Abstract
Short sleep duration or insomnia may lead to an increased risk of various psychiatric and cardio-metabolic conditions. Since DNA methylation plays a critical role in the regulation of gene expression, studies of differentially methylated positions (DMPs) might be valuable for understanding the mechanisms underlying insomnia. We performed a cross-sectional genome-wide analysis of DNA methylation in relation to self-reported insufficient sleep in individuals from a community-based sample (79 men, aged 39.3 ± 7.3), and in relation to shift work disorder in an occupational cohort (26 men, aged 44.9 ± 9.0). The analysis of DNA methylation data revealed that genes corresponding to selected DMPs form a distinctive pathway: “Nervous System Development” (FDR P value < 0.05). We found that 78% of the DMPs were hypomethylated in cases in both cohorts, suggesting that insufficient sleep may be associated with loss of DNA methylation. A karyoplot revealed clusters of DMPs at various chromosomal regions, including 12 DMPs on chromosome 17, previously associated with Smith-Magenis syndrome, a rare condition comprising disturbed sleep and inverse circadian rhythm. Our findings give novel insights into the DNA methylation patterns associated with sleep loss, possibly modifying processes related to neuroplasticity and neurodegeneration. Future prospective studies are needed to confirm the observed associations.
Similar content being viewed by others
Introduction
Lack of sleep and sleep disturbances are extremely common in the general population1,2. According to the population-based surveys conducted in Finland, up to one-third of the general adult population experiences occasional difficulties in sleep2 and almost one-fourth reported sleeping 6 hours or less per night2,3. Chronic lack of sleep affects health; specifically, it increases the risks of cardio-metabolic disorders such as type 2 diabetes and cardiovascular diseases, as well as mental disorders4. The symptoms of insomnia have become widespread, especially in the working population: in 2007, a report based in Finland revealed that insomnia-related symptoms were frequent among 9% of workers, and occasional in up to 45.3%3. Night and early morning shift work is a common source of insufficient sleep, since it leads to circadian misalignments, misbalances homeostasis, and truncates total sleep time by 1–4 hours5. The detrimental effects of shift work on sleep can result in shift work disorder (SWD) – a medical condition characterized by complaints of excessive sleepiness and/or insomnia as primary symptoms, accompanied by a reduction in sleep duration during the working period6. It is a common condition affecting over one-third of shift workers7,8,9.
The effect of sleep deprivation on the transcriptome and methylome has previously been studied both in experimental animal models and in selected samples10,11,12,13,14,15. Sleep deprivation induces notable changes in the brain transcriptome of rats, affecting protein synthesis, synaptic plasticity, and metabolism10,16. For example, Archer et al.17 found a reduction of rhythmic transcripts and major changes in the transcriptome once sleep was mistimed. Simply missing a single night’s sleep alters both the transcriptional and the DNA methylation profiles of core circadian clock genes13. Indeed, Bhatti et al.18 found a significant decrease in average methylation among the nightshift workers compared to the methylation profiles of dayshift workers.
Despite the extensive ‘omics’ studies conducted in human cohorts during the last decades, the biological mechanisms underlying the negative consequences of chronic lack of sleep in workers are not fully understood and the inter-individual variation of such consequences is known to be quite large. Our previous work19 focused on intrinsic genetic risk factors for intolerance to shift work, and found an association between job-related exhaustion and a variant downstream of the melatonin receptor 1A gene with a proposed mechanism of changes in DNA methylation at the gene promoter, when exposed to the risk environment (shift work).
In this study, we investigated DMPs in blood leukocytes that reflect the systemic stress triggered by insufficient sleep in two complementary samples of cases and controls from (i) individuals selected from a community-based sample (DILGOM, a sub-study of the population-based FINRISK) and from (ii) an occupational cohort of shift workers (Airline). In (i), cases were selected based on their self-reported evaluation of sleep insufficiency, meaning seldom or hardly ever sleeping enough. In (ii), cases were selected based on the presence of SWD symptoms specifically during shift work periods: symptoms of insomnia, sleepiness, and objectively measured reduction in total sleep time.
Results
The DILGOM sample included a total of 517 unrelated individuals from Helsinki, Finland, recruited for the Dietary, Lifestyle, and Genetic determinants of Obesity and Metabolic syndrome study in 2007, with extensive information on traits and lifestyle factors. For our study, we focused on the FINRISK survey question “Do you, in your opinion, sleep enough?” Based on the answer, we dichotomized our study group into the cases and controls (See Materials and Methods section “Study samples” for detailed information).
