Abstract
Dysregulation of the gut microbiome is associated with several life-threatening conditions and thus might represent a useful target for the prevention of dementia. However, the relationship between the gut microbial population and dementia has not yet been fully clarified. We recruited outpatients visiting our memory clinic to participate in this study. Information on patient demographics, risk factors, and activities of daily living was collected, and cognitive function was assessed using neuropsychological tests and brain magnetic resonance imaging scans. Faecal samples were obtained, and the gut microbiome was assessed by terminal restriction fragment length polymorphism (T-RFLP) analysis, one of the most well-established and reliable 16S ribosomal RNA-based methods for classifying gut microbiota. Patients were divided into two groups, demented and non-demented. Multivariable logistic regression models were used to identify the variables independently associated with dementia. The T-RFLP analysis revealed differences in the composition of the gut microbiome: the number of Bacteroides (enterotype I) was lower and the number of ‘other’ bacteria (enterotype III) was higher in demented than non-demented patients. Multivariable analyses showed that the populations of enterotype I and enterotype III bacteria were strongly associated with dementia, independent of the traditional dementia biomarkers. Further studies of the metabolites of gut microbes are needed to determine the mechanism underlying this association.
Similar content being viewed by others
Introduction
Globally, 47 million people were living with dementia in 2015, and this number is projected to triple by 20501. Dementia is an important healthcare problem in Japan, where by the mid-2010s, 4 million people, representing 15% of the over-65 population, had received a diagnosis of dementia2. Therefore, a comprehensive strategy for dementia research has been introduced to improve future healthcare in Japan3. Assessment of the risk of dementia from various viewpoints is useful because a multifactorial approach is important to determine the essential mechanisms underlying the disease.
Recently, differences in the gut microbiome have been found to be associated with several life-threating conditions, such as obesity, cardiovascular diseases and inflammatory diseases4,5,6. The gut microbiome can be defined as all of the species within the ecosystem and it is the largest reservoir of microbes in the human body, consisting of ~1014 cells7. Furthermore, recent research has identified a novel association between the gut microbiome and dementia7,8, suggesting that the gut microbiome may modulate host brain function via a microbiome–gut–brain axis9. The existence of such an axis has been hypothesised because changes in the gut microbiome have been shown to be part of the mechanism linking high levels of fat consumption and other unbalanced diets with impaired cognition9. More specifically, disruption of the neuro-inflammatory system may be caused by gut microbes10, which could lead to the deposition of amyloid β in the brain11,12. This may be a key component of the pathogenesis and progression of dementia, and particularly of Alzheimer’s disease. Nevertheless, the mechanism is still unclear and the composition of the gut microbiome differs among individuals according to their race and diet. Previous studies have revealed an association between the gut microbiome and cardiovascular disease in Japanese patients5,6; however, it remains to be established whether the composition of the gut microbiome is associated with dementia in the Japanese population.
In the present study, we aimed to investigate the relationship between the composition of the gut microbiome and dementia in Japanese patients, using a comprehensive assessment of cognitive function. We hypothesised that there would be differences in the composition of the gut microbiome between demented and non-demented patients.
Results
Patient characteristics
Informed consent was obtained from 181 patients who visited the memory clinic at the National Center for Geriatrics and Gerontology (NCGG) during the study period. Of these, 53 were excluded owing to missing data or faecal samples (n = 27), an incomplete neuropsychological assessment (n = 22) or a decline in the condition of the patient (n = 4). Therefore, we analysed 128 eligible patients (female: 59%, mean age: 74.2 ± 8.7 years, mean Mini Mental State Examination [MMSE] score 24). The patients were stratified by their level of cognitive function: 94 were classified as non-demented and 34 as demented. There were 14 participants in the demented group that had a CDR of 0.5 and a MMSE of less than 20.
