Abstract
Dictyostelid cellular slime molds (dictyostelids) are key components of soil microbes. The Qinghai-Tibet Plateau is characterized by unique and important forest types because of the considerable range in elevation which exists. During the period of 2012, 2013 and 2016, 12 species of dictyostelids were yielded from samples collected in this region, including two new species and three new records for China. Six other species were new records for this region. Ontogeny, morphology, ultrastructure and systematic molecular analyses (SSU & ITS) of D. minimum and D. multiforme confirm that they are Goup 4 new species. The ornamentation of the surface of dictyostelids’ spores is the first time to be observed until now. In the SSU phylogenetic tree generated in the present study, Synstelium, not assigned to order and family before, was assigned to the clade Acytosteliaceae in the Acytosteliales firstly. To our knowledge, the study reported herein is the first investigation of dictyostelid biodiversity carried out at elevations above 2000 m. Sorocarp size, sorus size, spore length, ratio of sorus and sorophore, and ratio of sorus and spore size were positively correlated with increasing elevation and no linear correlated with forest type, according to the results of linear regression analysis.
Similar content being viewed by others
Introduction
Dictyostelids, the second largest group of slime molds, have both animal-like (protozoan) and fungus-like characteristics. The vegetative phase consists of single-celled amoeboid forms that live in the soil, where they feed upon bacteria and other microbes, grow, and multiply until the available food supply is exhausted. When this happens, the amoeboid forms aggregate together in large numbers to form multi-celled pseudoplasmodia, which then give rise to fruiting bodies (sorocarps) that consist of supportive stalks and unwalled sori containing propagative spores1,2.
Although the first dictyostelid was described by Brefeld in 18693, relatively little work was done on the Dictyosteliaceae until 1941, when Harper, Arndt and Raper began their studies of these organisms2. The first ecological survey was carried out in the forests of southern Wisconsin in the United States, when Cavender and Raper4 sampled six sites located along a moisture gradient. Their samples were processed with the use of a quantitative method of isolation5. This experiment showed that dictyostelids are affected by environmental factors, especially moisture6. It is now known that there are a number of factors which can affect the distribution and abundance of dictyostelids, including the physiographic regime sampled7,8, soil pH, soil type, climatic conditions, forest type6,9,10, elevation7,10,11,12, and latitude13. Some species of dictyostelids have been grouped into abundance categories, including very common, common, rare, and very rare9,14,15,16.
Environmental factors such as elevation and pH appear to have a predominant effect on patterns of biodiversity in dictyostelids, while the effects of forest management are secondary7. Species biodiversity is generally very low under dry conditions, although Romeralo et al.17 found Polysphondylium violaceum Bref. to be prominent in drier vegetation types. In the Iberian Peninsula, dictyostelids abundance was reported to be highest in colder and wetter environments, which suggests that this group favors relatively cold places with high levels of water availability6.
Dictyostelids have evolved a number of different structures in response to the different environments in which they occur. Some species (e.g., Dictyostelium septentrionalis [Cavender 1978], now D. septentrionale) form a thick sorophore to help keep this structure erect as an adaptation for fruiting in cool temperatures; other species (e.g., D. rhizopodium Raper & Fennell, now Hagiwaraea rhizopodium [Raper & Fennell] S.Baldauf, S.Sheikh & Thulin) form root-like basal crampons in order to help the sorocarp remain erect longer in tropical habitats; and members of the genus Acytostelium produce small sorocarps, which is probably an adaptation associated with their narrow niche requirements17.
According to the traditional morphology-based classification, dictyostelids were placed in the class Dictyosteliomycetes under the phylum Protozoa. This class was considered to include one order, two families and four genera18 that are distinguished morphologically by differences in sorophore composition and branching pattern. However, data from a phylogenetic analysis based on 18S ribosomal RNA (18S rRNA) and α-tubulin, indicated that none of these three genera are monophyletic, with the dictyostelids divided into four groups19 and eight groups20, respectively. However, Sheikh et al.21 proposed a new classification based on unique 18S rRNA sequence signatures. These data provided a new insight into the taxonomy of the dictyostelids, and as a result of this new classification, two families, 9 genera and 92 new combinations were recognized at the level of species and variety.
The first records of dictyostelids from China were reported by Ronglin Bai22, who listed five species, including four species of Dictyostelium and one species of Polysphondylium, isolated from samples of soil and fallen leaves of broadleaf forests collected in Beijing, Jilin Province, and Shanxi Province, China. In the same year, Yeh and Chien23 reported the occurrence of D. brefeldianum H.Hagiw., D. giganteum B.N. Singh, P. violaceum and P. pallidum Olive (now Heterostelium pallidum [Olive] S.Baldauf, S.Sheikh & Thulin) in samples collected from nine localities located in broadleaf deciduous forests in Taiwan, China. In addition, several new records and new species of dictyostelids have since been reported from a number of localities in China, including Taiwan, Jilin, and Tibet, Heilongjiang, Jiangsu, Inner Mongolia, Hainan, Shanxi and Hubei provinces23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41.
The Qinghai-Tibet Plateau, the highest plateau in the world, is located in the west of China between 73.19–104.47°E and 26.00–39.47°N. This region has long been known as the roof of the world. Most of the plateau is characterized by relatively high elevations, often in excess of 4000 m, but some portions reach only about 2000 m. The range of different elevations leads to a number of unique vegetation types. Studies of dictyostelids in this region are exceedingly limited and consist of a single previous report of a species new to science35.
The primary objective of the study reported herein was to increase our knowledge of dictyostelid biodiversity and abundance at high elevations (>2000 m) and in ecologically complex habitats. Samples for isolation of these organisms were collected from nine sites on the Qinghai-Tibet Plateau. The dictyostelids recovered from these samples were studied, both for their morphological features and also with molecular markers. The species biodiversity of dictyostelids on the Qinghai-Tibet Plateau is discussed in relation to previous studies and concepts relating to the overall ecology of these organisms.
