Abstract
Nitrogen (N) and potassium (K) are essential macronutrients for plants growth; however, the mechanism by which K mediates negative effects on ammonium-sensitive plants is still poorly understood. We hypothesized that K supplies may enhance antagonistic ammonium stress while improving nitrate nutrition function, which wheat seedlings were grown in sand culture in the presence of two N forms (ammonium; nitrate) supplied at two rates (2, 10 mmol L−1) and three K levels (0.5, 5, 15 mmol L−1). We found that a high N rate increased plant biomass under nitrate nutrition, while it had a negative effect under ammonium nutrition. Compared with nitrate, biomass was depressed by 54% or 85% for low or high N rate under ammonium. This resulted in a reduction in gas exchange parameters and a subsequent decrease in growth variables and nutrient uptake, whereas these parameters increased significantly with increasing K levels. Moreover, in principal components analysis, these variations were highly clustered under nitrate nutrition and highly separated under ammonium nutrition. Our study shows a clear positive interaction between K and N, suggesting that high K supply relieves ammonium stress while improving growth vigor under nitrate nutrition by enhancing nutrient uptake and assimilate production in wheat plants.
Similar content being viewed by others
Introduction
Nitrogen (N) is a key element required for plant growth, and is one of the most important yield-limiting nutrients in crop production in all agro-ecological regions of the world. N is commonly taken up from the soil in one of two inorganic forms: ammonium (NH4+) and nitrate (NO3−)1,2,3. Different N forms can affect the physiological and metabolic processes of plants, such as nutrient uptake, enzyme activity, photosynthesis rate, respiration rate, water balance, and signaling pathways, thus eventually influencing plant growth and crop yield3,4,5,6. Although NH4+ is an intermediate in many metabolic reactions, it can result in toxicity symptoms in many higher plants when supplied as the sole N source1,7,8,9.
Sole NH4+ supply provokes negative effects on NH4+-sensitive plants. The effects of NH4+ and NO3− nutrition on plant growth have been intensively studied; however, the results are not consistent and depend mainly on plant species. Wheat and maize10, sugar beet11, beans12,13,14,15, tobacco16,17 and canola18, grow preferentially on NO3− nutrition; whereas, rice19,20,21, pine and larch22, grow preferentially on NH4+ nutrition. The mechanisms responsible for NH4+ toxicity have been the subject of much speculation, and proposals include proton extrusion associated with NH4+ uptake, cytosolic pH disturbances, decreased water use efficiency, shifts in plant carbohydrate status, the uncoupling of photophosphorylation, high energy costs of membrane transport, and displacement of crucial cations, such as K4,7,9,12,13,14.
NH4+ nutrition has been found to cause strong inhibition of potassium (K) uptake by plants4,16,17. K, which is an essential nutrient involved in many important plant physiological processes, can improve crop yield and quality and enhance stress tolerance23,24. Thus, N and K requirements and management of these essential nutrients for crop production have become a focus of research into the interactions between N and K in terms of factors such as form and rate. Currently, the imbalanced fertilizer use is common in field production in many developing countries. The practice often leads to an excess of soil N combined with a serious and continual depletion of soil K, mainly due to the application of excessive N and inadequate K25. Over-application of N is a serious problem in intensive agricultural production areas because this leads to enrichment of reactive N constituents in the environmental, soil acidification and also affects the transformation of soil N forms, with consequent impairment of ecosystems26,27,28. It is generally accepted that, regardless of the application of N fertilizer or not, the progress of N form transformation is inhibited by the interaction between NH4+ and NO3−, with higher NH4+ and lower NO3− status maintained in acidic soil29,30 and low oxygen or waterlogged conditions31,32.
Wheat is one of the most important cereal crops worldwide and grows preferentially under NO3− nutrition; however, its production is challenged by waterlogging of N forms, which is reported to cause yield losses between 15 and 20%33, and frequently occurs in regions with heavy rainfall and high ground water levels. Waterlogging causes significant reduction in gas diffusion and thereby, creates high NH4+ conditions in soil31,32, leading to a substantial decrease in wheat growth and crop yield33. Most studies have shown that the application of NH4+ as the sole N source decreased biomass in wheat compared with that achieved under NO3− nutrition10,34,35. In addition, changes in NH4+/NO3− ratios and K supply levels under soil culture conditions influence the yield and nutrient uptake of wheat plants36. Thus, we hypothesized that plant growth and crop yield are influenced not only by the amount of available N in the soil, but also by the N forms, and that the process can be regulated by K supply.
Although the individual effects of different N forms on plant growth have been widely studied, the combined effects of various levels of N forms and K supply on wheat growth and crop yield are largely unknown. Similarly, little is known about the effects of K supply relative to N forms on the photosynthetic process and nutrient uptake in wheat plants. In this study, we investigated the effects of different levels of N forms and K supply on growth in wheat plants, primarily by investigating biomass, growth, gas exchange, and N and K uptake.
