Abstract
Agaricus bisporus is in general cultivated on wheat and rice straw in China. However, millet straw is a potential alternative resource for Agaricus bisporus cultivation, but this has hardly been studied. In the present study, the feasibility of millet straw based mushroom production was analyzed by three successive trials. Mature compost demonstrated high quality with total nitrogen, pH, and C/N ratio of 2.0%, 7.5, and 18:1 respectively, which was suitable for mushroom mycelia growth. During composting, 47–50% of cellulose, 63–65% of hemicellulose, and 8–17% lignin were degraded, while 22–27% of cellulose, 14–16% of hemicellulose, and 15–21% of lignin were consumed by A. bisporus mycelia during cultivation. The highest FPUase and CMCase were observed during mushroom flushes. Endo-xylanase had the key role in hemicellulose degradation with high enzyme activity during cultivation stages. Laccase participated in lignin degradation with the highest enzyme activity in Pinning stage followed by a sharp decline at the first flush. Yield was up to 20 kg/m2, as this is similar to growth on wheat straw, this shows that millet straw is an effective resource for mushroom cultivation. Actinobacteria, Bacteroidetes, Chloroflexi, Deinococcus-Thermus, Firmicutes, and Proteobacteria were the dominant phyla, based on 16S rRNA gene sequencing during composting. The key environmental factors dominating bacterial communities of the samples were determined to be pH value, cellulose content, and hemicellulose content for prewetting and premixed phase of basic mixture (P0); moisture content for phase I (PI); and nitrogen content, lignin content, and ash content for phase II (PII), respectively.
Similar content being viewed by others
Introduction
Agaricus bisporus, commonly known as button mushroom or white mushroom, is one of the most popular edible mushroom species and widely cultivated all over the world, especially in Europe, North America, China and Australasia1,2. In European countries, commercial production of A. bisporus is cultivated on a composted mixture based on wheat straw, horse or chicken manure, gypsum, and water3,4. The composting process involves two phases (I and II). In phase I (PI), the straw is first wettened with water and subsequently mixed with the other compounds. The stage is about 5–7 days in European countries and 15–21 day in China4,5,6. During this stage, the compost temperature increases to 80 °C due to thermophilic microorganisms. Subsequently, a pasteurization process (phase II, PII) is performed. The compost is conditioned at 45–50 °C for about 4–9 days until the ammonia level is non-toxic to A. bisporus mycelia, after which the temperature is reduced to about 25 °C. At the end of PII, compost can be used for (optimal) A. bisporus growth4,7,8,9.
As a world-wide commercial production mushroom species, A. bisporus can be cultivated on various composts made by different raw materials, but is always carbon and nitrogen source based. In many countries, wheat straw and horse manure are used as primary carbon and nitrogen source respectively, while chicken manure is in some cases used as an additional nitrogen source3,4. Nowadays, China has become the greatest producer of button mushroom with annual production in 2016 of about 3 million tons, according to the statistical data from the China Edible Fungi Association. In China, wheat and rice straw, and chicken manure are used as primary carbon and nitrogen source, respectively, while bean meal is used as an additional nitrogen source6,10,11.
During the composting and mushroom cultivation process, carbohydrates of plant cell walls are hydrolyzed by which monosaccharides are released which support mushroom growth3,12. Plant cell walls are mainly composed of cellulose, hemicellulose and lignin13,14. Fate of lignocellulose derived components and their degrading enzymes during composting and mushroom cultivation were reported for wheat straw based compost1,15,16,17.
Most mushroom farms in China are located in Shandong, Fujian, Gansu provinces. However, many new mushroom farms are established in the Northern part of China where rice and wheat are not widely cultivated. Local plant straws are important as an alternative carbon source to save the transport costs. Millet (Panicum miliaceum), is the cereal that is grown in the (semi)- arid areas of Northern China and therefore we tested in this study whether it can be used to produce compost for A. bisporus.
Millet just like wheat, belongs to the family Poaceae in the plant group of Monocots, and ranks sixth among the world’s most important cereal grains18. In China, millet is widely cultivated in arid and semi-arid regions with an annual production of about 1.78 million tons19. Therefore, millet straw is sufficiently available as a potential source of commercial mushroom cultivation in Northern China. However, no studies on millet based composting have currently been reported.
The aim of the present study was to determine the feasibility of mushroom cultivation using millet straw as the primary carbon source. Changes of carbohydrate compositions, lignocellulolytic enzyme levels, and bacterial diversity during composting and cultivation process were investigated.
Results
Physico-chemical properties of millet straw
As the first step to determine whether millet straw is suitable to create compost for A. bisporus, its physico-chemical properties were compared with wheat. Millet straw was collected from Inner Mongolia and wheat straw samples gathered from Xinjiang and Jiangxi of China. The data are summarized in Tables 1 and S1. Total nitrogen (w/w%) of millet straw samples was 0.55%, which is similar to that of both wheat straw samples. Moreover, ash content (w/w%) of millet straw samples was shown to be 4.50% which was about half of that of wheat straw samples. Cellulose, hemicellulose (together forming the total carbohydrate fraction), and lignin content (w/w%) of millet straw samples was 36.68%, 17.31%, and 19.38%, respectively, which were also close to those of wheat straw samples. As the composition of millet straw is similar to that of wheat and the ash content is lower, it suggests that this straw can be a good substrate to make compost.
Physico-chemical properties of the compost
Three independent composting experiments were performed using millet straw as the major carbon source. Changes in compost composition during composting and mushroom cultivation were constantly monitored using onion mesh bags and basket cultivation methods15,20. The physico-chemical properties of compost samples during composting phases and mushroom cultivation stages of these three experiments are shown in Table 2. This includes total carbohydrate and lignin, total nitrogen, ash, content C/N ratio, pH, and moisture content (w/w%). During composting in the 3 experiment, total carbohydrate level declined from 43–45% to 27–32%, while lignin and total nitrogen increased from 20% to 25–27% and 1.2–1.4% to 1.8–2.2%, respectively. Mushroom cultivation can be divided in 5 stages (see materials and methods). During each stage samples were taken. The levels of the three components only changed slightly during each stage, and reached the final values of about 15–21% (total carbohydrate), 23–25% (lignin), and 2.1–2.3% (nitrogen), respectively after 3rd flush. To determine the C/N ratio, Carbon content was measured by the dry ashing method21. At the end of composting PII the C/N ratio had decreased from 33–37 to 18–19. During the composting stages, ash content continuously increased finally reaching 26–29%, while the final ash content and C/N ratio after mushroom cultivation was 32–39% and 15–18, respectively. The pH value was 7.3–7.7 at the end of PII and underwent a mild continuous decline during mushroom cultivation resulting in pH 6.2–6.4 after the 3rd flush. Moisture content at end of P0, PII, and 3rd flush were 74–77%, 68–71%, and 58–63%, respectively.