Results
After being processed, the samples collected from the Qinghai-Tibet Plateau yielded 16 isolates representing 12 species of dictyostelids (Fig. 1, Table S1). Three of these (Dictyostelium brevicaule Vadell & Cavender, D. vermiforme Vadell & Cavender, Cavenderia fasciculata [F. Traub er al.] S.Sheikh, Thulin & S.Baldauf) were reported for the first time from China. Six of these (D. brefeldianum H.Hagiw., D. crassicaule H.Hagiw., C. antarctica [Cavender et al.] S.Sheikh, Thulin & S.Baldauf, C. aureostipes [Cavender] S.Sheikh, Thulin & S.Baldauf, C. exigua (H.Hagiw.) S.Sheikh, Thulin & S.Baldauf, and Heterostelium tikalense [Vadell & Cavender] S.Sheikh, Thulin & S.Baldauf) are new records for the Qinghai-Tibet Plateau. Five of the six species (D. crassicaule, C. antarctica, C. aureostipes, C. exigua, and H. tikalense) had been recorded from China from only one previous record in Guizhou Province, Hubei Province, Liaoning Province, Taiwan Province and Jilin Province, respectively. The remaining two isolates are species new to science, and these have been given the names D. minimum and D. multiforme (Figs 2–5). Phylogenetic studies of the nuclear ribosomal small subunit (SSU) rDNA showed the two new species D. minimum and D. multiforme are members of Group 4 (Fig. 6, Table S2), based on the concepts of Schaap et al.19, Romeralo et al.20, and Sheikh et al.21.
Map showing the collecting sites in China and the nine collecting sites on the Qinghai-Tibet Plateau. Information on the species of dictyostelids recovered and the proportion of the total each species represented is provided for each of the nine sites. The different color from red to blue of elevation scale represented different elevations from 8800 m to 0 m. (i) the Sejila Mountain National Forest Park, Nyingchi; (ii) Medog; (iii) Bome; (iv) Zayu; (v) Lulang; (vi) Lhasa; (vii) Yushu; (viii) Huzhu Beishan National Forest Park; (ix) the Jiuzhaigou National Forest Park, Aba.
Morphological features of Dictyostelium minimum. (A) Spores; (B) Spores (SEM), arrow refers to globose bulges; (C) Spore (TEM), arrows refer to the uneven cell wall; (D) Cells of sorophore (TEM); (E,F) Base of sorophore; (G) Tips of sorophores; (H,I) Sori and tip of sorophore (SEM); (J) Aggregations; (K) Sorocarps; (L) Sorocarp (SEM). Abbreviations: n, nucleus; m, mitochondria; cw, cell wall. Bars: A = 10 µm; B,D = 2 µm; C = 0.5 µm; E,F,G = 50 µm; H = 5 µm; I = 4 µm; J = 500 µm; K = 1 mm; L = 50 µm.
Morphological features of Dictyostelium multiforme. (A) Spores; (B) Sorophore; (C,D) Tip of sorophores; (E,F,G) Base of sorophore; (H) Aggregations; (I) Sorogen (J,K) Sorocarps; (L) Spores (SEM), arrows refer to ridges of spore surface; (M) Sorus (SEM); (N) Spore (TEM), arrow refers to the uneven cell wall; (O) Cells of sorophore (TEM). Abbreviations: n, nucleus; m, mitochondria; cw, cell wall. Bars: A = 10 µm; B = 200 µm; C,D,E,F,G = 50 µm; H,I = 200 µm; J = 500 µm; K = 1 mm; L = 4 µm; M = 50 µm; N = 833 nm; O = 2.5 µm.
Taxonomy and molecular phylogeny
Dictyostelium minimum Li Y., P. Liu et Y. Zou, sp. nov. (Fig. 2)
MycoBank: MB823443
When cultured at 17 C on non-nutrient agar with Escherichia coli, sorocarps white, erect, stout, very short, only 95–720 μm high (average 324 μm), solitary, non-phototropic. Sorophore stout, tapering from base to tip, usually consisting single tier of cells from the middle portion to the tip, consisting of two or several tiers of cells from the middle portion to the base, base clavate or acuminate with accessory structures and two or several tiers of cells. The interior of the sorophore is hollow according to the SEM observations of the sorophore. Sori white, globose or citriform, 33–148 μm (average 72 μm) diam. Spores globose, without polar granules, 3.7–5.4 μm diam. Cell aggregations mound-like, without radiate streams, usually 39–73 × 29–57 μm (average 52 × 42 μm). Pseudoplasmodia not migrating without sorophore formation.
Etymology
This name refers to the small size of the sorocarps.
Holotype
HMJAU MR244. Isolated in 2012 (Strain 2794) from a soil sample collected from a mixed forest of Picea asperata, Pinus densata, Quercus semecarpifolia, Pterocarya stenoptera, and Betula delavayi, located at an elevation of approximately 3100 m in Lulang, Tibet.
Known distribution
Currently known only from China.
Commentary
There are primarily five species of dictyostelids that have globose spores42. These are Dictyostelium rosarium Raper & Cavender, D. globisporum Yu Li & P.Liu, Raperostelium ibericum (Romeralo et al.) S.Sheikh, Tieghemostelium lacteum (Tiegh.) S.Sheikh, Thulin & S.Baldauf var. papilloideum (Cavender) S.Sheikh, Thulin & S.Baldauf, and T. lacteum (Tiegh.) S.Sheikh, Thulin & S.Baldauf var. papilloideum (Cavender) S.Baldauf, S.Sheikh & Thulin. The spores of D. globisporum, R. ibericum, and T. lacteum all have polar granules, which are lacking in D. minimum. Although D. rosarium has inconspicuous polar granules, the aggregations in this species have radiate streams and form clustered sorocarps. The sorocarps of T. lacteum var. papilloideum (0.6–1.4 mm) are larger than those of D. minimum, the sorophores are thinner than those of D. minimum, and the pseudoplasmodia of T. lacteum var. papilloideum migrate without sorophore formation.