Demented vs. non-demented patients
Compared with non-demented patients, demented patients were more likely to be female and to show fewer activities of daily living (ADL) and lower cognitive function (Table 1). Dementia patients scored lower on ADL, the Mini-Nutritional Assessment–Short Form (MNA-SF), MMSE, Raven’s Coloured Progressive Matrices, Frontal Assessment Battery and Logical Memory subtests I and II of the Wechsler Memory Scale-Revised; and scored higher on the Clinical Dementia Rating (CDR) scale, Dementia Behaviour Disturbance Scale, Zarit Caregiver Burden Interview and Alzheimer’s Disease Assessment Scale–cognitive subscale. Brain abnormalities, such as silent lacunar infarcts (SLIs) and cerebral microbleeds (CMBs), and high voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD) scores, were frequent on magnetic resonance imaging (MRI) scans of patients with dementia. There were no significant differences in the laboratory findings, pulse wave velocity or ankle brachial index between the two groups, other than in estimated glomerular filtration rate (eGFR; demented 61.7 vs. non-demented 70.7 mL/min/1.73 m2, P = 0.028, Table S1). Demented patients were more likely to be taking anti-dementia drugs and anti-hyperglycaemic drugs than non-demented patients (25.7% vs. 6.5%, P = 0.005; 23.5% vs. 8.6%, P = 0.035, respectively). However, there were no significant differences in the numbers of patients taking other medications, such as anti-hypertensive drugs, statins, anti-thrombotic drugs, proton pump inhibitors/H2 blockers or aperients, between the two groups (Table S1).
Composition of the gut microbiome
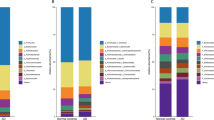
The dendrogram comparing the gut microbiome between demented and non-demented patients showed two major clusters (Fig. 1). Cluster 1 contained more patients with dementia than cluster 2 (40.4% vs. 18.5%, P = 0.012, Fisher’s exact test). Terminal restriction fragment length polymorphism (T-RFLP) comparisons of the gut microbiome composition showed that this differed between demented and non-demented patients (Fig. 2A). More specifically, demented patients had fewer microbes of enterotype I and more of enterotype III than non-demented patients, suggesting a lower prevalence of Bacteroides and a higher prevalence of ‘other’ bacteria (P < 0.001, Fig. 2B). We also found that Lactobacillales and Bifidobacterium were slightly more frequent (Fig. 3). Furthermore, the Firmicutes/Bacteroidetes (F/B) ratio was higher in the demented patients than the non-demented patients (median, interquartile range; 2.1, 1.3–3.0 vs. 1.4, 0.8–2.3, P = 0.013). The diversity of the gut microbiome was also assessed using the Shannon index and Simpson tests (Fig. S1). The Shannon index was significantly lower in the non-demented patients than the demented patients (1.89, 1.79–1.95 vs. 1.92, 1.88–2.03, P = 0.028) and the Simpson test showed a similar trend (0.82, 0.80–0.83 vs. 0.83, 0.81–0.85, P = 0.086).
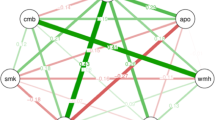
Regarding the relationships between MRI findings and the gut microbiome, the F/B ratio in patients with SLI was significantly higher than in those without (2.3, 1.5–3.6 vs. 1.5, 0.9–2.3, P = 0.028), but there were no significant differences with regard to white matter hypersensitivity (WMH), CMBs and cortical superficial siderosis (CSS) (Tables S2–5). Similarly, both the prevalence of ApoE ε4 and the numbers of patients taking anti-dementia drugs were not associated with the prevalence of either enterotype or the F/B ratio (Tables S6, S7).
Multivariable analysis
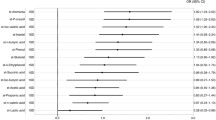
Stepwise multivariable logistic regression analyses (model 1 included enterotype I and model 2 included enterotype III) showed that female sex, enterotype, ApoE ε4, SLI or CMBs, high VSRAD score, and the use of anti-dementia drugs are independently associated with the presence of dementia (Table 2). These statistical models were appropriately fitted as multivariable logistic regression analyses (sensitivity, specificity, and area under the receiver operating characteristic curve [AUC]; model 1; 85%, 85%, 0.93; model 2; 88%, 86%, 0.93; respectively). However, when the analyses were repeated without including enterotype as a variable, the AUCs were lower (model 1; 85%, 79%, 0.89; model 2; 79%, 82%, 0.86; respectively). Although the sample size was too small to ensure complete reliability of the logistic regression analysis, a lower prevalence of Bacteroides and a higher prevalence of ‘other’ bacteria were associated with higher odds ratios than the traditional dementia biomarkers ApoE ε4, SLI and high VSRAD score.