Results
Wheat growth
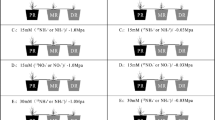
Significant differences in plant biomass were observed between those supplied with NH4+ and NO3− forms of N at two rates (Table 1). N supplied at the high rate in the form of NO3− significantly increased the biomass of different organs. In contrast, NH4+ resulted in a reduction in plant biomass that was exacerbated by high NH4+. However, the plant biomass increased with K levels under both NH4+ and NO3− nutrition. Compared with NO3− supply in the absence of K supply, the biomass of root, stem, leaf, and panicle in plants fed NH4+ was reduced by 67%, 54%, 53% and 40% at the low N rate and by 87%, 88%, 88% and 77% at the high N rate, respectively. These results were also clearly supported by the images collected during the culture stage (Fig. 1). Regardless of the N form, the high N rate significantly decreased the root:shoot ratios. In contrast to the effects of NH4+, the root:shoot ratios of plants supplied NO3− increased with K levels, and the highest ratio was observed under low NO3− treatment. A clear positive interaction was observed among N forms and rates and K levels on the biomass of the different organs except the panicle; however, there were no significant differences in the root:shoot ratios among the different K levels.
In accordance with the effects on biomass, the other growth variables (root volume, number of tillers, flag leaf area, total leaf area and specific leaf weight) also changed with the N forms and rates in the presence of K, with the exception of the number of tillers at different K levels and specific leaf weight under treatment with the different N forms (Table 2). Overall, NO3− dramatically increased the growth variables, with greater increases under high NO3− treatment than those under low NO3− treatment. In contrast to the effects of NO3− alone, the high N rate had a negative effect on growth variables in plants fed NH4+, whereas no significant differences in root volume, number of tillers and specific leaf weight were observed between the N rates.
Gas exchange and relative chlorophyll content
There were significant differences in gas exchange parameters (Pn, gs, Ci, and Tr) and relative chlorophyll content (SPAD value) between plants under NH4+ and NO3− nutrition at different N rates and K supply conditions (Table 3). The Pn, gs, and Tr values increased significantly with the K levels, while Ci decreased. However, under high NH4+ nutrition, the high N rate restrained Pn, gs, and Tr by 6%, 12% and 7%, respectively, while position effects were observed under NO3− nutrition. Under NO3− nutrition, the SPAD of flag leaves was decreased by 11% under low N rate compared with that under the high N rate, while no significant differences in SPAD were observed between plants under NH4+ nutrition and K levels.
N and K content
The different N forms and rates supplied with K had a significant effect on both N and K concentration and accumulation in wheat at the harvest period (Figs 2 and 3). Independent of K levels, the N concentration of different organs (including root, stem, leaf, and panicle) were regulated by both N form and rate (Fig. 2a–d), with generally higher concentrations under a high N rate than under a low N rate. Furthermore, the organ N concentrations were higher under NH4+ nutrition than under NO3− nutrition. Moreover, in contrast to the results of NO3−, the organ N concentrations decreased with increasing K levels under NH4+ nutrition. On the other hand, under NO3− nutrition, the organ K concentrations under the high N rate were higher than those under the low N rate, while a negative effect of N rate on organ K concentrations was observed under NH4+ nutrition. Regardless of N forms and rates, the organ K concentrations increased with K levels (Fig. 2e–h).
Effects of different levels of N forms and K supply on the N (a–d) and K (e–h) concentrations in wheat roots, stems, leaves, and panicles. Wheat plants were supplied with ammonia (AN) and nitrate (NN) at 2 and 10 mmol L−1 and three different concentrations of potassium (K) solution (0.5, 5, 15 mmol L−1 K [K0.5, K5, K15]). Data represent the mean ± standard deviation of three replicates. Significant differences (P < 0.05) were determined by different lowercase letters according to a one-way or multi-way ANOVA followed by Duncan’s multiple range test. NS, no significant difference.
Effects of different levels of N forms and K supply on the N (a–d) and K (e–h) accumulations in wheat roots, stems, leaves, and panicles. Wheat plants were supplied with ammonia (AN) and nitrate (NN) at 2 and 10 mmol L−1 and three different concentrations of potassium (K) solution (0.5, 5, 15 mmol L−1 K [K0.5, K5, K15]). Data represent the mean ± standard deviation of three replicates. Significant differences (P < 0.05) were determined by different lowercase letters according to a one-way or multi-way ANOVA followed by Duncan’s multiple range test. NS, no significant difference.
The accumulation of N and K was similar to pattern of changes in the K concentration (Fig. 3). Overall, compared with the low N rate, the average N accumulation in root, stem, leaf and panicle was decreased by 35%, 48%, 23% and 38%, respectively, by the high N rate under NH4+ nutrition and without K. The average K accumulation was also decreased by 10%, 19%, 35% and 36%, respectively. However, the accumulation of both N and K in organs was increased by the high N rate under NO3− nutrition.