This species belongs to Dictyostelid Group 419,20,21 in SSU rDNA phylogeny (Fig. 6). It forms a clade together with D. multiforme. In the ITS phylogenetic tree (Fig. 7), D. minimum forms a clade with D. crassicaule and D. pseudobrefeldianum H.Hagiw. However, the most noteworthy difference between them is the presence of globose spores in D. minimum. The SSU and ITS rDNA sequences for D. minimum are available at GenBank, with the accession numbers MG490369 and MG490372, respectively.
Dictyostelium multiforme Li Y., P. Liu et Y. Zou, sp. nov. (Fig. 3)
MycoBank: MB823442
When cultured at 23 C on non-nutrient agar with E. coli, sorocarps white, solitary or gregarious, erect or semi-erect, without branches or rarely with one branch. Semi-erect sorocarps mostly 0.68–1.80 mm long, sometimes with sinuous sorophores which have an “S” shape in the central portion, other semi-erect sorophores straight, consisting of several tiers of cells. Erect sorocarps mostly 0.21–1.13 mm long, sorophores straight, consisting one or two tiers of cells. Sorophore tips clavate, mostly 12.0–26.9 µm, with two or several tiers of cells, semi-erect, the tips with dense refractile matrices of slime present. Sorophore base clavate, acuminate, or conical, mostly 9.4–20.1 µm, with one or two tiers of cells, with basal support disk. The interior of the sorophore is hollow according to the SEM observations of the sorophore. Sori white, globose, mostly 90–250 µm diam. Spores oblong or elliptical, sometimes reniform, without polar granules, mostly 5.8–12.6 × 3.6–5.0 µm. Cell aggregations mound-like, minutum-type, without radiate streams. Pseudoplasmodia not migrating without sorophore formation.
Etymology
This name of the species refers to the presence of several types of sorocarps.
Holotype
HMJAU MR245. Isolated in 2013 (Strain 4007, 4009) from a sample of animal dung, the sample collected at an elevation of 4275 m in Yushu, Qinghai Province.
Known distribution
Currently known only from China.
Commentary
This species is similar to Dictyostelium barbibulus Perrigo & Romeralo, D. microsorocarpum Yu Li & Xiao L.He, D. mucoroides Bref. var. stoloniferum Cavender & Raper, and D. crassicaule, with the significant differences among them discussed below. However, the sorophore base of D. barbibulus is 50–80 µm, which is wider than that of D. multiforme. Moreover, the sorocarp of D. barbibulus is erect. The aggregation of D. microsorocarpum is radiate, whereas the aggregation of the new species is mound-like, and the sori of D. microsorocarpum are smaller than those of the new species. The sorocarps of D. mucoroides var. stoloniferum are phototropic, while the new species does not have this feature. The aggregations of D. crassicaule are radiate and the sorophore tips are capitate, whereas D. multiforme has mound-like aggregations, clavate sorophore tips and more narrow sorophore bases than is the case for D. crassicaule.
This species belongs to Dictyostelid Group 419,20,21 in the SSU rDNA phylogeny (Fig. 6). It forms a clade along with D. minimum. Morphologically, the spore shape and size, sorophore cell tiers and sorocarp appearance are different between D. multiforme and D. minimum. In the ITS phylogenetic tree (Fig. 7), D. multiforme is in a different clade with D. minimum which form a single clade in Group 4. The SSU and ITS rDNA sequences for D. multiforme are available at GenBank, the accession numbers are MG490370 (Strain 4007), MG490371 (Strain 4009), MG490373 (Strain 4007,) and MG490374 (Strain 4009), respectively.
Ontogeny
Life cycle of Dictyostelium minimum
Spore germination (Fig. 4A) begins with the appearance of a minute pore dissolved in the spore wall after 15 h on agar. At the same time, several myxamoebae (Fig. 4B) are released from the spores. Myxamoebae are colorless, transparent, and irregular. When a “critical mass” of myxamoebae occurs, myxamoebae aggregate to one center to form a single aggregation (Fig. 4C), with no cell streams 36 h after the spores have been inoculated on agar. The aggregations (Fig. 4D) grow larger and rise up in the center after 39 h. The aggregations culminate (Fig. 4E) and began to form pseudoplasmodia with indistinct sorophores 42 h after inoculation. The sorophore (Fig. 4F) begins to form and grow longer at about 45 h. Two hours later, the slug (Fig. 4G) begins to form and with no movement in this process. Three hours later, the sorogen is produced and is prostrate on the surface of the agar, and the young sorocarp begins to fruit (Fig. 4H). The top of the sorogen became tapered, then changes to globose, and the sorophore becames longer and curved on the agar (Fig. 3I). Fifty-six h after inoculation, the sorocarps (Fig. 4J) fruit; the sorocarps are very small, solitary, and non-phototropic.
The entire life cycle of D. minimum extends over 3 d. The spores germinated and released myxamoebae 15 h after inoculation. The aggregations formed 36 h after inoculation. Pseudoplasmodia formation required 42 h. Then, eight hours later, the sorogen formed. After having been inoculated 56 h previously, the sorocarps fruited.