Absolute changes of total carbohydrate and lignin during composting and cultivation level
In the former paragraph we described the relative changes in total carbohydrate and lignin levels. To obtain insight in the absolute changes we determined the fresh and dry weight of the compost samples and calculated the absolute changes using the data from Table 2. Changes of cellulose, hemicellulose, and lignin contents were visualized in Fig. 1 and Table 3. At the end of PII, 32–33% of the dry matter was consumed, which included the loss (consumption) of cellulose, hemicellulose, and lignin with 47–50%, 63–65%, and 8–17%, respectively. During mushroom cultivation, 6–12% of dry matter, 22–27% of cellulose, 14–16% of hemicellulose, and 15–21% of lignin were most likely primarily consumed by A. bisporus. So, the total loss from P0 till the cultivation is, 38–46% of dry matter finally including about 73–77% of cellulose, 78–81% of hemicellulose, and 28–37% of lignin (Table 3).
Lignocellulosic enzyme activities during composting and cultivation of A bisporus
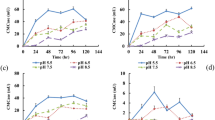
The lignocellulose compounds are degraded by enzymes made by the microbiome in the compost as well as A. bisporus. We tested the activity of several enzymes during the composting processes well as during cultivation of the mushrooms. We selected the following enzymes because they are known to degrade one of the lignocellulose compounds. Further it is known the A. bisporus can make these enzymes. Whether the microbiome responsible for the composting makes similar enzymes will be tested. The enzymes include filter paper cellulase (FPUase, A), carboxymethyl cellulase (CMCase, B) that both can degrade cellulose, endo-xylanase (C) and mannanase (D) can degrade hemicellulose, and laccase (E) and manganese peroxidase (MnP, F) can degrade lignin. The data are shown in Fig. 2. Cellulose and hemicellulose are fastest during the composting process and their degradation continues during the cultivation of the mushrooms (Fig. 1). This is in contrast with the measured enzyme activities. The enzymes degrading cellulose (Fig. 2A,B) as well as those degrading hemicellulose (Fig. 2C,D) have the highest activity during mushroom cultivation and have a markedly lower activity level during composting. This suggests that in addition to these 4 enzymes the microbiome involved in composting secretes additional enzymes that can degrade these carbohydrates. During both composting and mushroom cultivation lignin is degraded at a markedly lower speed than the 2 carbohydrates Fig. 1. The 2 lignin degrading enzymes are made at a high level during mushroom cultivation but are almost not active during the composting. Therefore, it seems probable that also in this case the microbiome uses other enzymes for the degradation of lignin.
Bacterial communities during composting
Bacterial communities during composting were determined by high throughput sequencing based on V3-V4 region of 16S rRNA genes. The sequence information and calculated microbial diversity index are summarized in Table 4. The number of sequences per sample ranged from 44010 to 57742, and the OTUs of each sample ranged from 323 to 567. In mature compost samples of T2 and T3, the bacterial taxa represented 17 phyla, 37 classes, and 85 orders. The composition of the bacterial communities at different stages (P0, PI, and PII) demonstrated substantial differences, while those in the same stage, but from different trials were rather similar (Fig. 3A). Bacteroidetes, Firmicutes, and Proteobacteria were the dominant phyla in P0 samples, and Prevotella was the dominant genus in all the three trials (Fig. 3B). Firmicutes, Bacteroidetes, Proteobacteria, Deinococcus-Thermus, and Actinobacteria were the dominant phyla in PI (Fig. 3A). Proteobacteria, Chloroflexi, Actinobacteria, and Firmicutes were the most abundant bacteria taxa in PII (Fig. 3B). The db-RDA analysis on genus level between the environmental factors and bacterial communities was visualized in Fig. 3C. Samples in different stages were separated into three clusters of P0, PI, and PII, respectively. The key environmental factors dominating bacterial communities of the samples were determined to be pH value, cellulose content, and hemicellulose content for P0; moisture content for PI; and nitrogen content, lignin content, and ash content for PII, respectively.
Bacterial community analysis during composting. (A) Relative abundance of bacterial communities (phylum level). (B) Heat map analysis (genus level). (C) db-RDA analysis between environmental factors and bacterial groups (OTU level). OTU: operational taxonomic units; P0: end of premix; PI: end of phase I; PII: end of phase II; T1, T2, T3: different trials. The heatmap plot indicates the relative abundance of genera in different samples. The phylogenetic tree was calculated using the neighbour-joining method. The color intensity is proportional to the relative abundance of bacterial genera.
Yield and biological efficiency (BE)
The mushroom yield of the three trials was 22.10, 21.30, and 24.47 kg/m2 respectively without any supplements (Table 5). For each trial, the yield of 1st flush accounted for the highest proportion among the total, followed by 2nd flush. BE of the three trials was 68.4%, 70.42%, and 72.58%, respectively.
Scanning electron microscope analysis
Microstructural changes of millet straw during composting and mushroom cultivation are shown in Fig. 4. Longitudinal sections show that, cell wall structures were intact in raw materials (Raw) and P0 stage, but continuously degraded from PI to 3F stage (Fig. 4A). The sclerenchymatous cells (sc, mainly containing lignin) and the parenchyma cells (pc, mainly containing cellulose and hemicellulose) can be easily seen in of the millet straw raw material. Many needle-like crystals were found on the millet straw surfaces at Fl stage (Fig. 4A-Fl). Cellular frame structure of parenchyma cells became gradually blurred from PI on, while sclerenchymatous cell structures were still very clear. These results corresponded to the changes of the levels of cellulose, hemicellulose, and lignin as summarized in Table 3. Transverse section show that the cell wall of parenchyma cells became continuously loosened (Fig. 4B). They were digested by composting microorganisms and mushroom mycelia.
Scanning electron microscope analysis of millet straw in various stages. (A) Vertical section of straw; (B) transverse section of parenchyma cells; Raw: raw materials; P0: end of premix; PI: end of phase I; PII: end of phase II; Fl: Filling; Pn: Pinning; 1F: end of 1st flush; 2F: end of 2nd flush; 3F: end of 3rd flush; sc: sclerenchymatous cells; pc: parenchyma cell.
