Abstract
The resilience of corals to ocean acidification has been proposed to rely on regulation of extracellular calcifying medium pH (pHECM), but few studies have compared the capacity of coral species to control this parameter at elevated pCO2. Furthermore, exposure to light and darkness influences both pH regulation and calcification in corals, but little is known about its effect under conditions of seawater acidification. Here we investigated the effect of acidification in light and darkness on pHECM, calcifying cell intracellular pH (pHI), calcification, photosynthesis and respiration in three coral species: Stylophora pistillata, Pocillopora damicornis and Acropora hyacinthus. We show that S. pistillata was able to maintain pHECM under acidification in light and darkness, but pHECM decreased in P. damicornis and A. hyacinthus to a much greater extent in darkness than in the light. Acidification depressed calcifying cell pHI in all three species, but we identified an unexpected positive effect of light on pHI. Calcification rate and pHECM decreased together under acidification, but there are inconsistencies in their relationship indicating that other physiological parameters are likely to shape how coral calcification responds to acidification. Overall our study reveals interspecies differences in coral regulation of pHECM and pHI when exposed to acidification, influenced by exposure to light and darkness.
Similar content being viewed by others
Introduction
The dissolution of anthropogenic carbon dioxide in the world’s oceans is simultaneously reducing seawater pH and the concentration of carbonate ions, a process commonly termed “ocean acidification”. As ocean acidification intensifies in future decades and beyond, changes in ocean carbonate chemistry are predicted to have negative impacts for many marine organisms. Biomineralizing marine organisms, such as reef-building corals, have been highlighted as particularly vulnerable to ocean acidification, due to the deleterious effects of ocean acidification on both rates of calcification and skeleton formation1,2. Understanding this vulnerability and any potential resilience to future ocean acidification hinges on an improved mechanistic vision of the physiological processes driving calcification3.
In reef corals, the aragonite skeleton forms in the extracellular calcifying medium (ECM) (=subcalicoblastic medium), which is spatially separated from the external seawater environment by the overlying calicoblastic (calcifying) epithelium and other overlying tissue layers. Current knowledge of ECM chemistry is limited, but several studies have shown that the pH of the calcifying medium (pHECM) is elevated with respect to the surrounding seawater. These include studies that have made direct measurements of pHECM by confocal microscopy using pH sensitive dyes4,5,6, studies that have inserted microelectrodes into the coral tissues7,8,9 and analysis of boron isotopes in the aragonite skeleton itself10,11. Because elevations in pH increase the relative concentration of CO32− in the total dissolved inorganic carbon (DIC) pool and thus the saturation state of aragonite (Ω) of the ECM, regulation of pHECM is considered to be one of the key steps in the biological control of biomineralization: setting up an elevated saturation state of aragonite in order to promote the calcification reaction12.
The importance of pHECM regulation in calcification physiology has led to intense research interest into how this parameter responds to ocean acidification. Studies have shown that pHECM declines in response to changes in seawater pH, potentially lowering Ω of the ECM which could be unfavourable to the maintenance of calcification rates5,8. However, research in the laboratory, field-based mesocosms and at natural CO2-vent sites have demonstrated that several coral species are capable of maintaining elevated pHECM despite severe decreases in external seawater pH5,13,14. Sometimes referred to as “pH upregulation” this trait has been suggested to be a possible physiological mechanism of resistance against ocean acidification5,15. However, there are surprisingly very few comparative studies that investigate the relative ability of different coral species to regulate pHECM under seawater acidification treatments. Previous comparisons of the capacity of corals to regulate pHECM against seawater acidification have mainly been conducted by compiling the results of different studies, which used different methodologies (e.g. boron isotopes, confocal microscopy)15,16. Few studies have directly compared the capacity of different coral species to regulate pHECM in a single controlled investigation apart from a recent study on Pocillopora damicornis and Acropora youngeii17.
Exposure to light or darkness is likely to be an important environmental factor in shaping the response of pHECM regulation to ocean acidification, as it is already known to affect coral calcification rates. Many corals are symbiotic with photosynthetic dinoflagellates (family Symbiodiniaceae18) and it is known that light can enhance calcification rates with respect to dark values (Light-Enhanced Calcification or LEC)19,20. More recently is has been shown that exposure to light can also influence the susceptibility of coral calcification rates to acidification21,22. Other work in the last decade has shown how light drives pH changes in coral endoderm intracellular pH (pHI) and how light-induced elevations in pHI mitigate endoderm cell acidosis at elevated seawater pCO223,24,25. However, there is currently little information on the effect of light and darkness on pHECM and intracellular pH (pHI) of the calicoblastic cells, particularly under conditions of acidification.
Here, we addressed these knowledge gaps in the current literature with a study that investigated how exposure to light and darkness affects pHECM and pHI regulation in different species of symbiotic coral under seawater acidification. We selected three species of coral that would potentially display differential responses to acidification. Two of these species, Stylophora pistillata (which is widely considered as resistant to ocean acidification26) and Pocillopora damicormis (for which responses to pCO2 are variable), belong to the scleractinian “robust” clade27. The third species, Acropora hyacinthus, is a member of a genus that is generally considered as vulnerable to ocean acidification and is a member of the “complex” clade28,29,30. The investigation was conducted under controlled laboratory conditions, allowing us to carry out in-vivo confocal microscopy to analyse pH regulation in both calcifying cells and the extracellular calcifying medium under different levels of acidification in darkness and at one irradiance level. To perform confocal analysis measurements were conducted on coral microcolonies grown on glass coverslips. In parallel experiments, we also investigated the effect of seawater acidification on calcification, photosynthesis and respiration rates in the three species using microcolonies suspended from monofilament threads.
Materials and Methods
Experimental set-up
Colonies of Stylophora pistillata, Pocillopora damicornis and Acropora hyacinthus were grown in the long-term coral culture facilities at the Centre Scientifique de Monaco where aquaria were supplied with flowing seawater from the Mediterranean sea (exchange rate 2% h−1), at a salinity of 38, under an irradiance of 175 µmol photons m−2 s−1 of photosynthetically active radiation (PAR) (400–700 nm) on a 12 h: 12 h light: dark cycle. Samples were prepared from mother colonies as nubbins suspended on monofilament threads (for measurements of calcification, photosynthesis and respiration) or microcolonies grown laterally on glass coverslips (for in vivo confocal microscopy of pHECM and pHI as described previously4). Temperature was monitored continuously by temperature sensors (Ponsel, France) and a monitoring system (Enoleo, Monaco) and maintained at 25 ± 0.3 °C (mean ± SD). Corals were fed daily with frozen rotifers and twice weekly with live Artemia salina nauplii.