The effects of the variations in NH4+, NO3− and NH4+ plus NO3− among the different N rate with K supply treatments were further evaluated by PCA (Fig. 4). The results showed that the growth and physiological parameters were significantly separated under the different N forms, with the first two principal components accounting for 81.92% (69.02% for PC1 and 12.90% for PC2), 92.15% (81.59% for PC1 and 10.56% for PC2) and 88.05% (68.65% for PC1 and 19.40% for PC2) of the total variations under NH4+, NO3− and NH4+ plus NO3−, respectively. According to the PCA, the total variations were highly clustered under NO3− nutrition. In contrast, the variations were less clustered under NH4+ nutrition, especially the N content and gas exchange parameters.
K+ uptake rate
As shown in Fig. 5, there was a significant difference in K+ uptake under the two N forms. Compared with NH4+ nutrition, the K+ uptake of wheat seedlings was increased by different K+ concentration under NO3− nutrition, while the K+ uptake rate under NO3− nutrition (4.4494) was increased by 36% compared with that under NH4+ nutrition (3.2818) based on regression analysis.
Discussion
Unlike plants such as rice19,21 and pine22, wheat is a typical low NH4+ tolerance plant, with toxicity symptoms manifested as a decline in growth and yield1,2,7. Generally speaking, wheat is not always confronted with a soil environment of high NH4+, except under special circumstances, such as heavy rainfall or irrigation and poor soil drainage combined with excess N fertilization31,32, which is especially common in wheat planting regions in the middle and lower of the Yangtze River Plain in southern China37.
N forms and rates affecting growth and photosynthetic CO2 fixation in wheat
Plant growth is clearly affected by the forms of N supplied as nutrient. In the present study, we showed that the biomass of both non-reproductive and reproductive organs was depressed by NH4+ supply, and resulted in lower root:shoot ratios, indicating the negative effects of pure NH4+ nutrition on wheat growth (Table 1). In accordance with this, Huang et al.35 reported that root and shoot biomass of wheat plants was lower when cultured in solution with NH4+ as a N source than that observed in the presence of NO3−. Furthermore, Wang et al.38 demonstrated that wheat yield was higher when using NO3− fertilizer than that achieved using NH4+ fertilizer under field conditions. Some studies showed that NH4+ nutrition resulted in differences in shoot:root ratios compared with those obtained under NO3− nutrition in wheat10, bean12,14,15 and canola18. These results were further confirmed in other studies of rice varieties5,19,20,39,40, which were found to be tolerant to pure NH4+ nutrition. In contrast to these findings, Walch-Liu et al.41 reported that the root:shoot ratios was higher under NH4+-fed than that under NO3−-fed in two tobacco cultivars. Moreover, the effect of N forms on root:shoot ratios is mediated by regulation of the N rate. Lu et al.17 identified a more marked positive relationship between root:shoot ratios under low (2 mmol L−1) or high (15 mmol L−1) NH4+ supply compared with those obtained under NO3− supply in tobacco plants. Zhou et al.42 also observed that higher root:shoot ratios were obtained with higher NH4+ supply (5 mmol L−1) than that using NO3− as a N supply, although lower N supply (1 mmol L−1) had no effect on the ratios in cucumber plants. Similar results were obtained in the present study, with consistently lower root:shoot ratios using NH4+ than those with NO3−, while high N reduced the ratio regardless of the N form (Table 1). However, dynamic changes in root:shoot ratios at various sampling points have also been observed in wheat35 and sugar beet11. These results indicate that the effects of N forms on root and shoot growth in different species and at different N rates and sample points are mediated primarily by altering the partitioning of fixed carbon (C) during photosynthesis in both root and shoot.
These results was consistent with those reported by Guo et al.3 and Gao et al.18, who suggested that the conflicting effects of N forms on plant growth are related to the gas exchange parameter Pn, which is associated with gs and also Ci. The lower biomass of different organs in wheat plants under NH4+ nutrition compared to those under NO3−nutrition was consistent with the observed differences in the gas exchange parameters (Table 3). Thus, it can be concluded that the reduced carbon assimilation in NH4+-fed wheat plants may have been important in contributing to reduced growth. The higher Pn and higher root:shoot ratios of the wheat plants under NO3−nutrition ensure greater availability and allocation of carbon to the root than to the shoot, thus improving plant growth. In addition, the inhibition of wheat growth in response to NH4+ application could be attributed mainly to a reduction in growth variables including root volume, number of tillers, and leaf area (flag leaf and total leaf), with the response intensified under high N conditions (Table 2). Similar results have been reported for sugar beet11 and tobacco41, in which the negative effect of NH4+ nutrition on leaf growth was explained by either increased chlorophyll content and chloroplast number and volume, reduced cell number and cell expansion, or by osmotic regulation43 and water uptake in bean plants12,13,14. However, differences in biomass production, gas exchange, root and leaf morphological and physiology observed under conditions of different N forms5,6,21,40,41,44, indicate that the N forms available affect plant growth and photosynthesis.