Life cycle of Dictyostelium multiforme
Elliptical spore germination (Fig. 5A) begins with the dissolution of the spore wall to release myxamoebae (Fig. 5B,C) after 21.5 h on agar. Myxamoebae are colorless, transparent, and irregular. Their shape changed as they moved about, feeding upon bacteria, growing larger and aggregating as a result of being attracted by cAMP to form a single mould-like aggregation (Fig. 5D) without radiated cell streams 28 h after the spores had been inoculated on agar. After the aggregating of more and more myxamoebae, the cell aggregations (Fig. 5E) rise up in the center begin to form pseudoplasmodia with indistinct sorophores 30 h after inoculation. Two hours later, the sorophores begin to form (Fig. 5F). Sorophores grow longer, which gives them the appearance of a fruiting sorophore (Fig. 5G) at 33 h, pseudoplasmodia form and undergo a very short period of movement. The sorophore is slightly curved, which gives it an “S” shape in the central portion. After the formation of the sorophore, the sori begin to grow gradually to form globose sori at 37 h (Fig. 5H). Forty-one h after inoculating spores on the agar, the sorocarps (Fig. 5I) finally fruit. Sorocarps are solitary, erect or semi-erect and lack branches.
The whole life cycle of D. multiforme extends of a period of less than 2 d (41 h). The spores germinated and released myxomoebae 21.5 h after inoculation. The aggregations formed 28 h after the inoculation, whereas the pseudoplasmodia formation needed 33 h. After that, the sorophores and sori begin to grow orderly and finally form fruiting sorocarps after 41 h. The formation of myxomoebae of this species is later than that of D. minimum; however, the formation of sorocarps from pseudoplasmodia in this species is shorter than that of D. minimum.
Ecology
Dictyostelids-elevation relationships
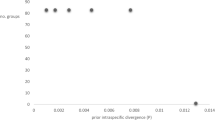
Environmental factors such as elevation clearly have an effect on the biodiversity and morphological features of species of dictyostelids. Considering the 11 species (15 isolates) of dictyostelids isolated in the present study, except Heterostelium tikalense, all have traditional simple sorophore, so the relationships between morphological characteristics of those 15 isolates and their elevations were analyzed. From the results of linear correlation analysis of elevation with the six predictors considered in the present study, increases in elevation led to increasing sorocarp size, sorus size, spore length, ratio of sorus and sorophore, and ratio of sorus and spore size (Fig. 8A–C,E,F). In contrast, increasing elevation was correlated with decreasing spore width (Fig. 8D).
Correlation of morphological features of 11 species of Dictyostelium and Cavenderia (15 isolates in total in this paper) with elevation. R sequared values for the linear regression are given in each panel. (A) Sorocarp size; (B) Sorus size; (C) Spore length; (D) Spore width; (E) Ratio of sorus and sorophore; (F) Ratio of sorus and spore size. y = −11170.711 + 1549.319 * sorocarp size + 23948.826 * sorus size + 142.497 * spore length − 1070.853 * spore width + 26827.390 * sorus / sorophore + 168933.603 * spore / sorus. “y = 0” represents the average elevation of those isolates, 3363 m (grey lines). “x = 0” reprensents the average of each predictors. The blue lines represent the trend of each predictors with elevations. Increases in elevation led to increasing sorocarp size, sorus size, spore length, ratio of sorus and sorophore, and ratio of sorus and spore size. In contrast, increasing elevation was correlated with decreasing spore width.
Dictyostelids-forest type relationships
In the present study, 13 isolates of dictyostelids were obtained from different forest types including mixed forest, broadleaf forest, coniferous forest, grassland, and alpine grassland. Another three isolates of dictyostelids were isolated from animal dung. After make an analysis of the morphological charactertistics of those dictyostelids with those forest types, we found there was no linear correlation of forest type with sorocarp size, sorus size, spore length, spore width, ratio of sorus and sorophore, and ratio of sorus and spore size.
Discussion
Effects of elevation on the biodiversity of dictyostelids
Ecological characteristics such as elevation clearly have an effect upon the biodiversity of dictyostelids. However, studies of the distribution and abundance of dictyostelids associated with similar habitats at high elevations (>2000 m) are exceedingly limited. Although Cavender et al.12 and Landolt et al.10 indicated that differences in elevation (<2000 m) and forest types affected the distribution patterns of dictyostelids in the Great Smoky Mountains National Park, a number of new species were recovered at high elevations in the park. These authors also commented on the apparent negative effect of elevation on dictyostelid abundance and a possible positive effect on species richness43. On the other hand, Paillet and Satre7 reported higher biodiversity of dictyostelids at higher elevations (<2000 m) in French Alps. The present study firstly investigated dictyostelid biodiversity in mountains at elevations >2000 m. Five species was recorded from samples collected at elevations of 2000–3000 m, four species at elevations of 3000–4000 m, and four species from samples collected at elevations of 4000–5000 m. This suggests that dictyostelids are probably not uncommon at higher elevations (>2000 m), although studies carried out at such elevations are exceedingly limited.
Effects of elevation on the morphology of dictyostelids
Elevation also affected the morphological features of the species of dictyostelids being considered. There was a positive correlation of increasing of elevation with sorocarp size, sorus size, spore length, ratio of sorus and sorophore, and ratio of sorus and spore size, although the R2 values for linear correlations of spore length were weak (Fig. 8). Dictyostelids carry bacteria during spore dispersal and thus can “seed” a new food crop, which is a major advantage if edible bacteria are lacking at the new site44. Presumably, the larger the size of the sorocarp and sori would allow them carry more bacteria if edible bacteria are not abundant enough in habitats such as those found at higher elevations on the plateau.
