectively. Moreover, there were no relationships between serum TBIL concentration and cognitive scores in HCs with higher TBIL concentration (>11.14 umol/L or >14.00 umol/L).
Stepwise multiple regression analysis further indicated that serum TBIL concentration in SCH patients was related to the immediate memory score. In contrast, serum TBIL concentration in SCH patients was not associated with other cognitive scores (i.e., subscales of visuospatial/constructional, attention, language, and delayed memory, and RBANS total score). Moreover, serum TBIL concentration was not associated with the cognitive scores in HCs.
Discussion
Our study showed that SCH patients had more severe cognitive impairment than HCs in most cognitive domains, except for the visuospatial/constructional domain, which is in line with our previous findings9,10,42. This finding is supported by several longitudinal and cross-sectional studies in first-episode SCH patients43,44,45. Interestingly, cognitive impairment has been found to be closely associated with the abnormality of leukoaraiosis that was influenced by the decreased BIL concentration in a sample of Korean adults38,39,40,41. Specifically, the change in BIL concentration may lead to microglia and astrocyte activation, myelination damage, and apoptosis of neuron cells in the brain37. These findings suggest that BIL might play a critical role in cognitive impairment. In the present study, serum TBIL concentration was positively correlated with the immediate memory score in SCH patients in a Chinese population. It is hypothesized that the immediate memory impairment may be mediated through antioxidant system defects resulting from decreased TBIL concentration26,27,46,47. A recent study has found a positive correlation of serum TBIL concentration with the cognitive score in MCI35. Serum concentration of BIL also was significantly associated with the recent memory score in SIVD patients36. Thus, the underlying mechanism responsible for the association of serum TBIL concentration with the immediate memory score could reflect the abnormality of antioxidant system that could further lead to neuronal and microglia impairment of SCH brain. Also, future studies should investigate the associations between other antioxidants and immediate memory score in first-episode SCH patients to confirm our findings.
Moreover, our findings indicated that serum TBIL concentration was significantly lower in SCH patients than that in HCs, which is consistent with previous studies18,19,20,21,22. Another study found that serum BIL concentration in HCs was approximately two-fold that in SCH patients23. Further studies have found that first-episode SCH patients had lower plasma TBIL concentration than HCs22,23. Compared to HCs, serum TBIL concentration also was significantly decreased in patients with major depressive disorder48. These findings further support the hypothesis that the defects of antioxidant defense system might be involved in the etiology of SCH26,27.
Several limitations should be noted in this study. First of all, our study had a relatively small sample size. These findings should be considered as preliminary. Second, it was a cross-sectional study design. Prospective studies with longitudinal follow-ups should be performed to confirm the association between serum TBIL concentration and immediate memory score in SCH patients. Thus, it is not clear whether there is a causal association of decreased serum TBIL concentration with the immediate memory deficits in SCH patients in this study. Furthermore, we did not differentiate direct and indirect BIL from TBIL because direct BIL was not measured. Additionally, although SCH patients were antipsychotic free for at least two weeks prior to this study, the effect of antipsychotics on serum BIL concentration could not be ruled out. Previous studies have indicated that antipsychotics and metabolic syndrome influence BIL concentration in SCH patients49,50. Thus, future studies should be performed in first-episode and drug-free SCH patients without metabolic syndrome. Finally, we did not collect other relevant data, including body mass index, alcohol intake, and smoking. Whether they might affect serum TBIL concentration and cognitive function in SCH patients, which should be further investigated in the future.
Collectively, we found that serum TBIL concentration was lower in SCH patients than that in HCs in a Chinese population. Serum TBIL concentration was positively associated with the immediate memory score in SCH patients. Our data further demonstrated that decreased TBIL concentration might reflect oxidant defense defects underlying the psychopathology of SCH. Also, the decline in serum TBIL concentration might play an important role in the immediate memory impairment in SCH patients. However, these findings should be regarded as preliminary due to little sample size, missing related data of antipsychotics use and metabolic syndrome, and absence of a longitude design. Thus, future studies should confirm our present findings in first-episode and drug-free SCH patients in a larger prospective sample.