For exposure to seawater acidification, colonies were transferred from the long-term culture facilities described above to eight seawater acidification aquaria supplied with Mediterranean seawater (exchange rate 60% per hour), at the same salinity, temperature and under the same irradiance conditions described above for culture facilities. This seawater acidification setup has been in continuous operation for several years and has been described in previous publications2,31. Each pH treatment was represented by two aquaria and coral colonies were randomly distributed as evenly as possible between them. Exposure to seawater acidification was conducted for a one-week duration. That is to say that each coral used in the investigation was transferred from long-term culture tanks to a tank in the seawater acidification set up for 1 week, before being removed and analysed for the appropriate physiological parameter. Because the number of replicates meant that all corals could not be analysed with in a single week, one week exposures in treatment tanks were repeated in time for 6 months to gather all the necessary data.
Control and maintenance of carbonate chemistry of the seawater pH treatments
In 6 of the 8 aquaria, carbonate chemistry was manipulated by bubbling with CO2 to reduce pH aquaria to the target values of pHT 7.2, 7.4 and 7.8 (Table 1). The two other aquaria were bubbled with CO2- free air (Tanks 1 and 2, pH 8.1, Table 1). Submersible pumps (EHEIM 3000, pump power 1800 l/h) ensured high water circulation in each aquarium. Aquaria were rigorously cleaned every week to prevent the growth of epiphytic algae and fouling communities or the accumulation of detritus.
pH electrodes (Ponsel-Mesure, France) calibrated to pH total scale and temperature sensors (Ponsel, France) were installed in the tanks and connected to a custom-made monitoring system (Enoleo, Monaco) which monitored pH and temperature continuously, and controlled CO2 bubbling rates and heating elements. pH measurements were also made using the indicator dye m-cresol purple (Acros 199250050) adapted from Dickson et al.32; the absorbance was measured using a spectrophotometer (UVmc2; Safas, Monaco). Measurements of total alkalinity (TA) were made according to protocols described in Dickson et al.32. TA was measured via titration with 0.03 N HCl containing 40 g NaCl l−1 using a Metrohm Titrando 888 Dosimat controlled by Tiamo software to perform automated titrations of 4-mL samples, and alkalinity was calculated using a regression routine based on Department of Energy guidelines33. For each sample run, certified seawater reference material supplied by the laboratory of A. G. Dickson (Scripps Institution of Oceanography, La Jolla, CA) was used to verify acid normality. Parameters of carbonate seawater chemistry were calculated from total scale pH, TA, temperature, and salinity using the free-access CO2SYS package34 using constants from Mehrbach et al.35 as refit by Dickson and Millero36. Spectrophotometric pH measurements and TA measurements were taken weekly, in addition to continuous monitoring by pH electrode during the 6-month period in which replicate week-long seawater acidification exposures were carried out. Mean values and standard errors of parameters of carbonate seawater chemistry in each treatment are given for the 6-month experimental period in Table 1.
Confocal microscope measurements of extracellular calcifying medium pH and intracellular pH in calcifying cells
Measurements of pH of the extracellular calcifying medium (pHECM) and intracellular pH (pHI) in calicoblastic cells in the light and dark were made on separate samples by inverted confocal microscopy (Leica SP5, Germany) and the ratiometric dye SNARF-1 (Invitrogen) according to methods we published previously4,17.
Samples grown laterally on glass coverslips were fitted in semi-closed perfusion chambers (PeCon, Germany) and mounted on the confocal microscope and supplied by perfusion with seawater drawn from the desired acidification treatment. A single irradiance level was provided at the same level as treatment aquaria (175 μmol photons m−2·s−1 PAR, which has also been used in previous investigations2,37), and temperature maintained at 25 °C. The chosen irradiance and temperature also corresponded to the light level provided during long-term culture of parent colonies and growth of the coral microcolonies and the acidification treatments.
A renewal rate of 50% per min of a 2.5-mL volume in the perfusion chamber ensured that seawater pH remained stable in both light and dark conditions2. The pH of the perfused seawater was checked by making confocal pH measurements in the seawater surrounding the corals to check that pH did not drift away from the target values used in treatments during the period of measurement (seawater pH values in the perfusion chamber are given in Supplementary 1). Measurement of oxygen in seawater in the perfusion chamber with a needle-type microsensor (PreSens, Germany) in light and darkness indicated oxygen levels also remained stable between values of 265–280 µmol l−1 under these conditions.
After being transferred from the treatment aquaria directly to the microscope, samples were first perfused with seawater from the desired experimental treatment for 20 min in either the light or dark. For pHECM, samples were then perfused with seawater from the desired treatment containing 45 µM cell-impermeable SNARF-1 for a 5 min loading period, before making five measurements of pHECM during a 10 min time window in light or dark. pH measurements were also taken in the seawater surrounding the corals in the perfusion chamber to confirm seawater pH remained stable during confocal analysis.
For measurements of pHI of calicoblastic cells, the procedure involved 10 min of dye loading by perfusion with seawater containing 10 mM cell-permeable SNARF-1 AM, followed by 10 min of seawater perfusion, during which pHI measurements were taken to check pHI was stable. Calibration of fluorescence of intracellular SNARF-1 AM and extracellular SNARF-1 (seawater and ECM) with pH was performed as described previously4 to the National Bureau of Standards (NBS) pH scale and total scale pH, respectively.
pHECM and calicoblastic cell pHI measurements were carried out at 40X magnification by excitation at 543 nm at 30% laser intensity, and fluorescence captured at emission wavelengths of 585 ± 10 nm and 640 ± 10 nm. For each measurement, several optical sections were captured in a Z-stack without contamination by chlorophyll autofluorescence by the symbiotic algae in the overlying tissues4,23. pHECM was measured in light and dark conditions in 5 samples from each treatment. Calicoblastic cell pHI was measured in 3 colonies from each treatment.