N forms and rates affecting nutrient uptake in wheat
N form and rate affect plant growth by regulating photosynthetic carbon fixation and distribution as well as plant nutrient uptake. Plant N concentrations in different organs (root, stem, leaves and panicle) were increased in response to NH4+ application with two N rates (Fig. 2). Brück and Guo15 reported that N concentrations in young, fully developed leaves under NH4+-fed were significantly increased by 59% compared to leaves under NO3−-fed in beans, and reduced in wheat, maize10 and rice19. Thus, plant N concentrations under NH4+ nutrition were consistently higher under NO3− nutrition, whether it is a prefer ammonium plant or a prefer nitrate plant, indicating that the growth of NH4+-fed plants was not limited by N availability. In PCA results, the growth and physiological parameters were significantly separated by the different N forms, with higher levels of variation clusters under NO3− nutrition than those under NH4+ nutrition, especially in terms of N content and gas exchange parameters (Fig. 4). These findings further indicated the influence of N uptake and assimilation on the responses of plants to N forms and rates in C fixation or biomass production. In addition, Guo et al.13,14 found that N uptake under NH4+-fed was significantly higher than that under NO3−-fed during the dark period, whereas N uptake under NH4+-fed was significantly lower compared to that under NO3−-fed during the light period with a split root system in bean plants. Thus, we suggest that plant N uptake may be influenced not only by N forms, but also by the environmental condition, especially changes in light/dark conditions.
Furthermore, as an important osmoticum, K+ was significantly decreased in wheat plants under NH4+ nutrition (Fig. 2). Similar results have been reported for tobacco17, in which K+ uptake was inhibited under NH4+ nutrition, while K+ transport in the xylem and K+ translocation in the phloem was still higher than that in plants under NO3− nutrition, although the process was improved more under high nutrient levels (6 mmol L−1 K and 15 mmol L−1 N) than under low levels (2.5 mmol L−1 K and 2 mmol L−1 N). Walch-Liu et al.41 found that the leaves of young, expanding and old tobacco plants under NH4+-fed showed 20%, 22% and 60% decreases in K concentrations, respectively, which indicated that K may mediate the effect of N forms on leaf morphogenesis. Most studies have revealed a negative effect of NH4+ nutrition on the osmotic regulation of leaves due to reduced K absorption41,45,46. These results suggest that this effect might be responsible for smaller leaf area and lower specific leaf weight as well as the reduction in root cell length and root morphological parameters frequently observed in rice plants under NH4+ nutrition6,32,39. Also, the K uptake by NH4+-fed vessels was significantly lower than that by NO3-fed vessels, while the K uptake rate was reduced by 466%13 and 1231%14, respectively, by the application of a split root system in bean plants. Similarly, in the present study, NH4+-fed resulted in a higher K+ uptake rate compared to that of NO3−-fed plants (Fig. 5). Thus, we suggest that plant K uptake may be influenced not only by the amount of available N in the environment, but also by the N forms.
Interaction between N and K supply in wheat
The interaction between K and N, especially K and N forms (both NH4+ and NO3−) on plant growth and development, has become a focus of research4,16,17,23,47,48. Our studies in wheat showed that N forms affect plant growth and the uptake of N and K nutrients; however, the supply level of K also has a significant influence on the regulation of plant growth, photosynthesis and nutrient absorption, with a positive interaction identified between N and K. This is consistent with earlier results of studies in wheat49, rice4, tobacco16,17 and beans13,14, in which NH4+ nutrition not only strong inhibited K uptake, but also had a marked influence on the flow and partitioning within plants, resulting in reducted water uptake and Tr, apparently due to reduced gs. This is consistent with the proposed theory that cycling of K in plants can act as an important signal for feedback control of nutrient uptake50. However, in this study, the growth variables, gas exchange parameters, and nutrient content of wheat plants were increased with increasing K supply, and the beneficial effects were observed under both NH4+ and NO3− nutrition. These results further implied that optimal K management may alleviate NH4+ stress or toxicity and improve the nutritional function of NO3− in wheat plants.
K is an essential macro-element of nutrition in plants and its uptake is strongly influenced by other elements, such as N. Elevating the K supply results in a significant reduction of NH4+ influx in rice plants4. Similarly, we observed that the growth of wheat plants under pure NH4+ nutrition was improved by K supply (Fig. 1), a phenomenon that demonstrates the influence of K supply on the tolerance of wheat to NH4+ stress or toxicity49. Based on the proposals of Szczerba et al.4 and Kong et al.49, we suggested that adequate K application is essential for plant growth, especially under conditions of high environmental NH4+ stress. The results of the present study also demonstrated that N and K interactions not only affect plant growth and nutrient uptake, but also significantly affect crop yield parameters, such as panicle biomass (Table 1). These results provide evidence that assimilate partitioning in plants can be improved by changes in K supply. Some studies suggest that the controversial effects of N forms and K supply on plant growth are related to the availability of photo-assimilates for production, transportation and distribution, further indicating that K plays an important role in yield formation23,47,48.