Substrate types
Dictyostelids have been recovered from animal dung45, forest soil17, grassland soil46, canopy soil47,48, and soil in an agricultural field49. In the present study, dictyostelids were recovered from both animal dung and forest soil. From these samples, four species (Dictyostelium brevicaule, D. minimum, D. sphaerocephalum, Cavenderia aureostipes) were recorded from mixed forest soil, three species (D. vermiforme, D. sphaerocephalum, C. fasciculata) from coniferous forest soil, three species (D. sphaerocephalum, C. exigua, Heterostelium tikalense) from broadleaf forest soil, two species (D. brefeldianum, D. sphaerocephalum) from alpine grassland soil, one species (C. antarctica) from grassland soil, and two species (D. crassicaule, D. multiforme) from animal dung. However, there were no positive linear correlations of forest type with sorocarp size, sorus size, spore size, ratio of sorus and sorophore, and ratio of sorus and spore size. There are the highest genus diversity and species diversity of dictyostelids in broadleaf forest and mixed forest seperately in this study.
Environmental conditions affect the distribution of dictyostelids
Dictyostelium minimum has very small sorocarps, and the sorophore is stout and thick, which is a character also found in species which occur in habitats characterized by cool temperatures (20 C or lower) in order to keep the sorocarp erect during slow development17. Presumably, the small size of the sorocarps would allow them to compete more successfully under conditions that are marginal for larger species, such as those microhabitats with a limited bacterial food supply10. In the present study, we used both 17 C and 23 C to culture the new species D. minimum; however, it grew well at 17 C and but was unstable and sometimes even unsuccessful at 23 C.
Dictyostelium sphaerocephalum tends to be more abundant in habitats where conditions are less favorable50,51,52. Examples include extremely cool, dry, or disturbed habitats15 such as in the tundra50,53. In our study, D. sphaerocephalum was also recovered at three collecting sites located at higher elevations (3000–4600 m). The occurrence of this species in alpine grassland soil, mixed forest soil and coniferous forest soil provide additional data to support the concept that D. sphaerocephalum is a truly widespread species7.
In contrast, D. crassicaule, C. exigua, and C. fasciculata were reported as restricted species in soils of the world’s forests43, herein they were all found again from forest soil and animal dung, which supports the point of view43 that animal vectors and plant associations have a major role in determining the distribution of dictyostelids.
Futhermore, the highest relative abundance of dictyostelids in this study are Localities (ii) Medog and (v) Lulang (Fig. 1). We found Medog has the highest annual temperature, and Lulang has the highest annual precipitation within localities of this study. Presumably, the species abundance of dictyostelids correlated with the environmental conditions such as temperature and precipitation a lot.
Ultrastructure of the two new species
From SEM and TEM images obtained for D. minimum and D. multiforme, we found that the spore wall was characterized by the presence of small bulges. The bulges of D. minimum are nearly globose and short. The ridged bulges of D. multiforme are deeper and longer than those of D. minimum. These data suggest that the surface of dictyostelid spore may differ with respect to ornamentation.
Molecular phylogeny
Detailed analysis involving the alignment of SSU of these two new species D. minimum and D. multiforme indicated that AAG and ACTG occur in the nucleotide positions 664–666 and 671–674, respectively (Fig. 6); this indicates that they clearly belong to genus Dictyostelium21. However, the globose spores of D. minimum add a new spore feature to be considered in the new classification of Dictyostelium. In the SSU rDNA phylogenetic tree (Fig. 6), D. minimum and D. multiforme were in the same clade. However, when the phylogenetic relationships of Group 4 species were analyzed with the ITS rDNA (Fig. 7), D. minimum and D. multiforme occurred in different clades.
In the SSU phylogenetic tree published by Sheikh et al.21, Synstelium was not assigned to any family or order; herein we found it was most closely related to clade Acytosteliaceae in the Acytosteliales. Consequently, Synstelium was recognized in the Acytosteliaceae and Acytosetliales in the present study. All other taxonomic levels (genus, family and order) are consistent with those given by Sheikh et al.21.
Three species (D. rosarium, Tieghemostelium lacteum, and Raperostelium ibericum) with globose spores are members of Group 4, Group 3 A, and Group 3 C, respectively21. In the present study, another species (D. minimum) with globose spores was obtained which belongs to Group 4 according to the new classification of Sheikh et al.21. These four globose spore speices all belongs to Dictyostliales of two families (Group 3 and Group 4). As such, it is not possible to use only a single morphology-based taxonomic feature such as spore shape to differentiate species in the dictyostelids.
Materials and Methods
Sampling
Samples used for isolation of dictyostelids were collected from nine sites on the Qinghai-Tibet Plateau in China during the period of 2012, 2013 and 2016. A total of 114 samples, including soil and animal dung, each approximately 30–50 g, were collected and placed in sterile whirl-pack plastic bags. In most instances, at least five samples were collected from each vegetation type at each locality. Afterwards, these samples were returned to the laboratory as soon as possible, following the recommendations of Cavender and Raper5. Each sample bag was numbered and the sample itself preserved at 4 C in the herbarium of the Mycological Institute of Jilin Agricultural University (HMJAU), Changchun, China.
Localities (Fig. 1, Figure S1) where samples were collected on the Qinghai-Tibet Plateau were (i) the Sejila Mountain National Forest Park, Nyingchi, Tibet, average elevation 3100 m, annual precipitation 650 mm, annual temperature 8.7 C; (ii) Medog, Tibet, a moist region, average elevation 1200 m, annual precipitation 900 mm, annual temperature 16 C; (iii) Bome, Tibet, elevation 2003–6648 m, annual precipitation 977 mm, annual temperature 8.5 C; (iv) Zayu, Tibet, elevation 1400–6740 m, annual precipitation 801 mm, annual temperature 12 C; (v) Lulang, Tibet, average elevation 3700 m, annual precipitation 1849 mm, annual temperature 12 C; (vi) Lhasa, Tibet, average elevation 3650 m, annual precipitation 200–510 mm, annual temperature 7.4 C; (vii) Yushu, Qinghai Province, average elevation 4200 m, annual precipitation 487 mm, annual temperature 2.9 C; (viii) Huzhu Beishan National Forest Park, Qinghai Province, average elevation 2100–4308 m, annual precipitation 477 mm, annual temperature 5.8 C; and (ix) the Jiuzhaigou National Forest Park, Aba, Sichuan Province, average elevation 2050–4964 m, annual precipitation 836 mm, annual temperature 6.1 C.