Calcification
Calcification was measured using the alkalinity anomaly technique38 using microcolonies suspended from monofilament threads. After 7 d of incubation in each pH treatment, colonies were chosen randomly from the tanks and placed in separate 50-mL plexi-glass beakers containing filtered (0.2 µm) seawater from the respective treatment tanks. Incubations were performed in light or darkness for 1 h with the same conditions of temperature (25 °C) and light (175 µmol photons m−2 s−1) as in the treatment aquaria, after which 20 ml of seawater was removed for TA measurement (procedure described above). Alkalinity anomalies were calculated correcting for treatment-specific blank beakers and taking sample displacement volume into account. Calcification rates derived from alkalinity anomalies were normalized to surface area, total protein and skeletal mass (see below). Light and dark calcification rates were measured on three coral microcolonies per treatment in the case of S. pistillata and P. damicornis, and six microcolonies per treatment for A. hyacinthus.
Photosynthesis and respiration rates and biomass parameters
Microcolonies were transferred directly from treatment aquaria in the light period to individual closed beakers. Each microcolony was suspended by its monofilament thread in the beaker and incubations were performed in the same conditions of temperature (25 °C) and seawater chemistry as in experimental tanks, but either under light conditions (175 μmol photons m−2 s−1) for photosynthesis, or dark conditions for respiration. Mixing was achieved with a magnetic stirrer and seawater pH and alkalinity were checked at the end of each experiment. An oxygen optode sensor system (oxy-4 mini, PreSens, Regensburg, Germany) was used to quantify oxygen flux. Data were recorded with OXY4v2_11FB software (PreSens). Before each measurement, the oxygen sensor was calibrated against air-saturated seawater (100% oxygen) and a saturated solution of sodium sulfite (zero oxygen). Rates of net photosynthesis and respiration were estimated by regressing oxygen data against time after an initial period of approximately five minutes in which rates were allowed to stabilize. Following analysis, samples were stored at −20 °C. Photosynthesis and respiration rates were derived taking sample displacement volume into account and normalized to skeletal mass. 5 coral microcolonies were measured per treatment.
Analysis of protein, surface area and skeletal mass
Frozen samples were placed in 0.5 N NaOH and tissues removed with a jet of pressurized nitrogen. The tissue slurry was then incubated at 90 °C for 10 min. Protein content was then determined using the bicinchoninic acid assay kit (BC Protein Assay, Interchim). The standard curve was established with bovine serum albumin and the absorbance was measured with a microplate reader (EpochTM, Bioteck, US) at 562 nm.
Following removal of tissues, the skeleton was collected, rinsed first in tap water and then in distilled water, oven-dried for several days at 60 °C and then weighed to determine skeletal mass. These skeleton samples were then used for measuring surface area. Colony surface area was measured using one of the common methods currently used, the paraffin wax method39. Briefly, coral skeletons were coated in paraffin wax by dipping in Paraplast wax (Sigma, France) at 65 °C. Surface area of the specimens was obtained by referring the weight of the paraffin wax coated on the specimen to the standard curve of paraffin wax versus surface area. The standard curve was generated by regressing weight of the paraffin wax to known surface area density blocks.
Statistical analysis
Data were analyzed using the programme SPSS v. 24 (IBM, France). Following Shapiro-Wilk’s tests and Levene’s tests to check the data adhered to the assumptions necessary for parametric analysis (normal distributions with homogenous variances), the parameters pHECM, pHI and calcification rate were analyzed by three-way ANOVA using species, seawater pH and light/darkness as independent variables. Photosynthesis and respiration rates were analysed by two-way ANOVA with seawater pH and species as independent variables. Posthoc analysis was carried out on significant main effects by Tukey tests. Significant interactions were analysed by simple effects analysis and pairwise comparisons with Bonferroni corrections. Results of ANOVAs are reported in Table 2. The results of simple effects analysis are reported in Table 2 and Supplementary 2.
Results
Extracellular calcifying medium pH in light and darkness under acidification
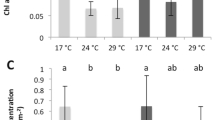
Corals were exposed to the pH treatments for 1 week, after which coral colonies of each coral species were analysed by confocal microscopy to determine pHECM in light and dark conditions (Fig. 1). pHECM values are also expressed as [H+] in Supplementary Fig. 3. There were significant effects of light/darkness, pH treatment and species on pHECM (Table 2 and Supplementary 2). Overall, we observed a general trend of declining pHECM with seawater acidification in all species in light and darkness. However, significant interactions were identified between species, seawater pH and light/darkness, indicating that there are differences in the way pHECM responded among the species in different conditions. In S. pistillata, pHECM varied from pH 8.28 ± 0.04 in light and 8.19 ± 0.05 in darkness at seawater pH 8.1, and falling to pH 7.82 ± 0.10 and 7.81 ± 0.08 at seawater pH 7.2. In this species pHECM values were not significantly different between light and darkness in any of the seawater pH treatments (Table 2 and S1). Under the irradiance used here, S. pistillata displayed higher values of pHECM than P. damicornis at seawater pH 8 and 7.2, but not A. hyacinthus (Supplementary 2). In darkness pHECM was higher in S. pistillata than the other two corals species in the seawater pH 7.4 and 7.2 treatments.
Effects of seawater acidification in light (open symbols) and darkness (closed symbols) on extracellular calcifying medium pH (pHECM) (a–c) and calcifying cell pH (pHI) (d–f) in Stylophora pistillata (column a–d), Pocillopora damicornis (column b–e) and Acropora hyacinthus (c–f). Dashed line represents equivalence with seawater pH. Data are means ± standard deviation. For pHECM (a–c), three way ANOVA and simple effects analysis identified conditions where pHECM is elevated in the light with respect to dark (indicated by asterisks). For calicoblastic pHI (d–f), three way ANOVA identified a significant positive effect of light on pHI across the species. See Table 2 and Supplementary 2 for statistical analysis and Table 1 for carbonate chemistry corresponding to each seawater pH.
In P. damicornis, light pHECM fell from pH 8.15 ± 0.18 to a low pHECM of 7.65 ± 0.07. Dark values were lower, falling from pHECM 8.12 ± 0.13 to 7.51 ± 0.14. In this species pHECM was significantly elevated in the light relative to dark in the seawater pH 7.8, 7.4 and 7.2 treatments.