We first investigated the effect of age and gender on DNA methylation in the complete DILGOM sample (N = 517). Age and gender were found to strongly affect the DNA methylation (Supplementary Fig. S1): 25.6% (122,721/479,954) and 7.9% (37,780/479,954) of all CpGs showed FDR-corrected P values < 0.05 for age and gender effect, respectively.
Thus, considering the reported effect of age and gender on the primary phenotype of our study (insufficient or disturbed sleep20,21), and, furthermore, the fact that most (81%) of the participants in the Airline sample were < 50 (n = 26), we focused on men < 50 years old in the DILGOM sample (n = 79) in the subsequent analyses. However, as age has an important effect on DNA methylation, it was included in all analyses as a covariate.
Methylome-wide analysis of DILGOM and Airline
In order to detect DNA methylation modifications in relation to insufficient sleep, we performed epigenome-wide association studies in two complementary samples: a population-based DILGOM sample (n = 79, age = 39.3 ± 7.3, from a sample total of N = 517) and an occupational Airline sample of shift workers (n = 26, age = 44.9 ± 9.0) by comparing genome-wide methylation between cases and controls in each sample.
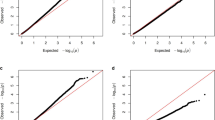
We identified 14,487 (DILGOM) and 19,303 (Airline) DMPs with uncorrected P values < 0.05. None of the sites survived an FDR-corrected threshold of 5%. After comparing the results for both groups, we identified 399 sites that were common to both samples and with the same direction of methylation in DILGOM and Airline (hyper- or hypomethylated sites), with 327 DMPs annotated to 317 genes. The majority 248 (78%) of the CpG sites were hypomethylated in cases compared with the controls (Supplementary Table S2).
Pathway analyses and database search of the overlap set
In order to identify the affected biological pathways, we used several freely available online resources (GoMiner, Panther, g:Profiler) and one commercially available pathway analysis tool, IPA. The comparison of results revealed two major groups of pathways – nervous system development (NSD) and cellular processes, with NSD (GO:0007399) common to all pathway tools (FDR-corrected P values: 0.0013, GoMiner; 0.0137, Panther; 0.00131, g:Profiler; uncorrected P value 1.54E-5, IPA). NSD comprised 92 DMPs annotated to 89 genes after duplicates were removed, in all tools (see Supplementary Table S3).
As an alternative strategy to search for the implications of the DMPs, we investigated the phenotype associations related previously for the set of 317 genes common to both groups, using the Ensembl genome browser (Human GRCh38.p10). Of these, 59 had no information about phenotype association; 182 were related to non-specific pathological conditions; 19 were associated with various sleep phenotypes (including circadian rhythms); 50 were associated with a genetic syndrome. Since 13 out of 50 genetic syndromes included some type of disturbances in visual processing (mostly retinal abnormalities), we performed an additional check of all 317 genes and discovered 8 genes that are associated with disturbances in visual processing (see Supplementary Table S4). Altogether, 79 out of 317 genes were associated with a specific syndrome, sleep problems, or visual disturbances.
The 317 genes were plotted in a karyoplot (Fig. 1), which revealed clusters of DMPs at various chromosomal locations. One of these clusters comprised 15 DMPs of genes on chromosome 17. Of these 15 genes, 12 have previously been associated with Smith-Magenis syndrome (SMS) in rodents or humans according to the rat genome database, and 3 genes are related to various retinopathies in animals and human patients (Fig. 2). The karyoplot identified several other specific regions of interest, including 1p36.12 (4 DMPs), 2p23.3 on chromosome 2 (4 DMPs), 3p21.31 on chromosome 3 (6 DMPs), 7q11.23 (4 DMPs), 11q13.1 (3 DMPs), and 19p12 (3 DMPs).