Isolation and cultivation
The isolation methods used in the present study followed those described by Cavender and Raper5. Each sample was weighed and diluted for an initial dilution of 1:10 by adding ddH2O. This dilution was shaken to disperse the material and to suspend the amoebae and spores of dictyostelids. Afterwards, a 0.5 mL aliquot of this dilution was added to each of five duplicate culture plates prepared with hay infusion agar2. Approximately 0.4 mL of a heavy suspension of the bacterium E. coli was added to each culture plate as a food source. The plates were incubated at temperatures of 17 and 23 C with a 12 h light and dark cycle. Each plate was examined at least once a day for two weeks after the appearance of initial aggregations. Each isolate was purified and cultivated for taxonomic studies and preservation on non-nutrient water agar plates with E. coli pregrown for 12–24 h. Spores from these plates were frozen in HL 5 media54 and stored at −80 C in HMJAU, Changchun, China.
Morphological features and life cycle observations
Dictyostelid isolates were identified with the use of the descriptions provided by Raper2, whose nomenclature also was followed except for those species recently assigned to new genera in the system of classification proposed by Sheikh et al.21. In the primary isolation plates, the locations of each early aggregating clone and sorocarp that developed were marked. The characteristic stages in the life cycle, including cell aggregation and the formation of pseudoplasmodia, and sorocarps were observed under a Zeiss dissecting microscope (Axio Zoom V16) with a 1.5× objective and 10× ocular. Slides with sorocarps were prepared with water as the mounting medium. Features of spores, sorophores, and sorocarps were observed and measured on the slides by using a Zeiss light microscope (Axio Imager A2), with 10× ocular and 10, 40, and 100× (oil) objectives. Photographs were taken with Zeiss Axiocam 506 color microscope camera.
Observation of spore germination
Hanging drop cultures as described by Keller and Schoknecht55 were prepared for the observation of spore germination. Spores obtained from a sorus were mixed with a droplet of sterile water on the undersurface of a 22-mm square cover glass. The cover glass was then inverted over a depression slide. Vaseline was used to ring the edges of the cover glass. Spores were freely suspended in the water droplet. Features of the myxamoebae were observed and photographed by a Zeiss laser confocal microscope (LSM 710).
Scanning electron microscopy
Spores of new species Dictyostelium minimum and D. multiforme were prepared for scanning electron microscopy according to the method of Boyde and Wood56. The collected spores were washed, fixed, dehydrated, dried, and prepared for study, then observed by with Hitachi scanning electron microscope (SU 8010).
Transmission electron microscopy
The sorocarps of new species D. minimum and D. multiforme were prepared for transmission electron microscopy according to standard techniques57. The sorocarps were collected with a prefixation in 4% glutaraldehyde for more than 4 h at 4 C, followed by postfixation in 2% osmium tetroxide for 2 h at 4 C, with both fixatives buffered in 0.05 N Na-cacodylate buffer at pH 7.4. Afterwards, the samples were dehydrated in water, ethanol and acetone, embedded in SPI-PON 812 for 12 h at 35 C, for 12 h at 45 C, and for 24 h at 60 C. After these processes had been completed, the sections to be observed were cut on a Leica EM UC7, stained in uranyl acetate and lead citrate, observed and photographed by a Hitachi transmission electron microscope (H-7650).
DNA isolation, PCR amplification and sequencing
After amoebae had cleared E. coli on the water agar media, the spores of dictyostelid isolates to be studied were collected with a sterile tip, then those spores were mixed with the lysed buffer of the NuClean Plant Genomic DNA Extraction Kit from CW Biotech (Beijing, China) and the following steps were carried out according to the instructions provided along with this kit. The genomic DNA solution was used directly for the PCR amplification. The SSU and ITS rDNA markers were amplified using the primers 18SF–A and 18SR–B, D542F and D1340R, and ITS1 and ITS4 (Table S3). PCR products were sent to Sangon Biotech Co., Ltd. (Shanghai) for sequencing.
Phylogenetic analysis
The newly-generated sequences were checked and then submitted to GenBank. Sequences for all closely related species were downloaded from GenBank (Table S2) for phylogenetic analysis. The ITS and SSU sequences were aligned and compared separately using the program Muscle v.3.658,59, then manually adjusted in MEGA 7.060. Maximum likelihood (ML) analyses were performed using RAxML v761. In the ML analyses, the best-fit substitution models were estimated using GTR submission model and a gamma correction for rate variation among sites (GTRGAMMA), using the CIPRES server. The statistical support of clades was assessed with 1000 rapid-bootstrap (BS) replications.
Ecological statistics analysis
Sorocarp size, sorus size, spore length, spore width, ratio of sorus and sorophore, and ratio of sorus and spore size data for the 11 species of Dictyostelium and Cavenderia being considered (15 isolates in total) in this study were inputted to the IBM SPSS 19.0 version software as predictors for linear regression analysis of those six predictors (sorocarp size, sorus size, spore length, spore width, ratio of sorus and sorophore, and ratio of sorus and spore size) with the two dependent variables of elevation and forest type, with each of the latter considered separately.