Methods
Subjects
The sample size was calculated by an online website (http://powerandsamplesize.com/) according to the findings of previous studies51,52. The minimum sample size of this study was calculated for 32 SCH patients and 46 HCs. However, 34 SCH patients and 119 HCs (about 1:4 ratio) recruited in this study were further determined according to the previous study reporting 1: 4 case/control ratio for matched case-control studies to find valid associations with regards to the power53.
We recruited 34 SCH patients (male = 21, female = 13) at the inpatient unit of the Affiliated Guangji Hospital of Soochow University. The inclusion criteria included the following: (1) age between 18 and 60 years, Han Chinese; (2) diagnosis of schizophrenia as confirmed by two psychiatrists using the Structured Clinical Interview for DSM-IV (SCID); (3) no previous exposure to antipsychotics at least 2 weeks prior to the beginning of this study; 4) able to provide the signed informed consent and participate in the psychopathology assessment.
For comparison, 119 HCs (male = 71, female = 48) were recruited at the same time from Suzhou local community. They were all Han Chinese. Two psychiatrists assessed their present psychiatric status, and collected personal or family history of psychiatric disorders using unstructured interviews. They did not have any psychiatric disorders as well as family history of psychiatric disorders.
A complete medical history and physical examination were obtained from all subjects. Any subjects with other medical illness i.e. substance abuse/dependence, liver disorder, cancer, diabetes, and pregnancy were excluded. The Institutional Review Board of the Affiliated Guangji Hospital of Soochow University approved the informed consent and protocol of this study, and all experiments would be carried out in accordance with the approved guidelines and regulations. All subjects must sign informed consent before they participated in this study.
Clinical measure
Each subject filled out a detailed questionnaire that recorded the general information, sociodemographic characteristics, and medical conditions. Other information was obtained from the available electronic medical records.
A clinical researcher used the RBANS to assess cognitive function in all subject54. The RBANS includes 12 subtests that are used to calculate a total score and 5 age-adjusted index scores. The test indices consist of attention, immediate memory, visuospatial/constructional, delayed memory, and language. Our research group has previously translated the English version of the RBANS into Chinese, and established its clinical validity and test-retest reliability in HCs and SCH patients55. The RBANS index scores were the standardized scores in this study.
Two psychiatrists used the PANSS to assess the severity of SCH psychopathology56. They had simultaneously attended a training session in the PANSS use before the beginning of this study. After training, a correlation coefficient greater more 0.8 was maintained for the PANSS total score by repeated assessments.
TBIL measurement
Serum samples were collected from the forearm vein between 7 and 9 AM following an overnight fast. The serum of samples was separated, aliquoted, and stored at −80 °C in a refrigerator before serum TBIL concentration was measured. We used a HITACHI automatic biochemistry analyzer (Mode: 7180, Japan) to measure serum TBIL concentration through the method of chemical oxidation using a commercially available kit (MedicalSystem Biotechnology, China). The sensitivity of this kit was 3.42 umol/L, and its intra- and inter-assay variation coefficients were 4% and 5%, respectively. For each sample, serum TBIL concentration was assessed in triplicate by our technician.
Statistical analysis
Since the sociodemographic and clinical characteristics including serum TBIL concentration were normally distributed in SCH patients and HCs (1-Sample Kolmogorov-Smirnov test), the comparisons between the two groups with regard to the sociodemographic and clinical characteristics were performed using analysis of variance (ANOVA) and Chi-squared test. The potential confounding variables were added as the covariates while the significant differences in the RBANS scores and serum TBIL concentration were observed between the two groups. Bonferroni correction were applied to each test to adjust for potential Type I error inflation due to multiple testing. Pearson’s correlation analyzed the correlations of serum TBIL concentration with the PANSS scores in SCH patients, and the associations of serum TBIL concentration with the RBNAS scores in SCH patients and HCs, respectively. Further stepwise multiple regression analysis was used to confirm the potential variables that might affect the RBANS scores in SCH patients and HCs. All statistical analyses were performed using the SPSS 17.0 for windows (SPSS Inc., Chicago, USA). The continuous variables were shown as mean and standard deviation (mean ± SD), and the statistical significance of all p values was set at 0.05 (2-sided).