DNA methylation and gene expression
We further investigated whether methylation at the identified DMPs correlates with the expression level of the corresponding gene. We performed correlation analyses of M-values with corresponding gene expression levels for 327 DMPs/317 genes/390 RNA transcripts. The methylation levels of 32 (10%) DMPs were correlated with the gene expression levels in DILGOM at P < 0.05 (Supplementary Table S5). None of the results were significant according to the Bonferroni-corrected threshold of 0.05/400 after multiple testing corrections. However, we identified an excess of DMPs whose methylated levels were correlated with the corresponding levels of gene expression at P < 0.0001 (observed 5 vs expected 1) and P < 0.01 (observed 12 vs expected 4). Four of these 32 sites found in DILGOM (cg27013755 BANP, cg03193489 TERF1, cg11214001 CAST, and cg14603227 NAV2) showed correlations of DNA methylation levels with RNA expression levels also in the Airline sample at P < 0.05.
Correlation analysis of M-values with sampling time
Since expression levels of some of the genes identified earlier have previously been found to fluctuate according to circadian time17, we studied the correlation of the methylation values with the time of sample collection. In DILGOM, we found M-values of 6/399 DMPs, to correlate with the sampling time at P < 0.05 (not significant after multiple testing corrections, see Supplementary Table S6). In Airline, we found 14 DMPs whose M-values correlated with the sampling time at P < 0.05 (not significant after multiple testing corrections, see Supplementary Table S7). None of these DMPs are identical to the ones found significant in the DILGOM sample.
Discussion
In this study, we identified suggestive deviations in DNA methylation of blood leukocytes associated with subjective sleep insufficiency in a population cohort and in an occupational shift work sample. We found a distinctive pattern of DMPs among individuals suffering from sleep disturbances. This pattern consisted of 399 DMPs, out of which 327 were annotated to genes that showed an enrichment of associations with the nervous system development pathway (NSD).
According to the Gene Ontology database, the NSD pathway is defined as the process of either the maturation of nervous tissue or its progression over time. It includes the processes of neurogenesis, regulation of nervous system development, synapse maturation, nerve development, and others. Our findings are in agreement with previous studies, in which DNA methylation differences induced by sleep disturbances were observed in pathways involved in synaptic plasticity and neuritogenesis12. However, earlier studies involved the analysis of DNA methylation in the brain tissue of rodents, whereas our study used blood samples from humans. Since the lack of sleep has a systemic effect on the human body, we suggest that blood samples are informative source for revealing systemic changes triggered by insufficient sleep.
The analysis of genome location of 317 genes indicated several clusters, most prominent on chromosome 17. Interestingly, 12 of these 18 genes located on chromosome 17 relate to SMS in rodents or humans (Fig. 2). SMS [MIM 182290] is a rare genetic disorder affecting 1 in 25 000 individuals, and in most cases is caused by a 3.7 Mb interstitial deletion in the short arm of chromosome 17. SMS affects the whole body, with disturbed sleep being one of the prominent symptoms (75–100% of cases according to Greenberg et al.22). Thus, the affected individuals have difficulty falling asleep at night and staying awake during the day, due to the inverted circadian rhythm of melatonin22. Some SMS patients have noted the absence of rapid eye movement (REM) sleep23. The mutations in RAI1, located in the 17p11.2 locus are known to be responsible for the inversion of the melatonin cycle24. However, not all SMS patients with RAI1 mutations or deletions experience sleep disturbances25, hence the SMS phenotype may be achieved by complex rearrangements of chromosome 17 that also involve its long arm26,27. The observation of DNA methylation changes in the group of genes related to SMS requires further investigation, possibly in occupational cohorts of shift workers, since shift work can lead to circadian misalignments and sleep loss5. Our findings nevertheless suggest that this region on chromosome 17, earlier linked to SMS, may also play an important role in regulation of sleep and circadian rhythm in the general population via epigenetic regulatory mechanisms.
In chromosome 3p21.31, we identified a cluster of 6 genes, of which 5 (LIMD1, SCAP, CDC25A, SLC38A3, and NEK4) are known to be associated with cell responses to stresses such as hypoxia (LIMD128 and SCAP29), UV-induced DNA damage (CDC25A30), potassium restriction (SLC38A331) or DNA damage (NEK432). Several earlier studies have highlighted the association of sleep deprivation with cellular stress33,34. Based on our results, we suggest that this association might also involve epigenetic mechanisms.
In 7q11.23, there was a small group of 4 genes including 3 genes related to anxiety and autism (STX1A35, GTF2IRD136, and MAGI237). Finally, on chromosome 19, we discovered a group of genes possibly involved in regulation of transcription: ZNF441, ZNF709, ZNF506, ZNF826, and ZNF43. In our set of 317 genes there were 11 zinc finger transcription factors with affected methylation levels (9 hypo- and 2 hypermethylated), and 5 of them are located on chromosome 19. Possibly, loss of methylation of transcription factors induced by sleep disturbances contributes to the overall change in gene expression17,38.