Discussion
There has been a recent focus on adverse composition of the gut microbiome as a novel risk factor for dementia6,7,8. In the present study, multivariable analyses adjusted for traditional risk factors revealed that a lower prevalence of Bacteroides and a higher prevalence of other bacteria are independently and strongly associated with dementia, and these associations are stronger than those for traditional dementia biomarkers. Similar associations have been found in previous studies of patients with carotid stenosis13 and coronary artery disease5. Bacteroides can regulate endothelial cell function and reduce inflammation7, which is consistent with our finding of an inverse relationship between the population of this genus and the presence of dementia. Conversely, Vogt et al. concluded that a larger population of Bacteroidetes and a smaller population of Bifidobacterium in the gut of patients with Alzheimer’s disease8 is suggestive of a counter-regulatory effect of Bacteroidetes and/or a repressive effect of Bifidobacterium. This discrepancy may be due to various differences between the studies, such as ethnicity, dietary composition and the criteria used to diagnose dementia. In the earlier study, a CDR of 0.5 was used as the criterion for the diagnosis of early dementia8, but we categorised this score as representative of mild cognitive impairment, implying that the patient is not demented but has a high risk of dementia. Thus, it is probably too early to draw a conclusion. Nevertheless, the relationships between some types of gut microbe and systemic arteriosclerotic diseases suggest common underlying mechanisms in the effects of gut microbial composition on multi-organ arteriosclerosis. The diversity of the gut microbiome is an interesting potential mediator, but we cannot evaluate its importance in detail because our methods were not capable of identifying the ‘other’ bacteria.
We speculate that there is a common microvascular arteriosclerotic and/or inflammatory mechanism underlying cardiovascular and cerebrovascular diseases, and dementia, and some changes in the gut microbiome could accelerate this mechanism. Microvascular arteriosclerosis and inflammation are known risk factors for such diseases4,6,10,11,13,14. The significant association between SLI and F/B ratio in the present study is consistent with such a mechanism. Previous studies have revealed that a metabolite released by gut microbiota, trimethylamine N oxide (TMAO), is associated with arteriosclerosis and cardiovascular disease15,16, directly increases platelet hyperactivity and potentially thrombosis17, and may be a modifiable environmental promoter of Alzheimer’s disease18. Because metabolites of this type promote arteriosclerosis, inflammation and the immunological response, further assessment of the metabolites released by gut microbes is warranted to elucidate such associations. The reduction in the release of such metabolites, secondary to normalisation of the gut microbiome, may help to prevent these life-threatening diseases.
This study demonstrates a clear relationship between the gut microbiome and dementia in Japanese patients. Although differences in the gut microbial composition is suggested by a smaller population of Bacteroides and a larger population of ‘other’ bacteria, further analysis of differences in the composition of the gut microbiome will be important to clarify the gut-brain connection. Our results are consistent with the hypothesis that gut microbes are involved in the development of dementia. Furthermore, multivariable analyses revealed that the sensitivity, specificity, and AUC for models 1 and 2 were sufficient for the purposes of identifying dementia. Furthermore, addition of enterotype to these analyses improved the scores, showing that enterotype is specifically associated with the presence of dementia. Typical data obtained using next-generation sequencing technology for a microbiome study are presented as OTU counts that are complex; only having positive values, being widely dispersed, and having a large number of zeros19. In the present study we did not use this approach, but instead categorised the microbiome into three enterotypes. Although none of the patients demonstrated enterotype II in the demented group, we do not regard zero-inflation as a serious potential problem for our study.
The strengths of the present study were the large sample size compared with previous studies8,12, and the nutritional and comprehensive cognitive assessments conducted, including the diagnosing of dementia using detailed neuropsychological tests, brain MRI and single photon emission-computed tomography (SPECT) images. We have shown that bacterial enterotypes are independent risk factors for dementia and are associated with higher odds ratios than traditional risk factors.