Discussion
In the present study, we focused on the feasibility of mushroom cultivation using millet straw as the primary carbon source. The mushroom yield of millet straw based compost was over 20 kg/m2 and in these cases no supplements were added. Previous studies showed that total yield of three flushes is about 25–35 kg/m2 when supplements are used22,23,24,25. The supplementation can accurately equilibrate the nitrogen content and the C/N ratio of compost, and increase production yields by up to 5–20%26,27,28. BE of our study was 68.40%, 70.42%, and 72.58%, respectively. BE of T1 was the lowest since the physico-chemical properties of compost at the end of PII were out of suggested ranges with 23% of C/N ratio and 1.76% of nitrogen content17,24,29. In previous study of wheat straw and chicken manure based compost, mushroom yield and BE were 18.90 kg/m2 and 88.7.9%, respectively26. It suggests that millet straw based compost is an effective cultivation medium for the button mushroom.
In European countries, wheat straw is preferred for mushroom compost since it is firm, does not lose its texture easily, and is available in large quantities. Moreover, barley, oat, and rye straw can also be used successfully. Of these three, rye straw most resembles wheat straw, while the other two are softer, absorb water readily, and lose their texture more quickly30. In China, rice and corn straw are also used successfully. Rice straw is closer to wheat straw, while corn straw more resembles barley or oat straw and loses its texture more quickly6,31,32. Up till now, few studies on millet straw have been reported for both its physico-chemical properties and application on mushroom cultivation.
Mushroom compost production is a process (divided into two stages PI and PII) involving the bioconversion of raw materials into a substrate supporting the growth of A. bisporus. In the present study, a 17-day PI stage was performed, which is about 2–3 times longer than that of European countries4,5,6. PI is characterized by high temperatures up to 80 °C. Thermophilic microbiota proliferate and degrade the carbohydrates and proteins, which results in the release of heat and ammonia. These reactions cause the raw materials (e.g. wheat straw and rice straw) to soften15,24. Since millet straw is thicker and longer than wheat straw, it is harder to be degraded. In our pre-experiments, a longer PI stage about 16–18 d can help the compost production reach the quality requirement for mushroom cultivation.
In the present study, we found that total carbohydrates and nitrogen of millet straw were close to not only that of wheat straw from Xinjiang and Jiangxi, but also those of wheat straw used in the Netherlands3. Moreover, contents of cellulose, hemicellulose, and lignin of millet straw were very close to those of wheat straw from Xinjiang and Jiangxi (Table 1). On the other hand, lignin content of millet straw (19.38%) was lower than that of wheat straw from Netherlands (26–27%)3,15. Based on present and previous studies, less than half of the total lignin was consumed during composting and mushroom cultivation16. Therefore, the difference of lignin of millet straw and wheat straw can not remarkably affect the mushroom yield. It suggests that millet straw demonstrates similar physicochemical properties with wheat straw, and can be used as high-quality raw materials for mushroom cultivation.
Since mushrooms consist of more than 92% water, moisture content is one of the key factors for mushroom cultivation. Water-holding capacity of the main raw materials is a very important parameter for both composting and mushroom cultivation30. In the present study, moisture content of the compost underwent a continuous decline from about 75% at the beginning to 60% at the 3rd flush, falling well in the recommended moisture variation range described by van Griensven30 of 75%, 72%, 67%, and 60% at the end of P0, PI, PII, and growing. Millet straw demonstrates similar water-holding capacity as wheat straw during the whole mushroom production process.
In the present study, total nitrogen and C/N ratio of the three trials were designed to be about 1.2–1.4% and 33:1–37:1, respectively, which is required for high quality compost30,33. At the end of PII, the physico-chemical properties of compost were the key factors to determine the mushroom yield, which need to be within specific ranges to obtain maximum mushroom yield33. The moisture, total nitrogen, pH, and C/N ratio should be in the range of 68–72%, 2.0–2.4%, 7.4–7.6, and 17–20:1, respectively17,24,29. After composting, total nitrogen, pH, and C/N ratio of T2 and T3 samples were about 2.1–2.2%, 7.4–7.7, and 18:1–19:1, respectively, suggesting that the two compost samples based on millet straw were of high quality and suitable for mushroom growth. At the P0 stage, T1 sample possessed high pH of 7.72 and C/N ration of 37:1, but low total nitrogen of 1.22%. After composting, the T1 sample was still not very well with high C/N ration of 23% and low total nitrogen of 1.76%. Since the millet straw was purchased from different local farmers of Inner Mongolia, the uniformity was not very high.
Lignin is a complex cross-linked phenolic polymer, particularly important in the formation of plant cell walls, and hard to be degraded34,35. In the present study, 8–17% and 15–21% of lignin was consumed during composting and mushroom cultivation, respectively. In contrast, about 3% and 15% of total lignin was respectively degraded during composting and mushroom mycelia growth in the wheat straw in previous study15. Wei et al.’s36 study bases on different types of straw composting from wheat, rice, corn and soybean revealed that the degrading ratio of lignin was continuously increased during composting. About 8–20% of total lignin of the assayed straw groups was degraded at the 20th day. In our study, a longer PI stage of about 2–3 times the length used in European studies was used to obtain high quality compost products. After the longer stage of PI, more lignin was degraded comparing with other studies.
In our study, 47–50% of cellulose and 63–65% of hemicellulose was degraded by composting microorganisms at the end of PII, while 50% of both cellulose and hemicellulose were metabolized in Jurak et al.’s report on wheat straw based compost15. On the other hand, very low laccase and MnP activities were detected at the end of PI and PII. This might be caused because most of the dominant microbes are thermophilic and function during the high temperature stages. Based on the results of the high throughput sequencing, microorganisms from genera Thermobifida, Rhodothermus, and Thermus were the dominant species during composting (Fig. 3). They have been reported to participate in lignin degradation37,38,39. Szekely, et al.40 reported that Thermus thermophilus was the dominant species during PI, while species of genera Thermobifida and Pseudoxanthomonas participated in the degradation of lignocellulose. It can also explain why about half of the cellulose and hemicellulose were consumed while all the tested lignocellulolytic enzyme activities were very low during composting especially during at PI stage.