The analysis of phenotypic associations of the overlap set of common genes revealed a large number of genes related to various disturbances in visual processing (31 genes, see Table 1), many of which are involved in retinal light transmission. For example, UNC119 is specifically expressed in the photoreceptors in the retina, and plays a role in photoreceptor neurotransmitter release through the synaptic vesicle cycle39. UNC119 is also located on chromosome 17, together with TSEN54 and CDK5R1, known for sight-threatening retinopathy in type 2 diabetes and disturbed retinal morphology, respectively. This finding is interesting considering the central role of light as an external zeitgeber for circadian rhythm.
In the overlap set of 317 genes, we discovered a group of 11 genes that are involved in both sleep and disturbances in visual processing (Table 1, last section). For example, variants in ERC2 have been found to be significant in genome-wide association studies for chronotype and daytime sleep phenotypes40,41. ERC2 was also found to be important for normal visual processing in mice, as its inactivation impairs synaptic encoding of visual stimuli42. Another example is GRIN2B – one of the subunits of the glutamate ionotropic receptor of NMDA type. In a study of rats43, those that were sleep-deprived had lower expression of GRIN2B in the hippocampus, leading to hippocampal synaptic impairment. NMDA receptors also play a role in photoreceptor functioning, as described in the study of rat models with retinitis pigmentosa44.
The majority of the DMPs (76%) were relatively hypomethylated, suggesting that a lack of sleep may be associated with a loss of methylation, consequently leading to systemic alterations of gene expression. This finding is concordant with a study of nightshift workers18, which revealed decreased DNA methylation in the blood of men lacking sleep. However, it is noteworthy that the effect of hypomethylation on transcription depends on the location of a given CpG (i.e. promoter region, gene body), which makes it difficult to draw any conclusions about increased or decreased gene expression.
A few limitations in this study warrant consideration. The first is the small sample size of the studied groups. Both samples sizes were not large enough to generate sufficient statistical power; however, combining results from the two independent cohorts was a major strength of our study. Second, in order to diminish the heterogeneity, based on our preliminary analysis (Supplementary Fig. S1), we focus only on men of a certain age in both studied samples. The third limitation is the use of blood samples for the study of sleep. Methylation is tissue-specific, so our findings must be interpreted with caution. However, disturbed sleep and circadian rhythm have systemic effects on the human body, as reported earlier17. Fourth, the phenotype data used in this study are based on self-reported sleep sufficiency, without objective measurements, which is a common limitation for many population surveys. Use of the self-reported criteria allowed us, however, to capture an essential part of the individual variability in the intrinsic need for sleep, which varies due to a number of factors, including genetic45,46. For example, the Airline control group, there were individuals who could sleep regularly for 6 hours or less, but did not complain about tiredness or symptoms of insomnia. Fifth, our study was cross-sectional, and future prospective studies are needed to establish the temporal relationship between sleep insufficiency and changes in DNA methylation. Finally, despite the homogeneity of the samples (men matched in age), there are, however, some important differences. The DILGOM respondents from the general population reported insufficient sleep due to various reasons, while the Airline group was dichotomized according to the symptoms of SWD specifically during shift work periods. In addition to the subjective experience of insufficient sleep, the cases in DILGOM reported also an increased amount of symptoms of insomnia, tiredness, and short sleep, while the diagnostic definition of SWD in the Airline sample included symptoms of insomnia, sleepiness, and reduction in total sleep duration. Thus, cases shared similarities at the level of sleep symptoms in these two complementary samples for insufficient sleep. It is, however, noteworthy that our study on men below 50 years of age cannot be generalized to wider or other populations.
Conclusion
In conclusion, our findings suggest that there is a distinctive pattern of genes showing a diversity of epigenetic modifications in relation to subjective sleep insufficiency in men below 50 years of age. These differences related to compromised neuroplasticity and neurodegeneration (involving genes, such as ERC2, MAGI2, CAST, and CDK5R1) might be triggered by insufficient sleep. The clarification of the processes behind the observed association requires further investigation, both in general population-based samples or larger occupational cohorts, and in experimental data.