Although the plants did not show any visible symptoms of mineral nutrient deficiency under different N and K treatments, there were marked morphological differences between wheat plants, especially the shoots, for which the erecting and tilting degree were improved under high N and low K conditions under NO3− nutrition compared with NH4+ nutrition (Fig. 1). Previous studies demonstrated that higher N rates reduced plant morphology traits, culm physical strength and lodging resistance in wheat51 and rice52, with every 2% increase in lodging resistance causing a 1% decrease in grain yield53. However, Zaman et al.54 found that optimizing K fertilizer improved stem strength and yield to alleviate the negative effects of higher N application. Kong et al.49 also reported that the additional provision of K+ considerably alleviated the negative effects of high NH4+, resulting in a 23% increase in culm mechanical strength and a 35% increase in the N remobilization efficiency in wheat plants. It has also been reported that the consequences of N metabolism55, N use efficiency48 and C-N balance56 are improved by K fertilizer application. Similarly, the results of the present study suggest that a positive synergistic interaction between K and N on assimilate production, nutrient uptake, yield formation and stress tolerance47,57. Thus, an understanding of the roles of the interaction between N (especially in NH4+) and K in the regulation of physiological and biochemical mechanical and yield formation is required to improve sustainable productivity in wheat plants.
In conclusion, wheat grown in anaerobic soils showed growth inhibition when the predominant form of nitrogen is NH4+ or a mixture of NH4+ and NO3−, and under conditions of limited K availability. NO3− treatment increased root growth, N uptake and photosynthetic productivity in wheat plants in comparison with NH4+ treated plants, and the effects were enhanced by K supply, especially under high N conditions. Thus, our results indicate that increased K supply mediates nutrient balance between N and K uptake, and enhances NH4+ tolerance in wheat plants supplied with pure NH4+ nutrition. Our results provide the basis for the development of new nitrogen fertilizer utilization schemes for wetland wheat production.
Materials and Methods
Plant materials and growing conditions
A split-unit randomized complete block design, with N forms as the main unit and factorial combinations of N rates and K levels as subunits, was used in this study. The treatments were: two N forms (ammonium, as NH4+ [AN]; nitrate, as NO3− [NN]), two N rates (2 and 10 mmol L−1), and three K levels (0.5, 5 and 15 mmol L−1 [K0.5, K5 and K15]) replicated three times. The ‘Yangmai 16’ hard red winter wheat (Triticum aestivum L.) was used as a model. Briefly, after germination on moist filter paper, wheat seeds were disinfected with 10% H2O2 for 30 min then transferred to a 2 mmol L−1 CaSO4 solution for germination at 25 ± 5 °C. When the seedlings had an average of 2.5 visible leaves (0.5 g fresh weight, 17 cm plant height), they were transplanted to 0.75 L (top diameter 9.5 cm × bottom diameter 6.5 cm × 16 cm high) plastic pots (two seedlings per pot) containing clean quartz sand and transferred into quarter-strength Hoagland’s nutrient solution (for composition, see below) with 1 cm depth in tray. Seedlings were grown in a greenhouse under a natural photoperiod. Four days later, the seedlings were transferred to half-strength nutrient solution. After an additional four days, the seedlings were treated with full-strength nutrient solution containing 12 different treatments (AN2K0.5, AN2K5, AN2K15, AN10K0.5, AN10K5, AN10K0.5, NN2K0.5, NN2K5, NN2K15, NN10K0.5, NN10K5, and NN10K15). The composition of the other nutrients in the solutions was as follows: macronutrients (mmol L−1): 2 or 10 N as (NH4)2SO4 or Ca(NO3)2, 0.5, 5 or 15 K as K2SO4 and KH2PO4, 1 P as KH2PO4, 5 Ca as CaCl2 or Ca(NO3)2, 2 Mg as MgSO4; micronutrients (μmol L−1): 100 Fe as Fe-EDTA, 9 Mn as MnCl2·4H2O, 0.7 Cu as CuSO4·5H2O, 0.7 Zn as ZnSO4·7H2O, 45 B as H3BO3, 1.7 Mo as (NH4)6Mo7O24·4H2O, and 100 Si as Na2SiO3·9H2O. The Ca content in the NH4+ nutrient solution was compensated for by the addition of CaCl2. A nitrification inhibitor (dicyandiamide, DCD) was added to prevent oxidation of NH4+ at a dose of 5% of total nitrogen. Nutrient solutions were changed every 4 d, after drip washing the sand surface with 0.5 L tap water, and the pH was monitored daily and maintained at 6.00 ± 0.05 by adding either 0.1 mmol L−1 NaOH or HCl until the final harvest. Each treatment group consisted of six plants in a completely randomized design to minimize edge effects.