In A. hyacinthus, light pHECM fell from pH 8.18 ± 0.11 at seawater pH 8.1 to pH 7.79 ± 0.14 in seawater pH 7.2. Declines in darkness were notably much greater, dropping from pH 8.09 ± 0.14 to the lowest pHECM values measured in the investigation (pHECM 7.41 ± 0.03). Overall A. hyacinthus displayed the most pronounced difference in the response of pHECM to acidification between light and darkness, and pHECM was significantly different between light and dark conditions at seawater pH 7.8, 7.4 and 7.2.
pHI in the calicoblastic epithelium
All species exhibited a general trend of decreasing pHI with decreasing seawater pH in both light and darkness (Fig. 1). Three-way ANOVA identified no three-way interaction between seawater pH, species and light/darkness. However, a significant two-way interaction was found to occur between seawater pH and species, indicating that calicoblastic epithelium pH responded differently to seawater pH in the three species (Supplementary 2). Light and darkness was found to have a significant effect on pHI, with light values higher than dark values. At the irradiance used here, the highest pHI values in light and darkness were found in S. pistillata ranging from pH 7.47 ± 0.04 to 7.30 ± 0.02 in the light, and 7.45 ± 0.03 to 7.25 ± 0.07 in the dark. Lower values were found in P. damicornis, ranging from pH 7.40 ± 0.07 to 7.08 ± 0.05 in the light, and 7.36 ± 0.06 to 7.03 ± 0.07 in the dark. Even lower values were recorded in A. hyacinthus, ranging from pH 7.20 ± 0.06 in light to 7.04 ± 0.05, and 7.17 ± 0.01 to pH 6.93 ± 0.01 in darkness. P. damicornis appeared to undergo the greatest change in pHi across seawater pH treatments, however when the difference in pHI between seawater pH 8.1 and 7.2 is expressed in terms of proton concentration, similar changes in [H+] were also observed in A. hyacinthus in darkness (Supplementary 3). Conversion to [H+] also highlights the fact that A. hyacinthus displayed the greatest disparity in pHI of the three species between light and dark conditions in conditions of seawater acidification.
Calcification, photosynthesis and respiration
Calcification as measured by total alkalinity anomaly was normalised by colony surface area (Fig. 2), protein and skeletal mass (Supplementary 4). The pattern of the response of calcification to acidification was very similar between the three methods of normalization. Indeed, there was no change in relationship between protein and skeletal mass across the pH treatments (Supplementary 5). Calcification rate normalized to surface area was significantly affected by species, pH treatment and light/dark conditions. Significant interactions were found between species and pH, and also species and light/dark conditions, indicating that three species responded differently to the pH treatment and the presence of light (Table 2 and Supplementary 2). No significant interaction was found between pH treatment and light/dark conditions.
Effects of seawater acidification in light (open symbols) and darkness (closed symbols) on calcification (a–c) net photosynthetic rate (d–f) and respiration rate (g–i) in Stylophora pistillata (a–g), Pocillopora damicornis (b–h) and Acropora hyacinthus (c–i). Data are means ± standard deviation. See Table 2 and Supplementary 2 for statistical analysis and Table 1 for carbonate chemistry corresponding to each seawater pH.
In the light, calcification rates remained unchanged across the pH treatments in S. pistillata ranging from 95.23 ± 15.59 µg cm−2 h−1 at seawater pH 8.1 to 90.1 ± 10.94 µg cm−2 h−1 at seawater pH 7.2. However, calcification rate declined in P. damicornis and A. hyacinthus, ranging from 154.70 ± 19.55 to 85.16 ± 2.12 µg cm−2 h−1 in P. damicornis, and 87.30 ± 12.8 to 35.19 ± 4.38 µg cm−2 h−1 in A. hyacinthus. Relative to calcification rate at seawater pH 8.1, this represented a 55% and 40% decline in calcification in P. damicornis and A. hyacinthus at seawater pH 7.2 respectively.
Dark calcification rates were significantly lower than in light conditions in all species (Fig. 2; Table 2). Dark calcification rates declined in all three species with decreasing seawater pH. In S. pistillata and P. damicornis, these declines corresponded to a respective 41% and 53% decrease in calcification rate at pH 7.2 relative to pH 8.1. In A. hyacinthus, calcification ceased entirely at and below seawater pH 7.4 in darkness. In A. hyacinthus at seawater pH 7.2, increases in total alkalinity in the incubation chamber during calcification measurements indicated that dissolution occurred in this treatment in darkness.
Photosynthetic rates for the three coral species across the four seawater pH treatments are given in Fig. 2. Analysis by two-way ANOVA indicated that photosynthetic rates were significantly higher in A. hyacinthus than the others species (Table 2). Simple effects analysis (with Bonferroni correction) indicated that overall there was little effect of seawater pH on photosynthetic rate in the three species at the irradiance used here (Table 2), although photosynthetic rates were significantly elevated in S. pistillata at pH 7.2 relative to pH 8.1 (but not the other seawater pH levels).
Respiration rates also did not vary between pH treatments. However, respiration rates varied significantly between species (Table 2). Respiration rates were significantly higher in S. pistillata than the other two species.