Lignocellulolytic enzymes of composting microorganisms and mushroom A. bisporus play a key role in lignocellulosic utilization during the whole mushroom production. In the present study, cellulose was hydrolyzed by both FPUase and CMCase. The highest activities of the two enzymes during composting stages were observed at the end of PII. On the other hand, the highest degradation rates of cellulose (about 26–31%) happened during PI. This might be because the duration of PI was 17 d, and most cellulases during PI were thermophilic. The highest CMCase activity during mushroom cultivation detected at 1F was 11.5–16.0 U/g. Previous studies on wheat straw based composting revealed that CMCase activity at spawing, induction, 1F, 2F, and 3F phases were 0.6, 1.4, 13.0, 11.7, and 6.6 U/g respectively, which variation in time is similar to our results28. A similar trend was also observed by Jurak, et al.16. The CMCase activity increased from Fl to 1F, and underwent a slight decrease in 2F.
Degradation of xylan (the major component of hemicellulose) is a very complex process promoted by many enzymes called xylanolytic enzyme system, including endo-xylanase (endo-1, 4-β-xylanase, E.C.3.2.1.8), β-xylosidase (xylan-1, 4-β-xylosidase, E.C.3.2.1.37), α-glucuronidase (α-glucosiduronase, E.C.3.2.1.139), etc. Among them, endo-xylanases are key enzymes responsible for the hydrolysis of hemicellulose and act on the homopolymeric backbone of 1, 4-linked β-D-xylopyranose by which xylooligomers (endo-) are produced41,42. Endo-xylanase activity continuously increased during the mycelia growth and maintained high level of about 20 U/g during mushroom flushes. Arce-Cervantes, et al.28 also reported that the greatest endo-xylanase activity occurred at the end of 1st break in unsupplemented wheat straw based compost. This might be caused because pentose produced by endo-xylanases might provide nutrition for mushroom mycelia growth.
In the present study, Laccase activity reached the highest level during mushroom mycelia growth but significantly declined during the first mushroom flush. A previous study also suggested that multicopper enzyme and MnP were very important in Agaricus spp. during mycelia growth43. Another study also showed that the level of laccase activity was greatest at the initial stage of cultivation, lowest at 1st flush, and continuous increased during three flushes28. It is rather similar to our study. Laccase secreted by mushroom mycelia participates in metabolic processes before fruiting bodies appear44. That is why the levels of laccase activity declined rapidly after vegetative mycelia growth.
The cell walls of straw of grasses are composed of lignin, cellulose, and hemicellulose45. Microstructure of millet straw during mushroom production was visualized using SEM. Just like our observation, a previous SEM study on wheat straw based mushroom composing also revealed that many of the plant fibers became separated, but the final material still retained considerable structural integrity46. Needle-like crystals found on the millet straw surfaces at Fl stage were speculate to be calcium oxalate which was produced by mushroom mycelia and similar to earlier research on wheat straw composing46,47. The crystals can reduce calcium ion concentrations and improve adaptability of mushroom mycelia to the environment.
Based on 16S rRNA sequencing, 17 phyla, 37 classes, and 85 orders were obtained in the mature compost samples, which was similar to a previous study on PII which showed that 16 phyla and approximate 80 orders were present48. It’s worth mentioning that the number of bacterial communities detected by high-throughput sequencing were higher than those using DGGE, RFLP, and clone library sequencing methods6,40,49,50. Based on the Chao and Shannon indexes, Samples of T2 and T3 in PI possessed the highest bacterial community diversity. Bacterial community diversity of T1 samples in different stages presented highest level at PII and lowest level at PI. Previous literature also reveals that microbial communities continuously change to adapt to the nutrient and environmental changes during composting5,51.
In the present study, Prevotella was the dominant genus in P0 samples. Prevotella spp. most likely came from chicken manure since they are widely distributed in animal intestines52,53. The abundance of Prevotella spp. underwent a significant decline during PI, which might be caused by the high temperature at this stage. During PI, microorganisms from genera Ruminiclostridium, Bacillus, Thermobacillus, Lactobacillus, and Caldicoprobacter of phylum Firmicutes, genus Thermus of phylum Deinococcus-Thermus, genera Bacteroides and Rhodothermus from of phylum Bacteroidetes, and genera Thermobifida and Salinispora of phylum Actinobacteria were dominant species, many of which were thermophilic and relative to cellulose degradation (Fig. 3B). Previous studies on 6 days’ PI demonstrated that Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes were most abundant phyla48. Partanen, et al.54 reported that bacteria from the phyla Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria and Deinococcus-Thermus were enriched at different stages of the mushroom composting process. Bacteria from phylum Deinococcus-Thermus are known for their resistance to high temperature, and take part in lignocellulose degradation at high temperature.
Species from genus Anaerolineaceae of phylum Chloroflexi, genus Thermopolyspora of phylum Actinobacteria, genera Pseudoxanthomonas and Xanthomonadales of phylum Proteobacteria, and genus Truepera of phylum Dienococcus-Thermus were enriched in PII. A previous study using high-throughput sequencing declared that Firmicutes, Proteobacteria, and Actinobacteria were dominant phyla48. Szekely, et al.40 using DGGE and T-RFLP methods to analyze bacterial succession during mushroom composting, revealed that Pseudoxanthomonas, Thermobifida, and Thermomonospora species were the dominant genera in mature compost. They were supposedly related to cellulose-degradation. Zhang, et al.5 also reported that cellulolytic actinomycetal species from genus Thermopolyspora were highly enriched in the wheat straw based mature mushroom compost. In the current study, bacterial communities and dominant taxa of millet straw based mature compost were similar to those of wheat straw based ones, suggesting that it is feasible to produce high quality mushroom using millet straw as the primary carbon source.
Up to now, few studies reported on relations of environmental factors and bacterial communities. In the present study, pH of T1 samples at P0 was significantly higher than that of T2 and T3 (Table 2), which might cause the differences in physico-chemical properties, lignocellulose components and enzymes, bacterial communities, and mushroom yield. Ross and Harris55 declared that yield and quality of Agaricus mushrooms were dependent on the presence of thermophilic microorganisms during composting. A previous study based on yield revealed that moisture content during composting and aeration of the compost heap were significant factors during composting56. Microbial communities of compost interacted with the nutrient and environmental elements5,51. Many studies reported that nitrogen rich supplementation during PII can increase mushroom yield, indicated that nitrogen content is one of the key factors in the stage24,26,27,57.