K+ uptake rate measurement
The examine the effect of N forms on K+ uptake rate, K+ influx of intact wheat plants was determined by the depletion of nutrient solution directly using a K+ concentration gradient method with N forms supplied under hydroponic solution conditions. Wheat seedlings grown in 2 mmol L−1 CaSO4 solution were pre-equilibrated for 2 h, then immersed in labelling solution between 8:30 to 16:30 (8 h). The solution was identical to the growth solution, except that it contained different K+ concentrations (0, 0.05, 0.1, 0.2, 0.4, 0.5, 1, 2, 4, 5, 10 mmol L−1) with the addition of 2 mmol L−1 AN or NN. Each treatment group consisted of three plants and was replicated three times in a completely randomized design. The seedlings were grown under greenhouse conditions (air temperature 30 °C; relative humidity 50%) under a photosynthetic photon flux density (PPFD) of 1000 μmol photons m−2 s−1 at the leaf level.
Gas exchange measurements
At the heading stage, 60 days after treatments initiation, the light-saturated photosynthetic rates of newly expanded leaves (flag leaf) were measured simultaneously between 09:00 and 15:00 with an infrared gas analyzer (6400XT, Li-Cor, Lincoln, NE, USA). Leaf temperature during the measurements was maintained at 28 °C and a relative humidity of 50% under a PPFD of 1000 μmol photons m−2 s−1. Data were recorded after equilibration to a steady state.
Relative chlorophyll content measurement
The one-dimensional (1 D) chlorophyll index of the labeled leaf segments was determined using a portable relative chlorophyll meter (SPAD-502, Minolta Camera, Osaka, Japan) to measure absorbance at 650 nm. The chlorophyll index was determined as the mean of six SPAD-502 readings from the same leaf at the heading stage.
Root volume, dry weight and specific leaf weight measurements
Wheat plants were harvested and separated into root, stem (including sheath and culm), leaf and panicle sections. Sand was washed from the roots before the root volume was measured using the displacement method described by Sattelmacher et al.58. The dry weight was measured after all samples were oven-dried at 105 °C for 30 min and then at 70 °C to constant weight. The leaf area was determined using a photocopy of the leaf and calculated according to the paper area. The specific leaf weight was then calculated as the ratio of leaf weight to leaf area.
N and K concentration and accumulation measurements
To determine the total concentrations of N and K in different organs, dried and ground samples were digested with H2SO4-H2O2 at 260–270 °C. N concentrations were measured using an Auto-analyzer 3 digital colorimeter (AA3, Bran + Luebbe, Hamburg, Germany) and K concentrations were measured by Flame Photometry (FP6400, Shanghai Precision Scientific Instrument, Shanghai, China). The measurements were validated using certified standard reference materials obtained from the Institute for Environmental Reference Materials of the Ministry of Environmental Protection (Beijing, China). The total accumulation of N and K was calculated from the sum of organ N and K content (element concentration × dry weight) at harvest.
Statistical analysis
Samples were analyzed in triplicate and mean values were used in comparisons analysis. Variance analysis (ANOVA) was performed using the SAS 9.3 statistical software package (SAS Institute, Cary, NC, USA). Means were compared among treatments by the least significant difference (LSD) test with P < 0.05 considered to indicate statistical significance. Principal components analysis (PCA) was used to analyze the growth and physiological variations of NH4+, NO3− and NH4+ plus NO3− under treatment with different N rates and K supplies using SPSS Statistics 17.0 (IBM, Armonk, NY, USA) and plotted using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA).
References
Britto, D. T., Siddiqi, M. Y., Glass, A. D. M. & Kronzucker, H. J. Futile transmembrane NH4 + cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 98, 4255–4258 (2001).
Glass, A. D. M. et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 370, 855–864 (2002).
Guo, S., Zhou, Y., Shen, Q. & Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants - growth, photosynthesis, photorespiration, and water relations. Plant Biol. 9, 21–29 (2007).
Szczerba, M. W. et al. NH4 +-stimulated and -inhibited components of K+ transport in rice (Oryza sativa L. J. Exp. Bot. 59, 3415–3423 (2008).
Yang, X. et al. Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol. 53, 495–504 (2012).
Ding, L. et al. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci. 234, 14–21 (2015).
Kronzucker, H. J., Britto, D. T., Davenport, R. J. & Tester, M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 6, 335–337 (2001).
Britto, D. T. & Kronzucker, H. J. NH4 + toxicity in higher plants: a critical review. J. Plant Physiol. 159, 567–584 (2002).
Gerendás, J. et al. Physiological and biochemical processes related to ammonium toxicity in higher plants. J. Plant Nutr. Soil Sci. 160, 239–251 (1997).
Cramer, M. D. & Lewis, O. A. M. The influence of nitrate and ammonium nutrition on the growth of wheat (Triticum aestivum) and maize (Zea mays) plants. Ann. Bot. 72, 359–365 (1993).