However, our study also has several limitations. A causal relationship between differences in the gut microbiome and dementia could not be established because of the cross-sectional study design. A study with a relatively small number of patients may be at risk of being statistically under-powered, and indeed the absence of enterotype II among the demented patients may have affected the statistical interpretation. There is also the possibility of selection bias, because this study was performed in a single hospital-based cohort. High-throughput DNA sequencing technology would be useful to identify the specific genera or species of microbes collectively categorised as ‘other’ bacteria by the T-RFLP method. Other factors, such as the release of inflammatory biomarkers4, metabolites such as TMAO18, and nutritional and dietary parameters20, which may have acted as confounding factors, were not assessed. Because the MNA-SF score was lower in demented patients than non-demented patients, we will analyse the diet of the participants in more detail in a forthcoming further study of the gut microbiome. Analysis of the amyloid β precursor protein could also be useful, because a high serum concentration of this factor suggests inflammatory endothelial dysfunction and a risk of cognitive decline21. Subtypes of dementia, such as Alzheimer’s disease or frontotemporal lobar degeneration, have not been considered in the present study, because its aim was to determine the relationship between the gut microbiome and dementia; all-cause dementia was defined by simple categorisation on the basis of the MMSE and CDR scores. Likewise, the potential effects of anti-dementia drugs have not been fully considered because of this study design. However, specific types of dementia may have stronger or weaker associations with the composition of the gut microbiome. Further studies that take these factors into consideration are needed to complement our findings.
Conclusions
We have shown that components of the gut microbiome, in particular Bacteroides and ‘other’ bacteria, are independently associated with dementia, and these associations are stronger than those of traditional dementia biomarkers.
Methods
Study design
This was a single-center observational study designed to investigate the association between the composition of the gut microbiome and the clinical condition of the patient, assessed using ADL and cognitive function, named Gerontological investigation of microbiome: a longitudinal estimation study (Gimlet study). This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board at the National Center for Geriatrics and Gerontology (NCGG). Informed consent was obtained from all patients and their families before participating in this study. The study is registered with the UMIN Clinical Trials Registry (UMIN000031851).
Subjects
Between March 2016 and March 2017, we enrolled consecutive patients visiting the memory clinic at the NCGG who agreed to undergo medical assessment of their cognitive function and faecal examination to survey the gut microbiome. Patients were eligible for the study if they: (1) were able to undergo brain MRI; (2) provided informed consent in writing; (3) provided informed consent for the NCGG Biobank to store their clinical data, blood and faecal samples; and (4) were accompanied by a study partner who could assess the condition of the patient. We excluded patients if they: (1) were unable to undergo MRI examination, or the MRI scan could not be evaluated because of movement; (2) had local lesions, such as cerebral infarction, detected by MRI before enrolment, which could significantly affect cognitive functioning; (3) had a history of a major psychological disorder, or current serious or unstable alcohol or drug abuse; (4) had ≤6 years of education; (5) had a history of cancer of the digestive tract; or (6) were judged by an investigator to be ineligible to participate as a study subject (because of the presence of a brain tumour, encephalitis/meningitis, normal pressure hydrocephalus, Huntington’s disease, progressive supranuclear palsy, corticobasal degeneration, multiple system atrophy, subdural hematoma, multiple sclerosis or lower cognitive function due to head injury).
Baseline assessment
We assessed the following clinical parameters: (1) demographic characteristics, such as age, sex and years of education; (2) the presence of risk factors, such as hypertension, dyslipidaemia, diabetes mellitus, ischaemic heart disease, chronic kidney disease, smoking habits, history of stroke and alcohol consumption; (3) global cognitive function, using MMSE22 and CDR23; (4) neuropsychology, using the Alzheimer’s Disease Assessment Scale-cognitive subscale24, Raven’s Coloured Progressive Matrices25; Frontal Assessment Battery26 and Logical Memory subtests I and II of the Wechsler Memory Scale-Revised27; (5) laboratory variables, including ApoE ε4 as a risk factor for AD; (6) ankle brachial index and pulse wave velocity as indicators of arteriosclerosis28, and the ‘impact’ of pulse14; and (7) brain imaging (MRI and SPECT). The clinical samples and data were provided by the NCGG Biobank, which collects clinical data for research. Detailed information is provided in the Supplementary File.