In summary, millet straw based compost is suitable for A. bisporus mushroom cultivation. After 17-day PI and 9-day PII, physico-chemical properties of compost were as good as those of wheat straw based compost applied in factory production. Lignin was partially degraded during PI while in other studies this is not the case. This degradation was achieved because the duration of this stage was much longer than that used in European countries. FPUase, CMCase, endo-xylanase, mannanase, laccase, and MnP participated in lignocellulose degradation. Actinobacteria, Bacteroidetes, Chloroflexi, Deinococcus-Thermus, Firmicutes, and Proteobacteria were the dominant phyla during composting, while pH value, moisture content, and nitrogen content were deduced to be key environmental factors in P0, PI, and PII stages, respectively.
Materials
Composting and cultivation
Composting and mushroom cultivation were performed in Chengde Xingchunhe Agricultural Co. Ltd., Chengde, Hebei province, China. Millet straw was purchased from Chifeng, Inner Mongolia, China. Other raw materials were purchased from local markets. The compost formula was determined based on the commercial mushroom compost with the initial nitrogen content of 1.6% and as follow: millet straw (43 t), chicken manure (45 t), bean meal (3.6 t) and gypsum (4 t). The composting process followed the modified standards of the Netherlands including phase 0 compost (P0, including pre-wetting for 3 days and premix of basic mixture), phase I compost (PI, 17 days), and phase II compost (PII, 9 days)1,28,30. A turning of the compost will happen in the first fermentation tunnel (23 × 5.5 × 4.5 m) when the temperature rises up to 80 °C during PI. In the present study, four turning was done which was about every three or four days. The cultivation process included Filling (Fl, 19 days), Pinning (Pn, about 14 days), 1st flush (1F, about 4 days), 2nd flush (2F, about 7 days), and 3rd flush (3F, about 7 days). Commercial A. bisporus strain A15 (Sylvan, USA) was used in this study with the inoculation quantity of 0.5%. Commercial peat purchased from Jilin Province, Northeast China was used as the casing materials. The casing used should be with the humidity of 75% and the thickness of 35–40 mm. The casing formula was as follow: the fresh peat (1 m3), calcium carbonate (20 kg), lime (15 kg), water (25–28 L), and pH 7.8. Mushrooms were harvested every day at their optimal commercial development stage during three flushes. Yield and biological efficiency (BE) were subsequently calculated by mushroom kilograms per cultivated area and total fresh mushroom weight divided by initial total substrate dry weight, respectively26.
Sampling
In the present study, three successive trials (T1, T2, and T3) were conducted. During sampling, the onion mesh bag method as Jurak, et al.15 described was used. At the end of premix, samples from 10 random points (3.0 kg each) were taken and thoroughly mixed (named as P0 samples). Subsequently, 25.0 kg of the P0 samples were divided into five onion mesh bags (5.0 kg each) and randomly placed at 50 cm below the compost surface in the first fermentation tunnel. When turning happened, the compost in each bag was respectively mixed and transferred into another new bag. Another P0 samples (1.0 kg) from the rest were divided into equal division (50.0 g each) for laboratory studies including physical-chemical properties, enzymatic activities, electron microscope observation, and microbial diversity. At the end of PI and PII, compost in each bag was weighed, thoroughly mixed, and sampled (1.0 kg). After sampling, the rest PI samples were equally reconfigured into 5 bags and placed in the second fermentation tunnel as in the first one described above (27 × 5 × 4.5 m). During mushroom cultivation, both layer and basket cultivation were used in the standard mushroom houses. Mature compost with spawn was taken into polyethylene boxes (45 × 32 × 23 cm). Thirty boxes were used, and each of them contained 15.0 kg of the substrate20,25. At the end of Fl, Pn, 1F, 2F, and 3F, five boxes were randomly selected and weighted1,16. Substrate (200 g) at about 10 cm below the surface was taken from each box, thoroughly mixed, and storage for further laboratory studies.
Determination of physico-chemical properties
Raw materials and samples were analyzed for their pH values and contents of moisture, nitrogen, carbon, ash, cellulose, hemicellulose, and lignin. The pH was analyzed in a 1:10 (w/v) fresh substrate water extract using a pH meter. Moisture content was assayed using fresh samples by the dry weighing method17. Frozen fresh samples were freeze dried and milled (<1 mm) for determination of other contents3,16. Total nitrogen was determined using the modified Kjeldahl method58,59. Ash and carbon content were assayed using the dry ashing method21. The C/N ratio was obtained based on total nitrogen of the Kjeldahl method and carbon content of the dry ashing method21,29. Cellulose and hemicellulose contents were determined by High Performance Liquid Chromatography (HPLC) according to the laboratory analytical procedure (LAP) of the National Renewable Energy Laboratory (NREL) (version 08-03-2012)16,60,61,62. In brief, dry samples (0.5 g) were treated with 72% (w/w%) H2SO4 (3.0 mL) at 30 °C for 1 h. Subsequently, distilled water (84 mL) was added for the second hydrolysis in the autoclave at 121 °C for 1 h. The mixture was then filtered by porcelain filter crucibles with glass filters. The glucose, xylose, arabinose, mannose, galactose and rhamnose concentrations in the filtrates were determined by HPLC using a Shodex sugar SP0810 column. The cellulose content was calculated using a correction of 0.90 for glucose, and the hemicellulose content was calculated using a correction of 0.88 for the sum of xylose and arabinose content plus a correction of 0.90 for mannose, galactose and rhamnose. Lignin content (total lignin) was defined as a sum of Klason lignin residue and acid soluble lignin, and measured following the method described by Jurak, et al.15. All treatments were performed in triplicate and the test data were statistically analyzed using SPSS 19.0.
Assay for lignocellulosic enzyme activities
Lignocellulosic enzyme activities of every composting and mushroom cultivation phases were determined including filter paper cellulase (FPUase, total cellulase activity), carboxymethyl cellulase (CMCase, endoglucanase, endo-1, 4-β-D-glucanase, EC 3.2.1.4), endo-xylanase (endo-1, 4-β-xylanase, E.C.3.2.1.8), mannanase (mannan endo-1, 4-β-mannosidase, EC 3.2.1.78), laccase (p-diphenol:dioxygen oxidoreductase, EC 1.10.3.2), and manganese peroxidase (MnP, EC 1.11.1.13). Freeze dried and milled (<1 mm) sample powders were dissolved in physiological saline (1:10, g/v), and incubated in shake flasks at 25 °C, 220 rpm for 2 h. Subsequently, the suspensions were centrifuged at 4 °C, 12000 rpm for 10 min. The supernatant was collected as the crude enzyme extract and further assayed or stored at −80 °C.