Raab, T. K. & Terry, N. Nitrogen source regulation of growth and photosynthesis in Beta vulgaris L. Plant Physiol. 105, 1159–1166 (1994).
Guo, S., Brück, H. & Sattelmacher, B. Effect of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 239, 267–275 (2002).
Guo, S., Shen, Q. & Brueck, H. Effects of local nitrogen supply on water uptake of bean plants in a split root system. J. Integr. Plant Biol. 49, 472–480 (2007).
Guo, S. et al. Relationship between water and nitrogen uptake in nitrate- and ammonium-supplied Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 170, 73–80 (2007).
Brück, H. & Guo, S. Influence of N form on growth and photosynthesis of Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 169, 849–856 (2006).
Wang, G., Li, C. & Zhang, F. Effects of different nitrogen forms and combination with foliar spraying with 6-benzylaminopurine on growth, transpiration, and water and potassium uptake and flow in tobacco. Plant Soil 256, 169–178 (2003).
Lu, Y. X., Li, C. J. & Zhang, F. S. Transpiration, potassium uptake and flow in tobacco as affected by nitrogen forms and nutrient levels. Ann. Bot. 95, 991–998 (2005).
Gao, L. et al. Enhanced salt tolerance under nitrate nutrition is associated with apoplast Na+ content in canola (Brassica. Napus L.) and rice (Oryza sativa L.) plants. Plant Cell Physiol. 57, 2323–2333 (2016).
Guo, S., Chen, G., Zhou, Y. & Shen, Q. Ammonium nutrition increases photosynthesis rate under water stress at early development stage of rice (Oryza sativa L.). Plant Soil 296, 115–124 (2007).
Guo, S. et al. Effects of different nitrogen forms and osmotic stress on water use efficiency of rice (Oryza sativa). Ann. Appl. Biol. 153, 127–134 (2008).
Li, Y. et al. Ammonium enhances the tolerance of rice seedlings (Oryza sativa L.) to drought condition. Agr. Water Manage. 96, 1746–1750 (2009).
Malagoli, M. et al. Differences in nitrate and ammonium uptake between Scots pine and European larch. Plant Soil 221, 1–3 (2000).
Zhang, F. et al. Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 335, 21–34 (2010).
Zhao, S. et al. Long-term effects of potassium fertilization and straw return on soil potassium levels and crop yields in north-central China. Field Crop Res. 169, 116–122 (2014).
Vitousek, P. M. et al. Nutrient imbalances in agricultural development. Science 324, 1519–1520 (2009).
Ju, X. T. et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 106, 3041–3046 (2009).
Chen, X. et al. Producing more grain with lower environmental costs. Nature 514, 486–489 (2014).
Guo, J. H. et al. Significant acidification in major Chinese croplands. Science 327, 1008–1010 (2010).
Che, J. et al. High pH-enhanced soil nitrification was associated with ammonia-oxidizing bacteria rather than archaea in acidic soil. App. Soil Ecol. 85, 21–29 (2015).
Jiang, X. et al. pH regulates key players of nitrification in paddy soils. Soil Biol. Biochem. 81, 9–16 (2015).
Qian, X. et al. Nitrogen form effects on yield and nitrogen uptake of rice crop grown in aerobic soil. J. Plant Nutr. 6, 1061–1076 (2004).
Tran, T. T. et al. Root plasticity and its functional roles were triggered by water deficit but not by the resulting changes in the forms of soil N in rice. Plant Soil 386, 65–76 (2015).
Herzog, M., Striker, G. G., Colmer, T. D. & Pedersen, O. Mechanisms of waterlogging tolerance in wheat - a review of root and shoot physiology. Plant Cell Environ. 39, 1068–1086 (2016).
Cox, W. J. & Reisenauer, H. M. Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium, or both. Plant Soil 38, 363–380 (1973).
Huang, J. W., Grunes, D. L. & Welch, R. M. Magnesium, nitrogen form, and root temperature effects on grass tetany potential of wheat forage. Agron. J. 82, 581–587 (1990).
Shaviv, A. & Hagin, J. Interaction of ammonium and nitrate nutrition with potassium in wheat. Fert. Res. 17, 137–146 (1988).
Zhao, X. et al. Nitrogen runoff dominates water nitrogen pollution from rice-wheat rotation in the Taihu Lake region of China. Agr. Ecosyst. Environ. 156, 1–11 (2012).
Wang, Z. H., Miao, Y. F. & Li, S. X. Effect of ammonium and nitrate nitrogen fertilizers on wheat yield in relation to accumulated nitrate at different depths of soil in drylands of China. Field Crop Res. 183, 211–224 (2015).
Gao, Y. et al. Ammonium nutrition increases water absorption in rice seedlings (Oryza sativa L.) under water stress. Plant Soil 331, 193–201 (2010).
Gao, C. et al. Nitrate increase ethylene production and aerenchyma formation in roots of lowland rice plants under water stress. Funct. Plant Biol. 44, 430–442 (2017).