References
WHO. The world health report 2001-Mental Health: New Understanding, New Hope. (Geneva: WHO, 2013).
Lencz, T. et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 59, 863–871 (2006).
Lieberman, J. A. et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 50, 884–897 (2001).
Dickerson, F. et al. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res. 129, 45–53 (2004).
Rund, B. R. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr Bull. 24, 425–435 (1998).
Hughes, C. et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. 59, 137–146 (2003).
Harvey, P. D., Geyer, M. A., Robbins, T. W. & Krystal, J. H. Cognition in schizophrenia: from basic science to clinical treatment. Psychopharmacology. 169, 213–214 (2003).
Mclntosh, B. J. et al. Performance-based assessment of functional skills in severe mental illness: results of a large-scale study in China. J Psychiatr Res. 45, 1089–1094 (2011).
Wu, J. Q. et al. Altered interleukin-18 levels are associated with cognitive impairment in chronic schizophrenia. J Psychiatr Res. 76, 9–15 (2016).
Hui, L. et al. Association between DBH 5′-insertion/deletion polymorphism and cognition in patients with chronic schizophrenia. J Clin Psychiatry. 77, 379–385 (2016).
Green, M. F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 153, 321–330 (1996).
Shean, G. D. Recent developments in psychosocial treatments for schizophrenic patients. Expert Rev Neurother. 7, 817–827 (2007).
Granholm, E. et al. Neuropsychological predictors of functional outcome in Cognitive Behavioral Social Skills Training for older people with schizophrenia. Schizophr Res. 100, 133–143 (2008).
Harvey, P. D. Pharmacological cognitive enhancement in schizophrenia. Neuropsychol Rev. 19, 324–335 (2009).
Maines, M. D., Ibrahim, N. & Kappas, G. A. Solubilization and partial purification of heme oxygenase from rat liver. J Bio Chem. 252, 5900–5903 (1997).
Yamaguchi, T., Komoda, Y. & Nakajima, H. Biliverdin-IXa reductase and biliverdin-IXb reductase from human liver. Purification and characterization. J Bio Chem. 269, 24343–24348 (1994).
Kozaki, N. et al. Bilirubin as an anti-oxidant for surgical stress: a preliminary report of bilirubin oxidative metabolites. HPB Surg. 11, 241–248 (1999).
Yao, J. K., Reddy, R., McElhinny, L. G. & van Kammen, D. P. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatr Res. 32, 385–391 (1998a).
Yao, J. K., Reddy, R., McElhinny, L. G. & van Kammen, D. P. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 32, 1–8 (1998b).
Yao, J. K., Reddy, R. & van Kammen, D. P. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 97, 137–151 (2000).
Pae, C. U. et al. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 50, 54–56 (2004).
Reddy, R., Keshavan, M. & Yao, J. K. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res. 62, 205–212 (2003).
Vítek, L. et al. Serum bilirubin levels and UGT1A1 promoter variations in patients with schizophrenia. Psychiatry Res. 178, 449–450 (2010).
Miyaoka, T., Seno, H., Itoga, M., Maeda, T. & Horiguchi, J. Schizophrenia-associated idiopathic unconjugated hyperbilirubi-nemia (Gilbert’s syndrome): 3 case reports. J Clin Psychiatry 61, 299–300 (2000a).