FPUase and CMCase activities were assayed using filter paper (Whatman No. 1) and CMC (Sigma-Aldrich) as substrates, respectively. Filter paper (50 mg) or 1% CMC (0.5 mL) and Na-citrate buffer (1.5 mL, 50 mM, pH 4.8) were added to a test tube. Subsequently enzyme solution (0.5 mL) was added and thoroughly mixed, followed by a constant temperature bath at 50 °C for 30 min. After incubation, the released reducing glucose was determined following the 3,5-dinitrosalicylic acid reagent (DNS) method. In brief, DNS (1.5 mL) was added into the reaction tube, followed by boiling for 5 min and cooled to room temperature. Finally, after the colored solution was diluted with 20 ml of H2O, the absorbance at 540 nm was measured63,64,65. The endo-xylanase65,66 and mannanase67,68 activities were measured following the same method of CMCase using xylan (Sigma-Aldrich) and locust bean gum (Sigma-Aldrich) as substrates, respectively. Standard curves of absorbance at 540 nm vs reducing sugar (glucose, xylose, or mannose) content were made. One enzyme unit (U) was defined as the amount of enzyme required to releasing 1 μM of reducing sugar per minute under the assay conditions65. All determinations were performed in triplicate. Heat-inactivated enzyme solution was used as negative control.
Laccase activity was determined using 2,2′-azino-bis (3-ethylbencentiazolin-6- sulphonic acid) (ABTS, Sigma-Aldrich) as the substrate69,70. Enzyme solution (10 μL) was mixed with 0.6 mM ABTS solution (290 μL, in 50 mM sodium acetate buffer, pH 5.2) at 37 °C for 5 min, followed by an addition of 5% trichloroacetic acid (TCA, 700 μL) to end the reaction. The increase in absorbance was monitored at 420 nm to test enzyme activity. MnP activity was determined using 2,6-dimethoxyphenol (DMP) as the substrate with the reaction solution of enzyme solution (100 μL), sodium tartarate buffer (690 μL, 100 mM, pH 4.5), MnSO4 solution (100 μL, 10 mM), H2O2 solution (10 μL, 10 mM), and DMP solution (100 μL, 10 mM). After incubation at 30 °C for 5 min, the reaction was ended by 5% TCA (500 μL) and absorbance at 420 nm was determined43,71. One enzyme unit (U) of laccase and MnP was defined as the amount of enzyme that oxidized 1 μmol of ABTS and DMP per minute, respectively.
Scanning electron microscope analysis of millet straw
Fresh millet straw segments of different phases were randomly collected, fixated, dehydrated, replaced, dried, and covered with gold in an Emscope sputter coater (HITACHI E-1010, JPN). Subsequently, samples were examined using a scanning electron microscope (TESCAN TS 5136MM, CZE)32,46.
16S rRNA gene sequencing and analysis
The extraction of total genomic DNA of samples from P0, PI, and PII were carried out by using the Power Soil DNA extraction kit (MoBio Laboratories Inc., Carslab, USA)72. The bacterial 16S rRNA gene region V3-V4 was amplified with the primer set 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′)73,74. All PCR reactions were carried out in 20 μL with 0.8 μL forward and reverse primers (5 μM), 10 ng template, 4 μL buffer (5×), 2 μL dNTPs (2.5 mM), 0.4 μL polymerase, 0.2 μL BSA. The PCR products were quantified and then constructed DNA library which was sequenced using the Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA). Raw reads were abundance filtered by removing read length lower than 50 bp, Phred score lower than 20, or containing N bases75. Subsequently, clean reads were clustered with the Ribosomal Database Project (RDP) Bayesian Classifier and clustered into operational taxonomic units (OTUs) at 97% identity with consensus taxonomy by Quantitative Insights into Microbial Ecology software (QIIME version 1.6.0)73,76. Distance based redundancy analysis (db-RDA) on Euclidean distances was performed to estimate variability in the bacterial community structure explained by physical-chemical properties77. The Heatmap and dbRDA were computed using the Vegan package in R78.
References
Kabel, M. A., Jurak, E., Makela, M. R. & de Vries, R. P. Occurrence and function of enzymes for lignocellulose degradation in commercial Agaricus bisporus cultivation. Appl Microbiol Biotechnol 101, 4363–4369 (2017).
Sonnenberg, A. S., Baars, J. J., Gao, W. & Visser, R. G. Developments in breeding of Agaricus bisporus var. bisporus: progress made and technical and legal hurdles to take. Appl Microbiol Biotechnol 101, 1819–1829 (2017).
Jurak, E., Kabel, M. A. & Gruppen, H. Carbohydrate composition of compost during composting and mycelium growth of Agaricus bisporus. Carbohydr Polym 101, 281–288 (2014).
Vos, A. M. et al. Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express 7, 12 (2017).
Zhang, X. et al. Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour Technol 170, 183–195 (2014).
Wang, L. et al. Comparison of characterization and microbial communities in rice straw- and wheat straw-based compost for Agaricus bisporus production. J Ind Microbiol Biotechnol 43, 1249–1260 (2016).
Colmenares-Cruz, S., Sanchez, J. E. & Valle-Mora, J. Agaricus bisporus production on substrates pasteurized by self-heating. AMB Express 7, 135 (2017).
Mamiro, D. P. & Royse, D. J. The influence of spawn type and strain on yield, size and mushroom solids content of Agaricus bisporus produced on non-composted and spent mushroom compost. Bioresour Technol 99, 3205–3212 (2008).
Iiyama, K., Stone, B. A. & Macauley, B. J. Compositional changes in compost during composting and growth of Agaricus bisporus. Appl Environ Microbiol 60, 1538–1546 (1994).
Song, T. T. et al. Comparison of microbial communities and histological changes in Phase I rice straw-based Agaricus bisporus compost prepared using two composting methods. Scientia Horticulturae 174, 96–104 (2014).
Cai, W. M. et al. Microbial community structure of casing soil during mushroom growth. Pedosphere 19, 446–452 (2009).
ten Have, R., Wijngaard, H., Aries-Kronenburg, N. A., Straatsma, G. & Schaap, P. J. Lignin degradation by Agaricus bisporus accounts for a 30% increase in bioavailable holocellulose during cultivation on compost. J Agric Food Chem 51, 2242–2245 (2003).
Lyons, G. A., Sharma, H. S., Kilpatrick, M., Cheung, L. & Moore, S. Monitoring of changes in substrate characteristics during mushroom compost production. J Agric Food Chem 54, 4658–4667 (2006).