Walch-Liu, P., Neumann, G., Bangerth, F. & Engel, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J. Exp. Bot. 51, 227–237 (2000).
Zhou, J. et al. Nitrate increased cucumber tolerance to Fusarium wilt by regulating fungal toxin production and distribution. Toxins 9, 100 (2017).
Heuer, B. Growth, photosynthesis and protein content in cucumber plants as affected by supplied nitrogen form. J. Plant Nutr. 14, 363–373 (1991).
Li, Y. et al. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol. 53, 892–900 (2012).
Raab, T. K. & Terry, N. Carbon, nitrogen, and nutrient interactions in Beta vulgaris L. as influenced by nitrogen source, NO3 - versus NH4 +. Plant Physiol. 107, 575–584 (1995).
Wang, Z. & Li, S. Effects of N form and rate on vegetable growth and nitrate accumulation. Pedosphere 13, 309–316 (2003).
Gendy, A. G. E. et al. Effect of nitrogen and potassium fertilizer on herbage and oil yield of chervil plant (Anthriscus cerefolium L.). Indust. Crop Prod. 69, 167–174 (2015).
Grzebisz, W. et al. Potassium impact on nitrogen use efficiency in potato - a case study from the Central-East Europe. Plant Soil Environ. 9, 422–427 (2017).
Kong, L. et al. Effects of high NH4 + on K+ uptake, culm mechanical strength and grain filling in wheat. Front. Plant Sci. 5, 703 (2014).
Engels, C. & Marschner, H. Adaptation of potassium translocation into the shoot of maize (Zea mays) to shoot demand: evidence for xylem loading as a regulating step. Physiol. Plantarium 86, 263–268 (1992).
Crook, M. J. & Ennos, A. R. The effects of nitrogen and growth regulators on stem and root characteristics associated with lodging in two cultivars of winter wheat. J. Exp. Bot. 46, 931–938 (1995).
Zhang, W. J. et al. Effects of nitrogen application rate and ratio on lodging resistance of super rice with different genotypes. J. Integr. Agr. 13, 63–72 (2014).
Setter, T. L., Laureles, E. V. & Mazaredo, A. M. Lodging reduces yield of rice by self-shading and reductions in canopy photosynthesis. Field Crop Res. 49, 95–106 (1997).
Zaman, U. et al. Potassium fertilization may improve stem strength and yield of basmati rice grown on nitrogen-fertilized soils. Pak. J. Agri. Sci. 52, 437–443 (2015).
Hu, W. et al. Relationship between potassium fertilization and nitrogen metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll during the boll development stage. Plant Physiol. Biochem. 101, 113–123 (2016).
Hu, W. et al. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol Biochem 115, 408–417 (2017).
Kumar, V., Singh, V. K. & Tani, T. Influence of nitrogen, potassium and their interaction on growth and phenology of papaya cv. Pusa dwarf. J. Crop Weed 13, 60–63 (2017).
Sattelmacher, B. Methods for measuring root volume and for study root morphology. J. Plant Nutr. Soil Sci. 150, 54–55 (1987).
Acknowledgements
The present study was financially supported by the Natural Science Foundation of China (31801947), the Natural Science Foundation of Fujian Province of China (2018J01708), the Special Fund for Scientific and Technological Innovation of the FAFU (CXZX2017229), the MATS program of the Ministry of Agriculture of China (CARS-26-01A) and the International Magnesium Institute Program (2018-MR0201). The authors are grateful to Prof. Dr. Shiwei Guo in Nanjing Agricultural University and Dr. Tianyuan Yang in Anhui Agricultural University in China for critical evaluation and correction of the manuscript.
Author information
Authors and Affiliations
Contributions
J.G. and Y.Z. conceived and designed the experiments. J.G., Y.J. and H.C. performed the experiments. J.G., Y.J., H.C., L.Z., J.Y. J.Z. and Y.Z. collected and analysed the data. J.G., Y.J. and Y.Z. wrote the main manuscript text. J.G., X.H., X.Y., Y.L. and Y.Z. commented and improved the manuscript. All authors have reviewed and commented the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Jia, Y., Chen, H. et al. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci Rep 9, 1248 (2019). https://doi.org/10.1038/s41598-018-37838-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37838-3
This article is cited by
-
Enhancing Soil Health and Fruit Quality in Calafate Orchards Through Sustainable Amendments
Journal of Soil Science and Plant Nutrition (2024)
-
Mineral composition modulates Erwinia amylovora resistance in pear based on path analysis
European Journal of Plant Pathology (2024)
-
Drone remote sensing of wheat N using hyperspectral sensor and machine learning
Precision Agriculture (2024)
-
Ammonium treatment inhibits cell cycle activity and induces nuclei endopolyploidization in Arabidopsis thaliana
Planta (2024)
-
Integrated application of biochar and chemical fertilizers improves wheat (Triticum aestivum) productivity by enhancing soil microbial activities
Plant and Soil (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.