Comprehensive geriatric assessment
All participants underwent a comprehensive geriatric assessment using: (1) the Barthel Index29 to assess basic ADL, and the Lawton and Brody scale to assess instrumental ADL30; (2) MMSE and CDR to assess global cognitive function; (3) the Geriatric Depression Scale31 to exclude depressive status; (4) the Dementia Behaviour Disturbance Scale32 to assess behavioural and psychological symptoms; (5) the Vitality Index to measure vitality in elderly patients; (6) Zarit Caregiver Burden Interview33 to assess the burden of caregivers; (7) assessment of movement parameters, such as gait speed, timed up and go test34 and fall history; (8) assessment of other items, such as the presence of frailty and hearing loss; (9) assessment of social and lifestyle factors, such as using the MNA-SF to assess nutritional status35; and (10) assessment of current medication (anti-dementia drugs, anti-hypertensive drugs, statins, proton pump inhibitors/H2 blockers, anti-thrombotic drugs, anti-hyperglycaemic drugs and aperients).
Classification of cognitive function
We divided patients into two categories according to their MMSE and CDR scores: (1) a non-demented group (MMSE ≥20 and CDR <1) and a demented group (MMSE <20 and/or CDR ≥1), because these measures reliably indicate the presence of dementia. A CDR score of 0.5 is regarded as indicating the presence of MCI and possibly very mild dementia, meaning that the patient has a higher risk of dementia36. Therefore, we categorised a CDR score of 0.5 as representing mild cognitive impairment and included these patients in the non-demented group in the present study.
Brain imaging
Patients underwent 1.5T MRI of the brain (Philips Ingenia, Eindhoven, the Netherlands). MRI scans, including diffusion-weighted imaging, fluid-attenuated inversion recovery imaging, T2-weighted imaging, T2*-weighted gradient echo imaging, 3D T1-weighted sagittal and axial coronal views, and 3D time-of-flight MR angiography scans, were obtained. The presence and components of cerebral small vessel disease were categorised using the standards for reporting vascular changes on neuroimaging37, including recent subcortical small infarcts, SLIs, WMH, CMBs and CSS. We used VSRAD advance (Eisai Co., Ltd., Tokyo, Japan) software to quantify cortical and hippocampal atrophy using a standardised z-score.
Patients also underwent N-isopropyl-p-[123I]-iodoamphetamine-SPECT. We assessed the presence or absence of a reduction in blood flow in the area of the posterior cingulate gyrus and/or precuneus as a surrogate marker of Alzheimer’s disease38.
Gut microbiome
Faecal samples were collected at home while patients were consuming their usual diet and were frozen and preserved at −81 °C at the NCGG Biobank. After all the samples had been collected, the gut microbiome was analysed using T-RFLP analysis by the TechnoSuruga Laboratory (Shizuoka, Japan)39. Microbial DNA was extracted from the faecal samples and amplified by polymerase chain reaction. The resulting 16S rDNA amplicons were treated with BslI (New England BioLabs, Ipswich, MA, USA). T-RFLP analysis is one of the most well-established and reliable 16S ribosomal RNA-based methods, especially when considering its high throughput and reproducibility (see Supplementary Methods). First, T-RFLP was used to classify gut microbes into the following 10 groups: Prevotella, Bacteroides, Lactobacillales, Bifidobacterium, Clostridium cluster IV, Clostridium subcluster XIVa, Clostridium cluster IX, Clostridium cluster XI, Clostridium cluster XVIII, and ‘others’5. Second, we stratified the gut microbiome into three enterotypes: enterotype I included Bacteroides at >30%, enterotype II included Prevotella at >15% and enterotype III included the remaining bacteria, by reference to the Human Faecal Microbiome T-RFLP profile5,40. A recent metagenomic analysis defined these three major clusters of gut microbes in humans on the basis of the predominant bacterial genera present in faecal specimens5. This classification was based on the previously established phylogenetic profile similarities obtained by mapping metagenomic reads to 1,511 reference genomes. Multidimensional cluster analysis and principal component analysis showed three distinct clusters, which were designated enterotypes I to III40. Enterotype III can also be characterised by the presence of few Bacteroides and Prevotella, rather than by a dominant genus5.
Third, we assessed the F/B ratio5. The phylum Firmicutes includes the Lactobacillales and the Clostridium clusters, and the phylum Bacteroidetes includes Bacteroides and Prevotella.