Gruppen, H., Hamer, R. J. & Voragen, A. G. J. Water-unextractable cell wallmaterial from wheat flour. 1. Extraction of polymers with alkali. J Cereal Sci 16, 41–51 (1992).
Jurak, E., Punt, A. M., Arts, W., Kabel, M. A. & Gruppen, H. Fate of Carbohydrates and lignin during composting and mycelium growth of Agaricus bisporus on wheat straw based compost. PLoS One 10, e0138909 (2015).
Jurak, E., Patyshakuliyeva, A., de Vries, R. P., Gruppen, H. & Kabel, M. A. Compost grown Agaricus bisporus lacks the ability to degrade and consume highly substituted xylan fragments. PLoS One 10, e0134169 (2015).
de Andrade, M. C. et al. Dynamics of the chemical composition and productivity of composts for the cultivation of Agaricus bisporus strains. Braz J Microbiol 44, 1139–1146 (2013).
Changmei, S. & Dorothy, J. Millet - the frugal grain. Int J Sci Res Rev 3 (2014).
Habiyaremye, C. et al. Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, U.S.: A Review. Front Plant Sci 7, 1961 (2016).
Rzymski, P., Mleczek, M., Niedzielski, P., Siwulski, M. & Gasecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J Sci Food Agric 97, 923–928 (2017).
Radojičić, D. et al. The simplified method for the assessment of the potential for thermal energy recovery from the manufacturing processes of mushrooms compost. Sustain Cities Soc 32, 331–337 (2017).
Royse, D. J. & Chalupa, W. Effects of spawn, supplement and phase II compost additions and time of re-casing second break compost on mushroom (Agaricus bisporus) yield and biological efficiency. Bioresour Technol 100, 5277–5282 (2009).
Patyshakuliyeva, A. et al. Uncovering the abilities of Agaricus bisporus to degrade plant biomass throughout its life cycle. Environ Microbiol 17, 3098–3109 (2015).
Royse, D. J. Effects of fragmentation, supplementation and the addition of phase II compost to 2nd break compost on mushroom (Agaricus bisporus) yield. Bioresour Technol 101, 188–192 (2010).
Royse, D. J. & Sanchez, J. E. Supplementation of first break mushroom compost with hydrolyzed protein, commercial supplements and crystalline amino acids. World J Microbiol Biotechnol 24, 1333–1339 (2008).
Pardo-Gimenez, A., Zied, D. C., Alvarez-Orti, M., Rubio, M. & Pardo, J. E. Effect of supplementing compost with grapeseed meal on Agaricus bisporus production. J Sci Food Agric 92, 1665–1671 (2012).
Pardo-Gimenez, A. et al. Effect of supplementing crop substrate with defatted pistachio meal on Agaricus bisporus and Pleurotus ostreatus production. J Sci Food Agric 96, 3838–3845 (2016).
Arce-Cervantes, O. et al. Alternative supplements for Agaricus bisporus production and the response on lignocellulolytic enzymes. Sci Hortic 192, 375–380 (2015).
Zied, D. C., Pardo-González, J. E., Minhoni, M. T. A. & Pardo-Giménez, A. A reliable quality index for mushroom cultivation. J Agri Sci 3, 50–61 (2011).
van Griensven, L. J. L. D. The cultivation of mushrooms. (Mushroom Experimental Station, Horst, the Netherlands, 1988).
Guo, Y. P., Zhang, G. Q., Chen, Q. J. & Yang, K. Analysis on bacterial community structure in mushroom (Agaricus bisporus) compost using PCR-DGGE. Agri. Sci Technol 16, 1778–1784 (2015).
Zhang, R., Wang, H., Liu, Q. & Ng, T. Chemical and ultrastructural studies of lignocellulose biodegradation during Agaricus bisporus cultivation. Biotechnol Appl Biochem 61, 208–216 (2014).
Sharma, H. S. & Kilpatrick, M. Mushroom (Agaricus bisporus) compost quality factors for predicting potential yield of fruiting bodies. Can J Microbiol 46, 515–519 (2000).
Tien, M. & Kirk, T. K. Lignin-degrading enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 221, 661–663 (1983).
Davin, L. B. & Lewis, N. G. Lignin primary structures and dirigent sites. Curr Opin Biotechnol 16, 407–415 (2005).
Wei, Y. et al. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour Technol 271, 66–74 (2019).
Furukawa, T., Bello, F. O. & Horsfall, L. Microbial enzyme systems for lignin degradation and their transcriptional regulation. Front Biol 9, 448–471 (2014).
McGee, C. F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl Microbiol Biotechnol 102, 1075–1083 (2018).
Kertesz, M. A. & Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl Microbiol Biotechnol 102, 1639–1650 (2018).
Szekely, A. J. et al. DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequence-aided T-RFLP profile of mature compost. Microb Ecol 57, 522–533 (2009).
Juturu, V. & Wu, J. C. Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30, 1219–1227 (2012).
Walia, A., Guleria, S., Mehta, P., Chauhan, A. & Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7, 11 (2017).
Hilden, K., Makela, M. R., Lankinen, P. & Lundell, T. Agaricus bisporus and related Agaricus species on lignocellulose: production of manganese peroxidase and multicopper oxidases. Fungal Genet Biol 55, 32–41 (2013).
Wood, D. A. & Hammond, J. B. Ethylene production by axenic fruiting cultures of Agaricus bisporus. Appl Environ Microbiol 34, 228–229 (1977).
Rytioja, J. et al. Plant-polysaccharide-degrading enzymes from Basidiomycetes. Microbiol Mol Biol Rev 78, 614–649 (2014).
Atkey, P. T. & Wood, D. A. An electron microscope study of wheat straw composted as a substrate for the cultivation of the edible mushroom (Agaricus bisporus). J Appl Bacteriol 55, 293–304 (1983).
Whitney, D. & Arnott, H. J. Calcium oxalatecrystal morphology and development in Agaricus bisporus. Mycologia 79, 180–187 (1987).
Vieira, F. R. & Pecchia, J. A. An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting-phase II) for Agaricus bisporus cultivation. Microb Ecol 75, 318–330 (2018).
Siyoum, N. A., Surridge, K., van der Linde, E. J. & Korsten, L. Microbial succession in white button mushroom production system from compost and casing to a marketable packed product. Ann Microbiol 66, 151–164 (2016).
Ishii, K., Fukui, M. & Takii, S. Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J Appl Microbiol 89, 768–777 (2000).
