Abstract
The safety of minimally invasive distal pancreatectomy (MIDP) and open distal pancreatectomy (ODP) regarding oncological outcomes of pancreatic ductal adenocarcinoma (PDAC) remains inconclusive. Therefore, the aim of this study was to examine the oncological safety of MIDP and ODP for PDAC. Major databases including PubMed, Embase, Science Citation Index Expanded, and the Cochrane Library were searched for studies comparing outcomes in patients undergoing MIDP and ODP for PDAC from January 1994 to August 2018. In total, 11 retrospective comparative studies with 4829 patients (MIDP: 1076, ODP: 3753) were included. The primary outcome was long-term survival, including 3-year overall survival (OS) and 5-year OS. The 3-year OS (hazard ratio (HR): 1.03, 95% confidence interval (CI): 0.89, 1.21; P = 0.66) and 5-year OS (HR: 0.91, 95% CI: 0.65, 1.28; P = 0.59) showed no significant differences between the two groups. Furthermore, the positive surgical margin rate (weighted mean difference (WMD): 0.71, 95% CI: 0.56, 0.89, P = 0.003) was lower in the MIDP group. However, patients in the MIDP group had less intraoperative blood loss (WMD: −250.03, 95% CI: −359.68, −140.39; P < 0.00001), a shorter hospital stay (WMD: −2.76, 95% CI: −3.73, −1.78; P < 0.00001) and lower morbidity (OR: 0.57, 95% CI: 0.46, 0.71; P < 0.00001) and mortality (OR: 0.50, 95% CI: 0.31, 0.81, P = 0.005) than patients in the ODP group. The limited evidence suggested that MIDP might be safer with regard to oncological outcomes in PDAC patients. Therefore, future high-quality studies are needed to examine the oncological safety of MIDP.
Similar content being viewed by others
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death among men and women in the USA and will become the second most deadly cancer in the near future1,2. Surgical resection remains the main treatment for pancreatic cancer3. The first minimally invasive distal pancreatectomy (MIDP) was reported in 1994. With the development of advanced technology, minimally invasive techniques have increasingly been used in pancreatic surgery4. MIDP is regarded as a safe and feasible procedure for pancreatic surgery5,6,7. However, one study showed that almost one-third of European pancreatic surgeons considered MIDP inferior to open distal pancreatectomy (ODP) in terms of oncological outcomes8. Furthermore, almost 21% of pancreatic surgeons considered the minimally invasive approach contraindicated for pancreatic ductal adenocarcinoma (PDAC)9. Moreover, some studies have shown that MIDP was not usually performed in clinical practice10.
Previously, a Cochrane review was published that describes all types of pancreatic cancer11. However, the oncological safety of MIDP for PDAC is still not clear. At present, some high-quality studies focusing on PDAC have been published, including one propensity score-matched study performed in Europe12. Thus, we conducted a systematic review and meta-analysis to evaluate the oncological safety of MIDP for PDAC.
Materials and Methods
Literature search
A systematic literature search was performed in PubMed, Embase, Science Citation Index Expanded, and the Cochrane Library to identify and retrieve studies published from January 1994 to August 2018 that examined distal pancreatectomy for PDAC (last search on August 8, 2018). The following medical search headings were used: (“Pancreatic Cancer” or “Pancreatic ductal adenocarcinoma” or “Pancreatic adenocarcinoma”) and (“Left pancreatectomy” or “Distal Pancreatectomy” or “Pancreatectomy”) and (“Laparoscopy” or “Laparoscopic-assisted” or “Laparoscopic” or “Robotic” or “Robot-assisted” or “Mini-invasive” or “Minimally invasive”). The language of full text articles was limited to English. In addition, the references of all selected articles were screened for any potential eligible studies.
Study selection
Studies were included in the meta-analysis if they met the following criteria: (1) human study; (2) published in English; (3) if studies were reported by the same institution (and/or authors), the study with either the larger sample size or higher quality was included. Studies meeting the following criteria were excluded: (1) case reports, letters, editorials, expert opinions and abstracts; (2) benign or other malignant tumors were included without reporting PDAC separately.
Qualitative assessment of the studies selected
The risk of bias of included nonrandomized studies was evaluated according to the risk of bias in nonrandomized studies of interventions (ROBINS-I) tool13.