Nomenclature
According to the International Code of Nomenclature for algae, fungi, and plants, the electronic version of this article in Portable Document Format (PDF) will represent a published work. In addition, new names contained in this study have been submitted to MycoBank and will each be allocated a unique MycoBank number which will be accessible through MycoBank, Index Fungorum, GBIF and other international biodiversity initiatives where they will be made available to the Global Names Index.
References
Raper, K. B. A. In: The Fungi (eds Ainsworth, G. C., Sparrow, F. K., Sussman, A. S.) 9–36 (Academic Press, New York, 1973).
Raper, K. B. The Dictyostelids. (Princeton University Press, United States, 1984).
Brefeld, O. Dictyostelium mucoroides. Ein neuer Organismus und der Verwandschaft der Myxomyceten. Abh Seckenburg Naturforsch Ges 7, 85–107 (1869).
Cavender, J. C. & Raper, K. B. The Acrasiae in nature. III. Occurrence and distribution in forests of eastern North America. American Journal of Botany 52, 302–308 (1965).
Cavender, J. C. & Raper, K. B. The Acrasiae in nature. I. Isolation. American Journal of Botany 52, 294–296 (1965).
Romeralo, M., Moya-Larano, J. & Lado, C. Social amoebae: environmental factors influencing their distribution and diversity across south-western Europe. Microbial Ecology 61(1), 154–165 (2011).
Paillet, Y. & Satre, M. The biodiversity of dictyostelids in mountain forests: A case study in the French Alps. Pedobiologia 53, 337–341 (2010).
Cavender, J. C. & Hopka, C. Distribution patterns of Ohio soil dictyostelids in relation to physiography. Mycologia 78, 825–831 (1986).
Landolt, J. C. & Stephenson, S. L. Cellular slime molds in forest soils of West Virginia. Mycologia 82, 114–119 (1990).
Landolt, J. C., Stephenson, S. L. & Cavender, J. C. Distribution and ecology of dictyostelid cellular slime molds in Great Smoky Mountains National Park. Mycologia 98(4), 541–549 (2006).
Benson, M. R. & Mahoney, D. P. The distribution of cellular slime molds in southern California with taxonomic notes on selected species. American Journal of Botany 64, 496–503 (1977).
Cavender, J. C., Vadell, E. M., Landolt, J. C. & Stephenson, S. L. New species of small dictyostelids from the Great Smoky Mountains National Park. Mycologia 97(2), 493–512 (2005).
Perrigo, A., Baldauf, S. L. & Romeralo, M. Diversity of dictyostelid social amoebae in high latitude habitats of Northern Sweden. Fungal Diversity 58(1), 185–198 (2013).
Cavender, J. C. & Vadell, E. M. Cellular slime molds of Ohio. Ohio Biological Survey Bulletin New Series 16, 1–2 (2006).
Cavender, J. C. Cellular slime molds of Rocky Mountains. Mycologia 75, 897–903 (1983).
Hagiwara, H. Dictyostelid cellular some molds of Pakistan. I. Distribution and occurrence in soils of forests, cultivated fields and alpine pastures. Cryptogamic Flora of Pakistan. Bulletin of the National Science Museum, Series B 1, 87–98 (1992).
Romeralo, M., Baldauf, S. & Escalante, R. Dictyostelids Evolution, Genomics and Cell Biology. (Springer, Berlin Heidelberg, 2013).
Kirk, P. M., Cannon, P. F., Minter, D. W. & Stalpers, J. A. Dictionary of the fungi, 10th edition. (CABI, UK, 2008).
Schaap, P. et al. Molecular phylogeny and evolution of morphology in the social amoebas. Science 314, 661–663 (2006).
Romeralo, M., Cavender, J. C., Landolt, J. C., Stephenson, S. L. & Baldauf, S. L. An expanded phylogeny of social amoebas (Dictyostelia) shows increasing diversity and new morphological patterns. BMC Evolutionary Biology 11, 84 (2011).
Sheikh, S. et al. A new classification of the dictyostelids. Protist 169, 1–28 (2018).
Bai, R. L. A study on some species of Acrasiomycetes. Acta Mycologica Sinica 2, 173–178 (1983).
Yeh, Z. Y. & Chien, C. Y. Cellular slime molds in Taiwan I: Four newly recorded species. Biological Bulletin of National Taiwan Normal University 18, 69–86 (1983).
Hagiwara, H., Yeh, Z. Y. & Chien, C. Y. Dictyostelium macrocephalum, a new dictyostelid cellular slime mold from Taiwan. Bulletin of the National Science. Museum, Series B 11, 103–108 (1985).
Cui, J. T. Taxonomic studies on Dictyostelium in Northeast China. MS thesis, College of Agronomy, Jilin Agricultural University, China (1991).
Hagiwara, H., Chien, C. Y. & Yeh, Z. Y. Dictyostelid Cellular Slime Molds of Taiwan. Bulletin of the National Science Museum, Series B 18, 39–52 (1992).
Lin, S. P. & Yeh, Z. Y. Six species of dictyostelid cellular slime molds isolated from Yang Ming Shan district of Taipei. Biological Bulletin of National Taiwan Normal University 34, 69–80 (1999).
Fan, Y. C. & Yeh, Z. Y. Notes on a dictyostelid cellular slime mold new to Taiwan. Bio Formosa 36, 43–46 (2001).
Fan, Y. C. & Yeh, Z. Y. Notes on dictyostelid cellular slime molds of Taiwan 1. Dictyostelium minutum and Dictyostelium clavatum. Taiwania 47, 31–36 (2002).
Hsu, S. L., Fan, Y. C., Ma, M. S., Chou, M. H. & Yeh, Z. Y. Dictyostelium delicatum a new record of dictyostelid cellular slime molds to Taiwan. Taiwania 46, 199–203 (2001).