Tuomela, M., Vikman, M., Hatakka, A. & Itavaara, M. Biodegradation of lignin in a compost environment: a review. Bioresour Technol 72, 169–183 (2000).
Wei, S., Morrison, M. & Yu, Z. Bacterial census of poultry intestinal microbiome. Poult Sci 92, 671–683 (2013).
de Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107, 14691–14696 (2010).
Partanen, P., Hultman, J., Paulin, L., Auvinen, P. & Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol 10, 94 (2010).
Ross, R. C. & Harris, P. J. The significance of thermophilic fungi in mushroom compost preparation. Sci Hortic 20, 61–70 (1983).
Kariaga, M. G., Nyongesa, H. W., Keya, N. C. O. & Tsingalia, H. M. Compost physico-chemical factors that impact on yield in button mushrooms, Agaricus bisporus (Lge) and Agaricus bitorquis (Quel) Saccardo. J Agri Sci 31, 49–54 (2012).
Makela, M. R., Lundell, T., Hatakka, A. & Hilden, K. Effect of copper, nutrient nitrogen, and wood-supplement on the production of lignin-modifying enzymes by the white-rot fungus Phlebia radiata. Fungal. Biol 117, 62–70 (2013).
Stoknes, K., Beyer, D. M. & Norgaard, E. Anaerobically digested food waste in compost for Agaricus bisporus and Agaricus subrufescens and its effect on mushroom productivity. J Sci Food Agric 93, 2188–2200 (2013).
Bradstreet, R. B. Kjeldahl method for organic nitrogen. Anal Chem 26, 185–187 (1954).
Zhu, N. et al. Metagenomic and metaproteomic analyses of a corn stover-adapted microbial consortium EMSD5 reveal its taxonomic and enzymatic basis for degrading lignocellulose. Biotechnol Biofuels 9, 243 (2016).
Zurawski, J. V. et al. Bioavailability of carbohydrate content in natural and transgenic switchgrasses for the extreme thermophile Caldicellulosiruptor bescii. Appl Environ Microbiol 83 (2017).
Garcia-Maraver, A., Salvachua, D., Martinez, M. J., Diaz, L. F. & Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag 33, 2245–2249 (2013).
Ghose, T. Measurement of cellulase activities. Pure Appl Chem 59, 257–268 (1987).
Yu, X. et al. Measurement of filter paper activities of cellulase with microplate-based assay. Saudi J Biol Sci 23, S93–98 (2016).
Singh, A. D., Abdullah, N. & Vikineswary, S. Optimization of extraction of bulk enzymes from spent mushroom compost. J Chem Technol Biotechnol 78, 743–752 (2003).
Wu, H. et al. Purification and characterization of a cellulase-free, thermostable endo-xylanase from Streptomyces griseorubens LH-3 and its use in biobleaching on eucalyptus kraft pulp. J Biosci Bioeng 125, 46–51 (2018).
Zhao, W., Zheng, J. & Zhou, H. B. A thermotolerant and cold-active mannan endo-1,4-beta-mannosidase from Aspergillus niger CBS 513.88: Constitutive overexpression and high-density fermentation in Pichia pastoris. Bioresour Technol 102, 7538–7547 (2011).
You, J., Liu, J. F., Yang, S. Z. & Mu, B. Z. Low-temperature-active and salt-tolerant beta-mannanase from a newly isolated Enterobacter sp. strain N18. J Biosci Bioeng 121, 140–146 (2016).
Si, J., Peng, F. & Cui, B. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresour Technol 128, 49–57 (2013).
Yang, J., Ng, T. B., Lin, J. & Ye, X. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int J Biol Macromol 77, 344–349 (2015).
Heinfling, A., Martinez, M. J., Martinez, A. T., Bergbauer, M. & Szewzyk, U. Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl Environ Microbiol 64, 2788–2793 (1998).
Zhang, Q., Sun, J., Liu, S. & Wei, Q. Manure refinement affects apple rhizosphere bacterial community structure: a study in sandy soil. PLoS One 8, e76937 (2013).
Xu, N., Tan, G., Wang, H. & Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74, 1–8 (2016).
Mori, H. et al. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res 21, 217–227 (2014).
Ziegler, A. S. et al. Dynamics of the fouling layer microbial community in a membrane bioreactor. PLoS One 11, e0158811 (2016).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73, 5261–5267 (2007).
Tebby, C., Joachim, S., Van den Brink, P. J., Porcher, J. M. & Beaudouin, R. Analysis of community-level mesocosm data based on ecologically meaningful dissimilarity measures and data transformation. Environ Toxicol Chem 36, 1667–1679 (2017).
Zhang, Y. et al. Fertilization shapes bacterial community structure by alteration of soil pH. Front Microbiol 8, 1325 (2017).
Acknowledgements
This work was financially supported by Beijing NOVA Program (XX2015B025), Beijing Innovative Grant of Modern Agricultural Technology System (PXM2016-014207-000036), and Natural Science Foundation of Beijing Municipality (5162006).
Author information
Authors and Affiliations
Contributions
H.L.Z., G.Q.Z. and Q.J.C. designed experiments and wrote the manuscript. H.L.Z., R.Y., X.J.G., Y.X.S. and P.P.C. performed the measurements. H.L.Z., J.K.W. and Q.H.W. performed composting and mushroom cultivation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, HL., Wei, JK., Wang, QH. et al. Lignocellulose utilization and bacterial communities of millet straw based mushroom (Agaricus bisporus) production. Sci Rep 9, 1151 (2019). https://doi.org/10.1038/s41598-018-37681-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37681-6
This article is cited by
-
Practice, pathways, and performance for resource utilization of crop straw: A case study of Xinyang City in China
Environmental Science and Pollution Research (2022)
-
Bacterial Community Patterns in the Agaricus bisporus Cultivation System, from Compost Raw Materials to Mushroom Caps
Microbial Ecology (2022)
-
Bacterial Community Shifts in Casing Soil Before and After the Cultivation of Oudemansiella raphanipes
Journal of Soil Science and Plant Nutrition (2022)
-
Lignocellulolytic enzymes and bioethanol production from spent biomass of edible mushrooms using Saccharomyces cerevisiae and Pachysolen tannophilus
Biomass Conversion and Biorefinery (2022)
-
Cultivation of king eryngii (Pleurotus eryngii (DC. ex Fr.) Quel.) isolates on various local agro-residues
Biomass Conversion and Biorefinery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.