Discussion
In the current study S. pistillata displayed the greatest capacity to regulate pHECM against decreases in seawater pH, maintaining the highest offsets between pHECM and seawater pH in light and darkness. These S. pistillata pHECM measurements are consistent with previous confocal measurements of pHECM in this species at seawater pH 8 and 7.22. By contrast to S. pistillata, both A. hyacinthus and P. damicornis displayed different responses of pHECM to acidification in light and darkness. While A. hyacinthus regulated pHECM to similar levels observed in S. pistillata in the light, it experienced pronounced decreases in pHECM in darkness. P. damicornis also underwent greater declines in pHECM in darkness than in the light in the acidification treatments, although the difference between light and dark treatments was less pronounced than in A. hyacinthus. There are two main interpretations of these findings. Firstly, that exposure to light can have a role in mitigating the impact of acidification on pHECM. - this is evident in P. damicornis and A. hyacinthus in which light keeps pHECM more elevated under seawater acidification than in darkness. Secondly, as the effect of seawater acidification on pHECM varies between species, this suggests that the species investigated here vary in their capacity to regulate pHECM against seawater acidification, particularly in darkness where S. pistillata maintained higher pHECM than the other species. These findings are consistent with the widely held view that S. pistillata is a relatively resistant species to ocean acidification among coral species26 and with reports that Acroporid corals are physiologically less tolerant of ocean acidification28,29. However, comparisons of the capacity of pHECM regulation in the current study are limited by lack of knowledge of the relationship between pHECM, light exposure and photosynthetic activity in the three species. Currently, the literature reports that light elevates pHECM relative to dark levels (e.g. increases in pHECM in the light have been observed previously both in S. pistillata and Galaxea fasicularis at seawater pH 8.14,7), but it is not known what level of photosynthetic activity is required to reach maximum levels of pH in ECM. Furthermore, photosynthesis-irradiance curves were not conducted in the current study and thus it is not known whether coral photosynthesis was operating at maximum or lower rates in the three species under the irradiance used here. As such we cannot rule out the possibility that the relative performance of the coral species may differ under a different irradiance regime. This clearly constitutes an important area for further research.
Measurements of calicoblastic cell pHI in the acidification treatments revealed a similar pattern of response to pHECM among the species, suggesting that acid-base regulation of the ECM and calicoblastic cells is closely linked. S. pistillata maintained highest pHI in light and darkness, and greater declines in pHI were observed in P. damicornis and A. hyacinthus. The largest differences between light and dark pHI were observed at low pH in A. hyacinthus, consistent with what was observed for pHECM. Interestingly, analysis of pHi data by three-way ANOVA also revealed a small but significant, positive effect of light on calicoblastic pHI (in the range of 0.02-0.1 pH units). This was most apparent in A. hyacinthus in the lower seawater pH treatments (Fig. 1). This is surprising because previous research on isolated cells and coral microcolonies suggests that light increases pHI only in coral endoderm cells harbouring photosynthetic symbionts23,24,25. Indeed, previous work with P. damicornis has shown that light-driven increases in pHi of endoderm cells may help protect against acidosis at low seawater pH25. Here in the current study our findings suggest that light can also drive pHi increases in cells of the calicoblastic cell layer which doesn’t contain symbionts, but is adjacent to the endoderm layer. The light-driven shifts in calicoblastic cell pHI are much smaller in comparison to those observed in endoderm cells (which occur in the range of 0.3–0.4 pH units24). Light effects on calicoblastic pHI may not have been observed previously because light/dark comparisons were carried out at pH 8.14, and the increases in pHI observed here are most apparent at lower seawater pH. Also, these previous measurements of calicoblastic cells were carried out with a different experimental design and may not have had the statistical power to distinguish the small light and dark differences. In any case, in accordance with further research proposed above for the relationship of pHECM and photosynthetic activity, additional work is required to better characterize the effect of light on pHi regulation in intact corals in both the calicoblastic cells and other tissue layers.
Understanding of the mechanistic basis underlying potential differences in coral species to regulate pHECM and pHi in light and darkness is limited. Previous work with cnidarian endoderm cells invokes the role of Na+/H+ exchangers40. In calicoblastic cells, a Ca2+ATPase and a HCO3−/Cl− exchanger have been localized in S. pistillata, and have thus been proposed to have roles in acid-base regulation linked to calcification41,42. However, mechanisms of pH regulation are likely to vary between species. A previous study that identified differences between P. damicornis and Montipora capitata in the capacity to regulate pHi at elevated temperature suggests there are links between mechanisms of thermotolerance and pHi regulation43. Other research indicates a differential localization of transporters with possible roles in calcification in Acropora yongeii compared to S. pistillata44. For example, this latter study indicates an abundance of Na+/K+-ATPase in the apical membrane of the oral epithelium in A. yongei but not S. pistillata, while Ca2+ATPase was abundant in the endoderm of S. pistillata but not A. yongei. Additionally several proposed mechanisms explaining the role of light in elevating pHECM have been published previously4,7,45. Among these it has been suggested that pH increases in the coelenteron lumen due to symbiont photosynthesis may provide a favourable gradient for the removal of protons from the ECM46,47. In the case of the calicoblastic cells, it could be hypothesized that increases in coelenteron pH and the adjacent endoderm cell layer may also promote the movement of protons from the calicoblastic cells resulting in slight increases in calicoblastic pHI observed in the current study. As such, light-driven increases in pHi that may help endoderm cells buffer against seawater acidification may also mitigate decreases in pHi in calicoblastic cells. However, as it stands, little functional data are available for proton transport in corals or the role of ion transporters localized to the calicoblastic cells, and this is an essential avenue for future research to gain a better mechanistic understanding of calcification.
We conducted parallel experiments to gain insight into how acidification in light and darkness affects coral photosynthesis, respiration and calcification, as these metabolism parameters could potentially influence pH regulation. Generally, no significant effect of seawater acidification was observed on photosynthetic rates in the three species at the irradiance level used here, although photosynthetic rates were significantly elevated at seawater pH 7.2 relative to pH 8 in S.pistillata. Although elevated pHECM in light relative to dark conditions in P. damicornis and A. hyacinthus clearly indicates that photosynthesis has a positive effect on pHECM under acidification in these species, the general lack of response of photosynthetic rates in the three species observed here makes it difficult to attribute a role for this parameter in determining the relative sensitivity of pHECM and pHI to acidification. However, as we state above, it is necessary to fully characterize the photosynthesis-irradiance (PI) response of each species in each treatment before firm conclusions about the response of photosynthesis and its role in pH regulation can be made. In the meantime, we note that the insensitivity of photosynthesis observed here in the three species is in agreement with previous work on S. pistillata at the same irradiance used here2, and also meta-analysis carried out by Kroeker et al.48 conducted on eleven investigations of seawater acidification on a range of coral species which indicated that seawater pCO2 had no overall significant effect on photosynthesis. However the influence of seawater acidification on coral photosynthesis can be equivocal, with both negative49,50 and positive effects51 also having been reported.