Statistical analysis
Continuous, ordinal and categorical variables are expressed as mean ± standard deviation, median and interquartile range, and frequency or proportion (percentage), and were compared using the unpaired Student t-test, Wilcoxon rank-sum test and χ2 test, respectively. First, the composition of the gut microbiome was analysed using a dendrogram and the T-RFLP patterns were analysed using Euclidean distance and the Ward Method. Second, we divided patients into two groups according to the presence or absence of dementia and compared their clinical characteristics using the Wilcoxon signed-rank test and the χ2 test. Third, we compared the composition of the gut microbiome between the two groups using the results of the T-RFLP analysis. In particular, the numbers of patients with enterotypes I, II and III were compared between the dementia and non-dementia groups using the χ2 tests. We then evaluated the differences in particular taxa using the Wilcoxon signed-rank test. The diversity of the gut microbiome was also assessed using the Shannon and the Simpson tests.
Next, we used multivariable logistic regression models to identify the variables independently associated with dementia. Backward stepwise multivariable logistic regression analyses were performed, adjusting for patient demographics (age, sex and education years), gut microbiota (enterotype I was included in model 1, enterotype III in model 2, and the F/B ratio in both), risk factors (hypertension, diabetes mellitus, dyslipidaemia, chronic kidney disease, ischaemic heart disease, history of stroke, smoking habit, alcohol drinking habit and ApoE ε4), the MNA-SF, brain MRI findings (SLI, WMH, CMBs, CSS and VSRAD score), blood flow reduction on SPECT images (posterior cingulate gyrus and/or precuneus), and current medication (anti-dementia drugs and anti-hyperglycaemic drugs). The sensitivity, specificity and AUC were also calculated to evaluate the usefulness of the technique for identification of the presence of dementia. Odds ratios are presented with 95% confidence intervals. All comparisons were two-tailed, and P < 0.05 was considered to represent statistical significance. All data were analysed using the JMP 11.0 software package (SAS Institute Inc., Cary, NC).
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Livingston, G. et al. Dementia prevention, intervention, and care. Lancet. 390, 2673–2734 (2017).
National Institute of Public Health. Dementia prevalence in the urban area and correspondence to a disorder of activities of daily living attribute to dementia, https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201218011A (2012).
Saji, N. et al. Orange’s challenge: Developing wide-ranging dementia research in Japan. Lancet Neurol. 15, 661–662 (2016).
Jiang, C. et al. The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58, 1–15 (2017).
Emoto, T. et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 23, 908–921 (2016).
Yamashita, T. et al. Intestinal immunity and gut microbiota as therapeutic targets for preventing atherosclerotic cardiovascular diseases. Circ J. 79, 1882–1890 (2015).
Alkasir, R. et al. Human gut microbiota: The links with dementia development. Protein Cell. 8, 90–102 (2017).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 7, 13537, https://doi.org/10.1038/s41598-017-13601-y (2017).
Solas, M., Milagro, F. I., Ramírez, M. J. & Martínez, J. A. Inflammation and gut-brain axis link obesity to cognitive dysfunction: Plausible pharmacological interventions. Curr Opin Pharmacol. 37, 87–92 (2017).
Wu, S. C., Cao, Z. S., Chang, K. M. & Juang, J. L. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in drosophila. Nat Commun. 8, 24, https://doi.org/10.1038/s41467-017-00040-6 (2017).
Pistollato, F. et al. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 74, 624–634 (2016).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 49, 60–68 (2017).
Karlsson, F. H. et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 3, 1245 (2012).
Saji, N., Toba, K. & Sakurai, T. Cerebral small vessel disease and arterial stiffness: Tsunami effect in the brain? Pulse. 3, 182–189 (2015).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 472, 57–63 (2011).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 368, 1575–1584 (2013).
Zhu, W. et al. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 165, 111–124 (2016).
Xu, R. & Wang, Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 10(Suppl 3), 63 (2016).
Xu, L. et al. Assessment and selection of competing models for zero-inflated microbiome data. PloS One. 10(7), e0129606 (2015).
Tomata, Y. et al. Dietary patterns and incident dementia in elderly Japanese: The Ohsaki cohort 2006 study. J Gerontol A Biol Sci Med Sci. 10, 122–1328 (2016).
Saji, N. et al. Cilostazol may decrease plasma inflammatory biomarkers in patients with recent small subcortical infarcts: a pilot study. J Stroke Cerebrovasc Dis. 6, 1639–1645 (2018).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”; a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–198 (1975).
Morris, J. C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 43, 2412–2414 (1993).
Rosen, W. G., Mohs, R. C. & Davis, K. L. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 141, 1356–1364 (1984).
Raven J. Guide to using the coloured progressive matrices. London. H.K. Lewis (1965).