Yasukawa, R. et al. Increased urinary excretion of biopyrrins, oxidative metabolites of bilirubin, in patients with schizophrenia. Psychiatry Res. 153, 203–207 (2007).
Reddy, R. & Yao, J. K. Free radical pathology in schizophrenia: a review. Prostaglandins, Leukotrienes and Essential Fatty Acids. 55, 33–43 (1996).
Reddy, R. D. & Yao, J. K. Membrane protective strategies in schizophrenia: conceptual and treatment issues. 75–88 (Marius Press, Lancashire, 1999).
Semnani, Y., Nazemi, F., Azariyam, A. & Ardakani, M. J. E. Alteration of serum bilirubin level in schizophrenia. Int J Psychiatry Clin Pract. 14, 262–267 (2010).
Dadheech, G., Sharma, P. & Gautam, S. Oxidative stress-induced response of some endogenous antioxidants in schizophrenia. Ind J Clin Biochem. 27, 278–283 (2012).
Miyaoka, T. et al. Schizophrenia-associated idiopathic unconjugated hyper-bilirubinemia (Gilbert’s syndrome). J Clin Psychiatry 61, 868–871 (2000b).
Miyaoka, T., Seno, H., Itoga, M., Inagaki, T. & Horiguchi, J. Structural brain changes in schizophrenia associated with idio-pathic unconjugated hyperbilirubinemia (Gilbert’s syndrome): a planimetric CT study. Schizophr Res. 52, 291–293 (2001).
Miyaoka, T. et al. Proton magnetic resonance spectroscopy (1H-MRS) of hippocampus, basal ganglia, and vermis of cerebellum in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gil-bert’s syndrome). J Psychiatr Res. 39, 29–34 (2005a).
Miyaoka, T. et al. Fluid-attenuated inversion-recovery MR imaging in schizophrenia associated with idiopathic unconjugated hyperbilirubinemia (Gil-bert’s sybdrome). Eur Psychiatry. 20, 327–331 (2000b).
Miyaoka, T. et al. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 15, 249–252 (2005c).
Chu, Z. H., Wang, W. J., Han, L. Z. & Wu, L. The relationship between serum albumin, bilirubin and cognitive impairment in patients with mild cognitive impairment. The Journal of Practical Medicine. 31, 2818–2821 (2015).
Zhou, X., Wang, L., Liu, H. & Sun, Z. W. Relationship between serum bilirubin, uric acid and cognition impairment in patients with subcortical ischemic vascular disease. Chin J Neurol. 47, 305–310 (2014).
Brites, D. Bilirubin injury to neurons and glial cells: new players, novel targets, and newer insights. Semin Perinatol. 35, 114–120 (2011).
Sakakibara, R., Hattori, T., Uchiyama, T. & Yamanishi, T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 67, 658–660 (1999).
O’Sullivan, M. et al. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75, 441–447 (2004).
Prins, N. D. et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 128, 2034–2041 (2005).
Park, B. J. et al. Association between serum total bilirubin level and leukoaraiosis in Korean adults. Clin Biochem. 45, 289–292 (2012).
Zhang, X. Y. et al. Gender difference in association of cognition with BDNF in chronic schizophrenia. Psychoneuroendocrinology. 48, 136–146 (2014).
Laurel, A. T., Ross, M. G. N., Ashok, K. M., Anita, D. R. & Rashid, R. A. Changes in cognitive functioning following comprehensive treatment for first episode patients with schizophrenia spectrum disorders. Psychiatry Res. 113, 69–81 (2002).
Bozikas, V. P. & Andreou, C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry. 45, 93–108 (2011).
Addington, J., Brooks, B. L. & Addington, D. Cognitive functioning in first episode psychosis: initial presentation. Schizophr Res. 62, 59–64 (2003).
Wilk, C. M. et al. Test-Retest Stability of the Repeatable Battery for the Assessment of Neuropsychological Status in Schizophrenia. Am J Psychiatry 159, 838–844 (2002).