Methods and Materials
Study samples
Population-based study
Subjects were obtained from the DIetary, Lifestyle, and Genetic determinants of Obesity and Metabolic syndrome (DILGOM) study, which is an extension of the FINRISK 2007 study aimed at assessing risk factors for metabolic and cardiovascular diseases in the Finnish population47. The DILGOM general population subsample comprised 517 unrelated Finnish individuals from the Helsinki area, aged 25–74 years (54% females, age = 51.9 ± 13.8). Our study comparing DNA methylation for insufficient sleep in blood samples included only men aged 25–50 years, with available data on smoking status and alcohol consumption (n = 88). In order to select the cases for the present study, we used the FINRISK survey question “Do you, in your opinion, sleep enough?” as described in Aho et al.38. Individuals who answered “Seldom or almost never” comprised cases (n = 17), others were controls (answers “Yes, almost always” and “Yes, often” combined to one group, n = 62). Nine individuals answering “I cannot say” were excluded, leaving 79 men in total (age = 39.3 ± 7.3). In addition to the subjective report of sleep insufficiency, all cases reported at least one of the following sleep-related symptoms: symptoms of insomnia during the last month (“often or sometimes”), short sleep (sleep duration of 6 hours or less), or tiredness in the mornings (“rather” or “very tired”). Almost all cases (94%) reported two or three of these sleep-related symptoms. The majority of the DILGOM controls (84%) reported none or one of the above-mentioned sleep-related symptoms. The blood samples were collected between 7 a.m. and 1 p.m. (the exact time was recorded for each participant), after a fasting period of at least 10 hours.
Occupational shift work study
The occupational sample Airline included 42 workers aged 27–60 (21% females) from a Finnish airline company, whose shift work schedule included mornings (starting by 6 a.m.) and/or nights (at least 3 hours of work between 11 p.m. and 6 a.m.) in addition to evenings. The current study focused on 26 male shift workers (age = 44.9 ± 9.0) with DNA samples and available data on smoking status and alcohol consumption. SWD and non-SWD groups were defined using: (1) questions assessing the shift type and work day-specific symptoms of insomnia and sleepiness, and (2) a 3-week sleep diary and actigraphy monitoring to detect a work shift-related reduction of total sleep time, as required by International Classification of Sleep Disorders – Third Edition (ICSD-3) criteria6. To be deemed an SWD case (n = 17), a participant had to report symptoms of insomnia and/or sleepiness “often/continuously” related to a work shift only (i.e. not while on holiday), and had to have a reduced total sleep time. Male shift workers lacking significant insomnia and sleepiness symptoms constituted the non-SWD control group (n = 9). For the blood sample collection performed between 7 a.m. and 11 a.m., the participants had to be healthy and it should have been 7 days since the last infection episode occurred. The samples were never taken while leaving from a night shift. See Table 2 for detailed characteristics of the DILGOM and Airline participants.
DNA methylation and gene expression
Infinium HumanMethylation450K BeadChip methylation measurements
In both study samples, the DNA extraction, CpG methylation, and quality control procedures were performed in the same manner. For DILGOM, DNA was extracted from whole blood samples and CpG methylation was performed using Infinium HumanMethylation450k BeadChip (Illumina, Inc., San Diego, CA, USA), as described in Karlsson Linnér et al.48. For Airline, DNA was extracted from whole blood samples using the same standard methods. DNA methylation was performed using Infinium HumanMethylation450K BeadChip. We conducted post-array processing and normalization for Airline in the same manner, as described in Karlsson Linnér et al.48. Briefly, raw intensity data files were preprocessed, and M-values (log2 ratio of the intensities of methylated probe vs unmethylated probe) were generated as described in the R package “minfi”49. Methylation values were corrected for background, followed by normalization with a subset quantile normalization approach (SWAN)50. After manual inspection of the control probe signals, no outliers were detected; no sex discrepancies were identified after checking the sex prediction samples. The quality control procedure removed the following probes: (a) probes with a low (<95%) detection rate at P < 0.01; (b) known cross-reactive probes51, (c) probes located on sex chromosomes; (d) probes containing a SNP either at the CpG interrogation or at the single nucleotide extension51. After these steps, 479,953 and 433,479 probes remained for the DILGOM and Airline samples, respectively.