Dubois, B., Slachevsky, A., Litvan, I. & Pillon, B. The FAB: A frontal assessment battery at bedside. Neurology. 55, 1621–1626 (2000).
Wechsler D. Wechsler memory scale-revised. San Antonio, TX. Psychological Corporation (1981).
Saji, N. et al. Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res. 38, 323–328 (2015).
Mahoney, F. I., Barthel, D. W. & Callhan, J. P. Rehabilitation of the hemiplegic patient: A clinical evaluation. South. Med. J. 48, 472–480 (1955).
Lawton, M. P. & Brody, E. M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 9, 179–186 (1969).
Yesavage, J. A. et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 17, 37–49 (1982).
Baumgarten, M., Becker, R. & Gauthier, S. Validity and reliability of the dementia behavior disturbance scale. J Am Geriatr Soc. 38, 221–226 (1990).
Zarit, S. H., Reever, K. E. & Bach-Peterson, J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist. 20, 649–655 (1991).
Podsiadlo, D. & Richardson, S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 39, 142–148 (1991).
Vellas, B. et al. Overview of the MNA–Its history and challenges. J Nutr Health Aging. 10, 456–465 (2006).
Perneczky, R. et al. Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 14, 139–144 (2006).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2016).
Ito, K. et al. Prediction of outcomes in MCI with (123)I-IMP-CBF SPECT: a multicenter prospective cohort study. Ann Nucl Med. 27, 898–906 (2013).
Osborn, A. M., Moore, E. R. & Timmis, K. N. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ Microbiol. 2, 39–50 (2000).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature. 473, 174–180 (2011).
Acknowledgements
This study was supported by research grants from the Research Funding of Longevity Sciences (26-20, 27-21, 28-15, 30-1), the National Center for Geriatrics and Gerontology, grants from the NARO Bio-oriented Technology Research Advancement Institution project (Advanced integration research for agriculture and interdisciplinary fields), and grants from the Toyoaki Scholarship Foundation. We thank Maki Yamamoto, Yukie Ohsaki, Saori Yoshimura and Hitomi Kozaki (NCGG) for their technical and secretarial assistance, and the BioBank, NCGG, for the quality control of the clinical samples and data. We thank Rachel Baron, PhD and Mark Cleasby, PhD, from Edanz Group (www.edanzediting.com/ac) for editing drafts of this manuscript. We thank Hiroko Kawasaki, PhD from Biological Resource Center, National Institute of Technology and Evaluation (NITE) for valuable discussions.
Author information
Authors and Affiliations
Contributions
N.S. is the principal investigator and contributed to the concept, drafting and design of the protocol. S.N., K.M., T.H., T.T., T.S., A.K., K.T. and T.S. contributed to the design of the study, analysis of the data and review of the manuscript’s intellectual content.
Corresponding author
Ethics declarations
Competing Interests
Dr. Saji has received grants from the NARO Bio-oriented Technology Research Advancement Institution project (Advanced integration research for agriculture and interdisciplinary fields), the BMS/Pfizer Japan Thrombosis Investigator Initiated Research Program, and grants from the Toyoaki Scholarship Foundation. Dr. Saji, Dr. Niida and Dr. Sakurai have received research grants from the Research Funding of Longevity Sciences from the National Center for Geriatrics and Gerontology. Dr. Saji, Dr. Niida, Dr. Toba and Dr. Sakurai have received research funds for Comprehensive Research on Aging and Health from the Japan Agency for Medical Research and Development (AMED). Dr. Tsuduki has received grants from the NARO Bio-oriented Technology Research Advancement Institution project (Advanced Integration Research for Agriculture and Interdisciplinary Fields).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saji, N., Niida, S., Murotani, K. et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep 9, 1008 (2019). https://doi.org/10.1038/s41598-018-38218-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38218-7
This article is cited by
-
Gut microbiota, circulating cytokines and dementia: a Mendelian randomization study
Journal of Neuroinflammation (2024)
-
Research progress on intestinal microbiota regulating cognitive function through the gut-brain axis
Neurological Sciences (2024)
-
Gut inflammation associated with age and Alzheimer’s disease pathology: a human cohort study
Scientific Reports (2023)
-
Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis
Translational Neurodegeneration (2022)
-
Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions
Journal of Translational Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.