Zhang, X. Y. et al. Cognitive function, Plasma MnSOD activity, and MnSOD Ala-9Val polymorphism in patients with schizophrenia and normal controls. Schizophr Bull. 40, 592–601 (2014).
Peng, Y. F., Xiang, Y. & Wei, Y. S. The significance of routine biochemical markers in patients with major depressive disorder. Sci Rep. 6, 34402 (2016).
Garcia-Unzueta, M. T. et al. Alterations of liver function test in patients treated with antipsychotics. J Clin Lab Anal. 17, 216–218 (2003).
Karadag, F. et al. Relationship between serum bilirubin levels and metabolic syndrome in patients with schizophrenia spectrum disorders. Clin Psychopharmacol Neurosci. 15, 153–162 (2017).
Chow, S. C. Shao, J. & Wang, H. Sample size calculation in clinical research. (New York: Marcel Dekker, 2003).
Pae, C. U. et al. Decreased plasma antioxidants in schizophrenia. Neuropsychobiology. 50, 54–56 (2004).
Grimes, D. A. & Schulz, K. F. Compared to what? Finding controls for case-control studies. Lancet. 365, 1429–1433 (2005).
Randolph, C., Tierney, M. C., Mohr, E. & Chase, T. N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20, 310–319 (1998).
Zhang, B. H. et al. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) as a Screening Test in Chinese: Reliability and Validity. Chinese Mental Health Journal 22, 865–869 (2008).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 13, 261–276 (1987).
Acknowledgements
We are deeply grateful to all subjects. This study was supported by the National Natural Science Foundation of China (81771439 and 81501160), Jiangsu High-level Health Talent Project (LGY2018010 and QNRC2016228), Jiangsu Six Talent Peaks Project (WSN-165), Jiangsu Key Research and Development Program (BE2018662), Suzhou Key Diagnosis and Treatment Program (LCZX201820 and LCZX201515), the Prevention and Control Program of Suzhou Major Diseases (Gwzx201801), Suzhou Key Laboratory for Biological Psychiatry (SZS201722), Suzhou Key Medical Center for Psychiatric Diseases (Szzx201509), Suzhou Sci-Tech Bureau Program (SS201706 and SYSD2015136), Wenzhou Sci-Tech Bureau Program (Y20170077), Suzhou Health City “531” Action Plan, and Zhejiang Province Rising Star in Medicine.
Author information
Authors and Affiliations
Contributions
X.L.Y., Q.F.J., G.Y.Z., H.S.X. and L.H. contributed to the overall design of this study, conducted the statistical analysis, and wrote the first draft of manuscript. Q.F.J., G.Y.Z., C.X.J., X.Y.Y., Y.S.L., P.C., X.C.G., Z.K.Q. and G.Z.Y. recruited the subjects, performed the clinical assessment, and collected the serum samples. X.L.Y., Q.F.J. and G.Y.Z. were responsible for evolve the ideas and edit the manuscript. J.P.Z. and T.S. revised the language and contents of manuscript. H.S.X. and L.H. were responsible for write the protocol and manuscript, and provided the funding for this study. Each author approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, X.L., Jia, Q.F., Zhang, G.Y. et al. Association between decreased serum TBIL concentration and immediate memory impairment in schizophrenia patients. Sci Rep 9, 1622 (2019). https://doi.org/10.1038/s41598-018-38227-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38227-6
This article is cited by
-
A preliminary metabolomics study of the database for biological samples of schizophrenia among Chinese ethnic minorities
BMC Psychiatry (2024)
-
Risk factors for post-cerebral infarction cognitive dysfunction in older adults: a retrospective study
BMC Neurology (2024)
-
Sex differences between serum total bilirubin levels and cognition in patients with schizophrenia
BMC Psychiatry (2021)
-
Plasma bilirubin levels are reduced in first-episode psychosis patients and associates to working memory and duration of untreated psychosis
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.