Gene expression measurements
Gene expression data were extracted from Illumina HumanHT-12 Expression BeadChip (Illumina Inc., San Diego, CA, USA) for the DILGOM subsample of 79 men aged 25–50 and the Airline subsample of 12 male shift workers (7 cases with SWD, 5 controls with non-SWD). Gene expression detection and data processing, including quantile normalization, were conducted as described in Inouye et al.47.
Analyses
Methylome-wide analysis
White blood cell proportions for DILGOM and Airline were estimated from methylation data by the Houseman method implemented in the ‘minfi’ package in R52. In the DILGOM sample, we first evaluated the effect of age and gender on methylation in the entire sample. The effect of sleep insufficiency on DNA methylation was only analyzed in men aged 25–50 years, as described in “Study samples”. The subjects were divided into two groups based on their self-reported sleep insufficiency (insufficient sleep status). In the Airline sample, subjects (27–60 years old) were divided into two groups based on the severity of the symptoms of SWD. For testing the correlation between each CpG site’s M-value and insufficient sleep or SWD status, we used a multiple linear regression model adjusted for age, smoking status, alcohol consumption, estimated white blood cell subtype proportions, and methylation array batch. Hypomethylation or hypermethylation was defined from the value of the beta coefficient in our regression model, with hypomethylation defined by a negative value. We applied a false discovery rate (FDR) Benjamini-Hochberg correction method for multiple testing to the results. Analyses were performed using R software (R = 3.3.0). Differentially methylated positions (DMPs) (P < 0.05 cutoff level of significance) found common for both studied groups and having the same value (positive or negative) of the estimate in the regression model were annotated to genes, using Infinium HumanMethylation450k BeadChip annotation data.
Pathway analyses
To identify enriched gene ontology (GO) terms for the genes corresponding to DMPs identified in the previous step, we used GoMiner (https://discover.nci.nih.gov/gominer/index.jsp), Panther (http://www.pantherdb.org), and g:Profiler (https://biit.cs.ut.ee/gprofiler) online software tools. An unranked list of gene names served as the input and P < 0.05 (after Benjamini-Hochberg multiple testing correction) was used to indicate statistical significance. We also used QIAGEN’s licensed software Ingenuity® Pathway Analysis (IPA®, QIAGEN Redwood City,CA, USA, www.qiagen.com/ingenuity) with the default options for pathway analysis.
Database search
We used the Ensembl genome browser (https://www.ensembl.org/index.html, release 90, August 2017), National Center for Biotechnology Information (NCBI), the Rat Genome Database (RGD; http://rgd.mcw.edu), and UniProt (http://www.uniprot.org/) for the analysis of gene functions and associated medical conditions (phenotypes). Among the phenotypes, we focused primarily on the following keywords: a) sleep, b) syndrome, and post-hoc we also added c) visual/retinal abnormalities.
Gene expression analysis
The correlation analyses of M-values with corresponding gene expression were performed in SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.), using Pearson correlation.
Sampling time
The correlation analyses of M-values with sample time were conducted in SPSS, using Pearson correlation.
Study approval
Sample collection and study design for DILGOM and Airline were performed according to the principles of the Declaration of Helsinki and were approved by Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. All participants provided written informed consent.
Data Availability
The ethical approval limits the individual-level data availability from Airline and DILGOM cohorts, and prohibits the authors from making the data set publicly available. Data are available from the corresponding author (Tiina Paunio) upon ethical approval from the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District.
References
Pallesen, S., Sivertsen, B., Nordhus, I. H. & Bjorvatn, B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 15, 173–179 (2014).
Kronholm, E. et al. Prevalence of insomnia-related symptoms continues to increase in the Finnish working-age population. J. Sleep Res. 25, 454–457 (2016).
Kronholm, E. et al. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 12, 215–221 (2011).
Porkka-Heiskanen, T., Zitting, K. M. & Wigren, H. K. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol. (Oxf) 208, 311–328 (2013).
Akerstedt, T. Shift work and disturbed sleep/wakefulness. Sleep Med. Rev. 2, 117–128 (1998).
American Academy of Sleep Medicine International Classification of Sleep Disorders, 3rd edn (2014).
Waage, S. et al. Shift work disorder among oil rig workers in the North Sea. Sleep 32, 558–565 (2009).
Flo, E. et al. Shift work disorder in nurses–assessment, prevalence and related health problems. PLoS One 7, e33981 (2012).