Yeh, Z. Y. Biodiversity inventory of dictyostelid cellular slime molds in Taiwan. Mycotaxon 86, 103–110 (2003).
He, X. L. Preliminary Taxonomic Studies on Dictyosteliaceae in China. MS thesis, College of Agronomy, Jilin Agricultural University, China (2008).
He, X. L. & Li, Y. Three new records of dictyostelids in China. Mycosystema 27, 532–537 (2008).
He, X. L. & Li, Y. A new species of Dictyostelium. Mycotaxon 106, 379–383 (2008).
He, X. L. & Li, Y. A new species of Dictyostelium from Tibet, China. Mycotaxon 111, 287–290 (2010).
Liu, P. & Li, Y. Dictyostelids from Heilongjiang Province, China. Nova Hedwigia 94(1–2), 265–270 (2012).
Liu, P. & Li, Y. New records of dictyostelids from China. Nova Hedwigia 94(3–4), 429–436 (2012).
Liu, P. & Li, Y. Dictyostelids from Jilin Province, China. I. Phytotaxa 183(4), 279–283 (2014).
Liu, P. & Li, Y. Dictyostelids from Jilin Province, China. II. Phytotaxa 323(1), 077–082 (2017).
Ren, Y. Z., Liu, P. & Li, Y. New records of dictyostelids from China. Nova Hedwigia 99(1–2), 233–237 (2014).
Zhao, M. J., Liu, P., An, Y., Yao, Y. J. & Li, Y. Dictyostelium annularibasimum (Dictyosteliaceae, Dictyostelida), a new purple species from China. Nova Hedwigia 104(1–3), 351–358 (2017).
Liu, P. & Li, Y. New species and new records of dictyostelids from Ukraine. Mycologia 103(3), 641–645 (2011).
Swanson, A. R., Vadell, E. M. & Cavender, J. C. Global distribution of forest soil dictyostelids. Journal of Biogeography 26(1), 133–148 (1999).
Brock, D. A., Douglas, T. E., Queller, D. C. & Strassmann, J. E. Primitive agriculture in a social amoeba. Nature 469, 393–396 (2011).
Stephenson, S. L. & Landolt, J. C. Vertebrates as vectors of cellular slime moulds in temperate forests. Mycological Research 96, 670–672 (1992).
Rollins, A. W., Landolt, J. C. & Stephenson, S. L. Dictyostelid cellular slime molds associated with grasslands of the central and western United States. Mycologia 102, 996–1003 (2010).
Stephenson, S. L. & Landolt, J. C. Dictyostelid cellular slime molds in canopy soils of tropical forests. Biotropica 30, 657–661 (1998).
Stephenson, S. L. & Landolt, J. C. Dictyostelids from aerial “canopy soil” microhabitats. Fungal Ecology 4(3), 191–195 (2011).
Stephenson, S. L. & Rajguru, S. N. Dictyostelid cellular slime moulds in agricultural soils. Mycosphere 1, 333–336 (2010).
Cavender, J. C. Cellular slime molds in tundra and forest soils of Alaska including a new species. Dictyostelium septentrionalis. Canadian Journal of Botany 56, 1326–1332 (1978).
Stephenson, S. L., Landolt, J. C. & Laursen, G. A. Dictyostelid cellular slime molds from western Alaska and the Russian Far East. Arctic and Alpine Research 29, 222–225 (1997).
Romeralo, M., Landolt, J. C., Cavender, J. C., Laursen, G. A. & Baldauf, S. L. Two new species of dictyostelid cellular slime molds from Alaska. Mycologia 102, 588–595 (2010).
Cavender, J. C. Cellular slime molds in forest soils of eastern Canada. Canadian Journal of Botany 50, 1497–1501 (1972).
Cocucci, S. M. & Sussman, M. RNA in cytoplasmic and nuclear fractions of cellular slime mold amebas. The Journal of Cell Biology 45, 399–407 (1970).
Keller, H. W. & Schoknecht, J. D. Life cycle of a new annulate-spored species of Didymium. Mycologia 81, 248–265 (1989).
Boyde, A. & Wood, C. Preparation of animal tissues for surface-scanning electron microscopy. Journal of Microscopy 90(3), 221–249 (1969).
Hayat, M. A. Principles and techniques of electron Microscopy. Biological applications. Van Nostrand Reinhold 79(1), 73–75 (1970).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797 (2004).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 (2004).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology & Evolution 33(7), 1870 (2016).
Stamatakis, A. RAxML-VI-HPC: maximum likelihood-basedphylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006).
Acknowledgements
This study was supported by the National Key Technologies R & D Program (No. 2012BAC01B04), the National Natural Science Foundation of China (No. 31870015, 31300016, 31093440, 31493010 and 31493011), Science and Technology Development Program of Jilin Province (No. 20180101273JC), the Program of Creation and Utilization of Germplasm of Mushroom Crop of “111” Project (No. D17014), and the Science and Technology Research Programs of the Education Department of Jilin Province in the Thirteenth Five–Year Plan (No. JJKH20180671KJ). We wish to express our appreciation to Dr. Bao Qi, Master Ximing Tang and Yanqiu Zhang for collecting partial soil samples for us.
Author information
Authors and Affiliations
Contributions
Y.Z. and P.L. carried out the research, P.L. wrote the manuscript, Q.W. and Y.L. designed the research, S.L.S. revised the manuscript, S.L. contributed new analytical tools. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, P., Zou, Y., Li, S. et al. Two new species of dictyostelid cellular slime molds in high-elevation habitats on the Qinghai-Tibet Plateau, China. Sci Rep 9, 5 (2019). https://doi.org/10.1038/s41598-018-37896-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37896-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.