Respiration rates could also be relevant to pH regulation, because higher rates of CO2 production could potentially present a greater challenge to acid-base regulation of both calicoblastic cells and the calcifying medium. In this respect, if seawater acidification caused higher respiration rates in the corals, then negative effects on pH regulation might be expected. Again this was not the case here, as respiration rates did not significantly change between the pH treatments in the three species. Similar to photosynthesis, previously reported responses of coral respiration to seawater acidification can also be rather equivocal. Decreases in respiration rate under elevated pCO2 have been reported for Acropora millepora and massive Porites sp. corals, but a recent study by52 observed no effect of pCO2 on respiration in six (including P. damicornis) out of 8 species tested, in agreement with what was observed here in the current study.
In the current study, effects of seawater acidification were much more apparent on calcification rates than the other metabolism parameters. Generally, increasing seawater acidification had a negative effect on calcification, but the effects were species-specific and influenced by light and darkness. All three species exhibited light-enhanced calcification (LEC), a phenomenon that has been observed in numerous studies on corals20. In S.pistillata light calcification rates were unaffected by seawater acidification, however in darkness, calcification rates were significantly lower at seawater pH 7.4 and pH 7.2 relative to pH 8. In P. damicornis, light calcification rates also decreased under acidification, while dark calcification rates did not, and in A. hyacinthus both light and dark calcification decreased in acidification treatments. As calcification- irradiance response curves were not carried out during this investigation, it is not known whether these observed responses occurred at optimum light levels for calcification. However, the current data do indicate that calcification is more sensitive to acidification in darkness in S. pistillata and A. hyacinthus which is in line with previous studies in the literature21,53. Interestingly in A. hyacinthus, calcification ceased entirely in the dark at pH 7.4 and dissolution of the skeleton occurred at pH 7.2 in darkness. This is consistent with reports of skeletal dissolution in the coral Acropora millepora53 in darkness under elevated pCO2 conditions (1073uatm).
pHECM and calcification rates are anticipated to be linked, because increases in pHECM would be expected to increase the relative proportion of [CO32−] in the dissolved inorganic carbon pool and therefore Ω of the ECM thereby favouring calcification. Broadly, calcification rates in the three species followed a similar trend to pHECM, as calcification rates declined as pHECM decreased with increasing seawater acidification. The most dramatic declines in calcification rate were observed in darkness in A. hyacinthus which also exhibited that largest declines in pHECM. In the case of P. damicornis and A. hyacinthus, both pHECM and calcification rates were lower in darkness than in the light. These data fit the view that pHECM influences rates of skeleton formation4,15,54. There are however a number of inconsistencies between pHECM and calcification rate, suggesting the relationship between pHECM and calcification is more complex than previously thought. In P. damicornis, pHECM declined significantly between pH 8.1 and 7.8 in light and darkness, but no accompanying change was observed in calcification rates. We also made the same observation in S. pistillata with pHECM declining between the 8.1 and 7.8 treatments in light and dark, but with no accompanying decrease in calcification. Other interspecies comparisons have also shown how variation in coral calcification rates can bear little relation to changes pHECM in corals exposed to changes in seawater carbonate chemistry, including a recent study by Comeau et al. that used boron isotope systematics to determine pHECM on P. damicornis and another Acroporid (A. yongei)54.
The in vivo approach used in the current study allowed us to explore the effect of light and darkness on the relationship between pHECM and calcification rate, something that was not possible in the recent boron isotope study on P. damicornis and A. yongei54. We observed in S. pistillata, that although calcification rates were consistently higher in the light versus the dark both at pH 8.1 and in conditions of acidification down to seawater pH 7.2, light and dark pHECM were not different. By illustration, at seawater pH 7.4, light calcification was 10 times higher than dark calcification, but light and dark pHECM are identical. These data do not support the previously proposed idea that light-driven elevation of pHECM is involved in the mechanism underlying light enhanced calcification (LEC), at least in the case of S. pistillata4.
The difficulties in reconciling pHECM and calcification data in light and darkness maybe related to the fact that the pHECM measurements and calcification measurements were performed on different experimental material (i.e. microcolonies on glass coverslips and microcolonies suspended on thread). There may be differences in tissue thickness, symbiont densities and rates of exchange between ions in ECM and seawater in these two different types of microcolony preparation, which could influence their physiological response to acidification and light. Alternatively, inconsistencies between pHECM and calcification rate may arise from the fact that calcification is ultimately driven by numerous physiological factors in addition to pHECM which may also be responsive to acidification. Indeed recent research highlights three aspects of calcification physiology that could influence the calcification response to acidification: regulation of Ca2+ concentration in the ECM55,56, dissolved inorganic carbon concentration [DIC] in the ECM57 and the role of the organic matrix in the biomineralization process20. In the case of Ca2+ regulation, recently developed geochemical approaches indicate that maintenance of calcification rates under acidification may be related to the capacity of corals to elevate [Ca2+] in the ECM55. In this study, an acidification-tolerant P. damicornis was found to elevate [Ca2+] in the ECM with respect to seawater, whereas an acidification-sensitive A. youngei did not. It is therefore plausible that a differential ability to regulate ECM [Ca2+] in the corals of the present study may have influenced calcification rates. Turning to ECM [DIC], this parameter (together with [Ca2+] and pH) determines ECM Ω and could therefore influence calcification16,55,57,58. While recent geochemical studies indicate that resistance to acidification may only involve moderate increases in ECM [DIC]59, further research into the role of ECM DIC regulation in determining calcification rates is needed. Lastly, a growing body of research is elucidating the role of the organic matrix (OM) (which consists of proteins, sugars and lipids) in coral calcification. OM components have several proposed roles in the calcification mechanism including reducing the free energy required for aragonite crystal nucleation60 and controlling the growth of aragonite crystals20,61,62,63. As such, the production of the OM may also have a key role in determining calcification rates under acidification62. Indeed, previous research on S. pistillata indicates that this coral species increases OM production under acidification, possibly to promote calcification under less favourable conditions2. In addition to its role in calcification, the presence of organic matrix in the skeleton has also been reported to influence its solubility64,65, suggesting that interspecies differences in the composition and/or quantity of organic matrix may also affect the tendency of skeletons of certain species to undergo dissolution before others. This may explain why in the current study at seawater pH 7.2, we observed skeletal dissolution in darkness in A. hyacinthus and not P. damicornis, although both species maintained a similar pHECM (pH 7.5-7.4).