Di Milia, L., Waage, S., Pallesen, S. & Bjorvatn, B. Shift work disorder in a random population sample–prevalence and comorbidities. PLoS One 8, e55306 (2013).
Cirelli, C. & Tononi, G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 885, 303–321 (2000).
Benedict, C. et al. Acute sleep deprivation increases serum levels of neuron-specific enolase (NSE) and S100 calcium binding protein B (S-100B) in healthy young men. Sleep 37, 195–198 (2014).
Massart, R. et al. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Translational Psychiatry 4 (2014).
Cedernaes, J. et al. Acute Sleep Loss Induces Tissue-Specific Epigenetic and Transcriptional Alterations to Circadian Clock Genes in Men. J. Clin. Endocrinol. Metab. 100, E1255–61 (2015).
Ventskovska, O., Porkka-Heiskanen, T. & Karpova, N. N. Spontaneous sleep-wake cycle and sleep deprivation differently induce Bdnf1, Bdnf4 and Bdnf9a DNA methylation and transcripts levels in the basal forebrain and frontal cortex in rats. J. Sleep Res. 24, 124–130 (2015).
Skuladottir, G. V., Nilsson, E. K., Mwinyi, J. & Schiöth, H. B. One-night sleep deprivation induces changes in the DNA methylation and serum activity indices of stearoyl-CoA desaturase in young healthy men. Lipids in Health and Disease 15 (2016).
Cirelli, C., Gutierrez, C. M. & Tononi, G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron 41, 35–43 (2004).
Archer, S. N. et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl. Acad. Sci. USA 111, E682–91 (2014).
Bhatti, P. et al. Nightshift work and genome-wide DNA methylation. Chronobiol. Int. 32, 103–112 (2015).
Sulkava, S. et al. Common genetic variation near melatonin receptor 1A gene linked to job-related exhaustion in shift workers. Sleep 40 (2017).
Polo-Kantola, P. et al. Gender differences in actual and preferred nocturnal sleep duration among Finnish employed population. Maturitas 94, 77–83 (2016).
Mander, B. A., Winer, J. R. & Walker, M. P. Sleep and Human Aging. Neuron 94, 19–36 (2017).
Greenberg, F. et al. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2). Am. J. Med. Genet. 62, 247–254 (1996).
De Leersnyder, H. et al. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J. Pediatr. 139, 111–116 (2001).
Williams, S. R., Zies, D., Mullegama, S. V., Grotewiel, M. S. & Elsea, S. H. Smith-Magenis syndrome results in disruption of CLOCK gene transcription and reveals an integral role for RAI1 in the maintenance of circadian rhythmicity. Am. J. Hum. Genet. 90, 941–949 (2012).
Edelman, E. A. et al. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin. Genet. 71, 540–550 (2007).
Girirajan, S. et al. 17p11.2p12 Triplication and Del(17)q11.2q12 in a Severely Affected Child with Dup(17)p11.2p12 Syndrome. Clin. Genet. 72, 47–58 (2007).
Goh, E. S., Banwell, B., Stavropoulos, D. J., Shago, M. & Yoon, G. Mosaic microdeletion of 17p11.2-p12 and duplication of 17q22-q24 in a girl with Smith-Magenis phenotype and peripheral neuropathy. Am. J. Med. Genet. A. 164A, 748–752 (2014).
Foster, J. G., Wong, S. C. & Sharp, T. V. The hypoxic tumor microenvironment: driving the tumorigenesis of non-small-cell lung cancer. Future Oncol. 10, 2659–2674 (2014).
Li, J., Nanayakkara, A., Jun, J., Savransky, V. & Polotsky, V. Y. Effect of deficiency in SREBP cleavage-activating protein on lipid metabolism during intermittent hypoxia. Physiol. Genomics 31, 273–280 (2007).
Yanagida, J. et al. Accelerated elimination of ultraviolet-induced DNA damage through apoptosis in CDC25A-deficient skin. Carcinogenesis 33, 1754–1761 (2012).
Busque, S. M. & Wagner, C. A. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am. J. Physiol. Renal Physiol. 297, F440–50 (2009).
Nguyen, C. L. et al. Nek4 regulates entry into replicative senescence and the response to DNA damage in human fibroblasts. Mol. Cell. Biol. 32, 3963–3977 (2012).
Trivedi, M. S., Holger, D., Bui, A. T., Craddock, T. J. A. & Tartar, J. L. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One 12, e0181978 (2017).
Hirotsu, C., Matos, G., Tufik, S. & Andersen, M. L. Changes in gene expression in the frontal cortex of rats with pilocarpine-induced status epilepticus after sleep deprivation. Epilepsy Behav. 27, 378–384 (2013).
Nakamura, K. et al. Genetic and expression analyses reveal elevated expression of syntaxin 1A (STX1A) in high functioning autism. Int. J. Neuropsychopharmacol. 11, 1073–1084 (2008).
Young, E. J. et al. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 7, 224–234 (2008).
Zhang, N. et al. S-SCAM, a rare copy number variation gene, induces schizophrenia-related endophenotypes in transgenic mouse model. J. Neurosci. 35, 1892–1904 (2015).
Aho, V. et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci. Rep. 6, 24828 (2016).
Higashide, T. & Inana, G. Characterization of the gene for HRG4 (UNC119), a novel photoreceptor synaptic protein homologous to unc-119. Genomics 57, 446–450 (1999).
Jones, S. E. et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet. 12, e1006125 (2016).
Spada, J. et al. Genome-wide association analysis of actigraphic sleep phenotypes in the LIFE Adult Study. J. Sleep Res. 25, 690–701 (2016).
tom Dieck, S. et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J. Neurosci. 32, 12192–12203 (2012).
Kim, E., Grover, L. M., Bertolotti, D. & Green, T. L. Growth hormone rescues hippocampal synaptic function after sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1588–96 (2010).
Grunder, T., Kohler, K. & Guenther, E. Alterations in NMDA receptor expression during retinal degeneration in the RCS rat. Vis. Neurosci. 18, 781–787 (2001).
Partinen, M. et al. Genetic and environmental determination of human sleep. Sleep 6(3), 179–85 (1983).
Dashti, H. et al. GWAS in 446,118 European adults identifies 78 genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. bioRxiv, https://doi.org/10.1101/274977.
Inouye, M. et al. An immune response network associated with blood lipid levels. PLoS Genet. 6, e1001113 (2010).
Karlsson Linnér, R. et al. An epigenome-wide association study meta-analysis of educational attainment. Mol. Psychiatry (2017).
Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369 (2014).
Maksimovic, J., Gordon, L. & Oshlack, A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome Biol. 13, R44-2012-13-6-r44 (2012).
Chen, Y. A. et al. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics 8, 203–9 (2013).
Houseman, E. A. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13, 86 (2012).
Acknowledgements
This work was supported by grants from the Academy of Finland (Grant No. 290039), EVO (TYH2016242), and Biomedicum Helsinki Foundation (Young Scientist’s Grant). We would like to thank Tarja Stenberg for her encouraging approach regarding this project and for thoughtful discussions. Our warmest appreciation goes to Professor Antti Honkela for his aid with pathway analysis statistics. We are grateful to Katri Kantojärvi for her help with DILGOM data analyses. The authors wish to acknowledge the CSC – IT Center for Science, Finland, for computational resources.
Author information
Authors and Affiliations
Contributions
T.P. and A.L. designed and obtained funding for the study. S.P., P.V., M.H. and K.V. were responsible for assembling, collecting and verifying the Airline data, as well as assessing the SWD status. M.P. and T.P. were responsible for assembling and verifying the DILGOM data. L.M. and A.T. designed the molecular experiments, and generated methylation and expression data. N.P. and A.L. performed quality control and pre-processing of the DNA methylation data. A.J., A.L. and P.S. interpreted the methylation data and performed statistical analyses. S.S. interpreted the data and provided input for the final version of the manuscript. All authors reviewed the manuscript and provided editorial feedback.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lahtinen, A., Puttonen, S., Vanttola, P. et al. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep 9, 1193 (2019). https://doi.org/10.1038/s41598-018-38009-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38009-0
This article is cited by
-
Objective and subjective measures of sleep initiation are differentially associated with DNA methylation in adolescents
Clinical Epigenetics (2023)
-
Mediating effects of DNA methylation in the association between sleep quality and infertility among women of childbearing age
BMC Public Health (2023)
-
Longitudinal associations of DNA methylation and sleep in children: a meta-analysis
Clinical Epigenetics (2022)
-
Differential DNA methylation in recovery from shift work disorder
Scientific Reports (2021)
-
Ischemic Stroke and Sleep: The Linking Genetic Factors
Cardiology and Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.