Overall, it is therefore likely that the response of coral calcification to acidification may depend on several interacting aspects of calcification physiology in addition to pH regulation54,55,58,62,63. The interspecies differences identified in the current work suggest that mechanisms of calcification physiology may vary among scleractinians. One question is whether the different responses observed here were influenced by scleractinian phylogeny. Robust and complex clades are thought to have evolved calcification independently30 and thus ion transport mechanisms linked to calcification (such as those involved in pHECM regulation) might be expected to be different between these groups44. S. pistillata and P. damicornis are members of the robust clade, while A. hyacinthus belongs to the complex clade of scleractinia. The susceptibility of both pH regulation and calcification to acidification in A. hyacinthus does indeed suggest that this species is the outlier of the group. Furthermore, even under ambient seawater pH 8.1 conditions, A. hyacinthus displayed a markedly lower intracellular pH suggesting that its system of cellular acid-base balance is distinct from the other species. On the other hand, the pattern observed here does not easily correspond to the robust and complex groups, because both robust members (S. pistillata and P. damicornis) exhibit distinct responses themselves. As such, future investigation of more members of each clade is required before clear conclusions about clade-specific physiological traits can be made. Similarly, future work could investigate the potential influence of different symbiont types (i.e. different members of the family Symbiodiniaceae18) associated with the three coral species on their pH regulation. If the coral species investigated here associate with symbiont types that vary in their response to light and seawater acidification, then differences in symbiont communities in each coral may also influence pH regulation and the physiology of each coral species under acidification. Additionally, deciphering mechanistic differences in pH regulation and calcification between the species may be achieved using manipulations of seawater carbonate chemistry beyond what was performed in the current investigation. Here, we chose to investigate the impact of seawater acidification by CO2 enrichment due to its environmental relevance to climate change, but future work could potentially reveal interesting mechanistic differences by manipulating seawater bicarbonate and carbonate concentrations in a manner similar to some previous investigations17,45.
In summary, the current study reveals differences in the extent to which pHECM, pHI and coral calcification are impacted by acidification in light and darkness. An important caveat of our study was that it was conducted under controlled, laboratory conditions (which has advantages for mechanistic research), at a single light level and as such, the current data cannot easily be extrapolated to the performance of these corals in the field where numerous environmental parameters vary in concert. An important future challenge will be to use what we learn in the laboratory to orientate field-based investigations into coral calcification physiology in the natural environment. This will be an essential step in understanding the adaptive capacity of corals to a changing ocean, both in terms of their geological past and their future in coming decades.
References
Chan, N. C. S. & Connolly, S. R. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Chang. Biol 19, 282–290 (2013).
Tambutte, E. et al. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 6, 7368 (2015).
Weis, V. M. & Allemand, D. What Determines Coral Health? Science. 324, 1153–1155 (2009).
Venn, A. A., Tambutté, E., Holcomb, M., Allemand, D. & Tambutte, S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS One 6, e20013 (2011).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA 110, 1634–1639 (2013).
Ohno, Y. et al. An aposymbiotic primary coral polyp counteracts acidification by active pH regulation. Sci. Rep. 7, 40324 (2017).
Al-Horani, F. A., Al-Moghrabi, S. M. & de Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar Biol 142, 419–426 (2003).
Ries, J. B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim Cosmochim Ac 75, 4053–4064 (2011).
Cai, W. J. et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat Commun 7, 11144 (2016).
Reynaud, S. et al. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Chang. Biol. 9, 1660–1668 (2003).
Trotter, J. et al. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173 (2011).
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford University Press, 1989).
Georgiou, L. et al. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc Natl Acad Sci USA 112, 13219–13224 (2015).
Wall, M. et al. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Sci. Rep. 6 (2016).
McCulloch, M., Falter, J., Trotter, J. & Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2, 623–627 (2012).
Raybaud, V. et al. Computing the carbonate chemistry of the coral calcifying medium and its response to ocean acidification. J. Theor. Biol. 424, 26–36 (2017).
Comeau, S. et al. Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. Lond., B, Biol. Sci. 284 (2017).
LaJeunesse, T. C. et al. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 28, 2570–2580.e6 (2018).
Gattuso, J.-P., Allemand, D. & Frankignoulle, M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Am. Zool. 39, 160–183 (1999).
Tambutté, S. et al. Coral biomineralization: From the gene to the environment. J. Exp. Mar. Biol. Ecol. 58–78, https://doi.org/10.1016/j.jembe.2011.07.026 (2011).
Suggett, D. J. et al. Light availability determines susceptibility of reef building corals to ocean acidification. Coral Reefs 32, 327–337 (2013).
Dufault, A. M., Cumbo, V. R., Fan, T.-Y. & Edmunds, P. J. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc R Soc B 279, 2951–2958 (2012).
Venn, A. A. et al. Imaging intracellular pH in a reef coral and symbiotic anemone. Proc. Natl. Acad. Sci. USA 106, 16574–16579 (2009).
Laurent, J., Tambutte, S., Tambutte, E., Allemand, D. & Venn, A. The influence of photosynthesis on host intracellular pH in scleractinian corals. J. Exp. Biol. 216, 1398–1404 (2013).
Gibbin, E. M., Putnam, H. M., Davy, S. K. & Gates, R. D. Intracellular pH and its response to CO2-driven seawater acidification in symbiotic versus non-symbiotic coral cells. J Exp Biol 217, 1963–1969 (2014).
Krueger, T. et al. Common reef-building coral in the Northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open Sci. 4, 170038 (2017).
Bhattacharya, D. et al. Comparative genomics explains the evolutionary success of reef-forming corals. Elife 5, e13288 (2016).
Schoepf, V. et al. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS One 8, e75049 (2013).
Comeau, S., Edmunds, P. J., Spindel, N. B. & Carpenter, R. C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Ocean. 58, 388–398 (2013).
Romano, S. L. & Palumbi, S. R. Evolution of Scleractinian Corals Inferred from Molecular Systematics. Science. 271, 640 LP–642 (1996).
Liew, Y. J. et al. Epigenome-associated phenotypic acclimatization to ocean acidification in a reef-building coral. Sci. Adv. 4 (2018).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to Best Practices for Ocean CO2 Measurements. 191 (2007).
Dickson, A. G. & Goyet, C. eds Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. Version 2, https://doi.org/10.2172/10107773 (1994).
Pierrot, D. E., Lewis, D. W. & Wallace, R. MS Excel Program Developed for CO2 System Calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee. https://doi.org/10.3334/CDIAC/otg.CO2SYS_XLS_CDIAC105a (2006).
Mehrbach, C., Culberso, C. H., Hawley, J. E. & Pytkowic, R. M. Measurement of apparent dissociation constants of carbonic-acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973).
Dickson, A. G. & Millero, F. J. A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res. A 34, 1733–1743 (1987).
Moya, A. et al. Study of calcification during a daily cycle of the coral: implications for ‘light-enhanced calcification’. J. Exp. Biol. 209, 3413 LP–3419 (2006).
Chisholm, J. R. M. & Gattuso, J.-P. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Ocean. 36, 1232–1239 (1991).
Naumann, M. S., Niggl, W., Laforsch, C., Glaser, C. & Wild, C. Coral surface area quantification–evaluation of established techniques by comparison with computer tomography. Coral Reefs 28, 109–117 (2009).
Laurent, J. et al. Regulation of intracellular pH in cnidarians: response to acidosis in Anemonia viridis. FEBS J. 281, 683–695 (2013).
Zoccola, D. et al. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora pistillata. Biochim. Biophys. Acta 1663, 117–126 (2004).
Zoccola, D. et al. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 5, 9983 (2015).
Gibbin, E. M., Putnam, H. M., Gates, R. D., Nitschke, M. R. & Davy, S. K. Species-specific differences in thermal tolerance may define susceptibility to intracellular acidosis in reef corals. Mar. Biol. 162, 717–723 (2015).
Barott, K. L., Perez, S. O., Linsmayer, L. B. & Tresguerres, M. Differential localization of ion transporters suggests distinct cellular mechanisms for calcification and photosynthesis between two coral species. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00052.2015 (2015).
Comeau, S., Carpenter, R. C. & Edmunds, P. J. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc R Soc B 280 (2013).
Furla, P., Bénazet-Tambutté, S., Jaubert, J. & Allemand, D. Functional polarity of the tentacle of the sea anemone Anemonia viridis: role in inorganic carbon acquisition. Am. Physiol. Soc. 274, R303–R310 (1998).
Furla, P., Galgani, I., Durand, I. & Allemand, D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457 (2000).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol 19, 1884–1896 (2013).
Kaniewska, P. et al. Major Cellular and Physiological Impacts of Ocean Acidification on a Reef Building Coral. PLoS One 7, e34659 (2012).
Anthony, K. R. N., Kline, D. I., Diaz-Pulido, G., Dove, S. & Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 105, 17442–17446 (2008).
Strahl, J. et al. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp Biochem Physiol A Mol Integr Physiol 184, 179–186 (2015).
Comeau, S., Carpenter, R. C., Edmunds, P. J. & Norkko, H. editor: J. N. editor: J. Effects of pCO2 on photosynthesis and respiration of tropical scleractinian corals and calcified algae. ICES J. Mar. Sci. 74, 1092–1102 (2017).
Vogel, N., Meyer, F. W., Wild, C. & Uthicke, S. Decreased light availability can amplify negative impacts of ocean acidification on calcifying coral reef organisms. Mar Ecol Prog Ser 521, 49–61 (2015).
Comeau, S., Cornwall, C. E. & McCulloch, M. T. Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 7, 7573 (2017).
DeCarlo, T. M., Comeau, S., Cornwall, C. E. & McCulloch, M. T. Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc. R. Soc. B Biol. Sci. 285 (2018).
Sevilgen, D. S. et al. Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Sci. Adv. In press (2019).
Allison, N. et al. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat Commun 5, 5741 (2014).
McCulloch, M. T., D’Olivo, J. P., Falter, J., Holcomb, M. & Trotter, J. A. Coral calcification in a changing World and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8, 15686 (2017).
Schoepf, V., Jury, C. P., Toonen, R. J. & McCulloch, M. T. Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc. R. Soc. B Biol. Sci. 284 (2017).
Mann, S. Biomineralisation, Principles And Concepts in Bioinorganic Materials Chemistry (Oxford University Press, 2001).
Puverel, S. et al. Soluble organic matrix of two Scleractinian corals: Partial and comparative analysis. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 141, 480–487 (2005).
Mass, T. et al. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr Biol 23, 1126–1131 (2013).
Von Euw, S. et al. Biological control of aragonite formation in stony corals. Science. 356, 933–938 (2017).
Gautret, P., Cuif, J.-P., Stolarski Gautret, J. & Stolarski, J. Organic components of the skeleton of scleractinian corals-evidence from in situ acridine orange staining. Acta Palaeontol. Pol. 45 (2000).
Holcomb, M., Cohen, A. L., Gabitov, R. I. & Hutter, J. L. Compositional and morphological features of aragonite precipitated experimentally from seawater and biogenically by corals. Geochim Cosmochim Acta 73, 4166–4179 (2009).
Acknowledgements
This research was funded by the Government of the Principality of Monaco. We thank Dominique Desgre for assistance with coral culture and Eric Elia for technical assistance.
Author information
Authors and Affiliations
Contributions
S.T., E.T. and A.A.V. designed and conceived the experiments. E.T., N.C.S., N.T. and A.A.V. performed the experiments. D.A., S.T., E.T. and A.A.V. contributed reagents/materials/analysis tools. S.T., D.A., E.T. and A.A.V. analyzed data and wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Venn, A.A., Tambutté, E., Caminiti-Segonds, N. et al. Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci Rep 9, 2201 (2019). https://doi.org/10.1038/s41598-018-38168-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38168-0
This article is cited by
-
Spatial variability of and effect of light on the cœlenteron pH of a reef coral
Communications Biology (2024)
-
In vivo observation of lipid droplets in coral calcifying cells: fat stores to fuel the reef-building process?
Coral Reefs (2023)
-
Effects of variable daily light integrals and elevated CO2 on the adult and juvenile performance of two Acropora corals
Marine Biology (2022)
-
Recent ocean acidification trends from boron isotope (δ11B) records of coral: Role of oceanographic processes and anthropogenic CO2 forcing
Journal of Earth System Science (2022)
-
Intracellular pH regulation: characterization and functional investigation of H+ transporters in Stylophora pistillata
BMC Molecular and Cell Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.