Data extraction and synthesis
Each study was evaluated by two independent reviewers (Du-Jiang Yang and Jun-Jie Xiong) for inclusion or exclusion from the review. Disagreements between the reviewers were resolved by consensus and by consultation with a third reviewer (Hui-Min Lu) when necessary. Data were collected by two independent researchers using standardized forms. The study characteristics, quality assessment, and intraoperative and postoperative outcomes were included. The means of outcomes were used for the meta-analysis unless otherwise mentioned. Furthermore, the means and standard deviations or medians and ranges were reported14.
The following data were extracted from each study: author, year, country, study design, study duration, number of patients, age, sex, body mass index, tumor size, operation time, intraoperative blood loss, hospital stay, morbidity, postoperative pancreatic fistula (POPF) occurrence, mortality, positive surgical margin rate, lymph nodes harvested, perineural and lymphovascular invasion, multivisceral resection, positive lymph nodes, recurrence, adjuvant therapy and follow-up time.
Outcomes of interest and definitions
Minimally invasive was defined as a laparoscopic, robotic, laparoscopic-assisted or robot-assisted procedure. The primary outcome was overall survival (OS) time, which was defined as the time from the operation until death or the final follow-up evaluation. The secondary outcomes included operative time, intraoperative blood loss, hospital stay, morbidity, POPF occurrence, mortality, positive surgical margin, lymph nodes harvested, recurrence, perineural and lymphovascular invasion, multivisceral resection, positive lymph nodes, and adjuvant therapy. The operative time was defined as the interval from incision to suturing of the skin. Intraoperative blood loss was defined as the blood loss during surgery. The hospital stay was defined as the length from patient admission to discharge from the hospital. Morbidity was defined as all complications that occurred during the hospital stay or within 90 days after surgery. POPF occurrence was defined according to the International Study Group of Pancreatic Fistula (ISGPF)15. Mortality was defined as the number of deaths occurring during hospitalization or within 30 days after surgery. A positive surgical margin was defined as tumor in the transection and circumferential margins with a distance from the margin to the tumor of <1 mm12,16,17 or <0 mm18,19. Lymph nodes harvested was defined as the number of lymph nodes obtained during the operation. Recurrence was defined as local recurrence or distant metastasis. Adjuvant therapy was defined as the number of patients who received adjuvant therapy including postoperative radiation or chemotherapy.
Statistical analysis
A meta-analysis was performed using Review Manager Version 5.3 software (The Cochrane Collaboration, Oxford, UK). Variables are expressed as weighted mean differences (WMDs) or odds ratios (ORs) as appropriate, with their corresponding 95% confidence interval (CI). For continuous and categorical variables, treatment effects are expressed as WMDs and ORs with corresponding 95% CIs. For the survival analysis, we extracted data from the survival curve using a method reported in a previous study, and hazard ratios (HRs) were used for the quantitative analysis20. A Chi-square test was used to assess heterogeneity, with P < 0.1 considered significant. I2 values were used to evaluate statistical heterogeneity; an I2 value of 50% or more indicated the presence of heterogeneity21. The fixed-effects model was initially used for all outcomes, while the random-effects model was used if the test suggested rejection of the assumption of homogeneity22. Descriptive methods were used if the data were inappropriate for meta-analysis. A sensitivity analysis was performed by removing individual studies from the data set and analyzing the effect on the overall results to identify sources of heterogeneity. A funnel plot was constructed to evaluate potential publication bias based on the OS and positive surgical margin rate23.
Results
Description of the included studies
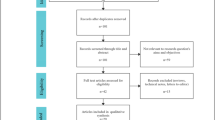
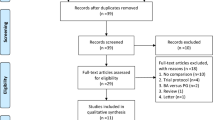
The reporting of this systematic review was in accordance with the PRISMA Statement24. A flow diagram of the study is shown in Fig. 1. In total, 2612 studies were identified from the electronic databases, and 954 studies were excluded because they were duplicate publications. Finally, the full texts of 40 studies11,12,16,17,18,19,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58 were screened for eligibility; however, 28 studies were excluded for various reasons11,25,27,28,29,30,31,32,33,34,35,38,39,41,42,43,45,46,47,48,50,51,52,53,54,55,57,58 (Supplementary Materials). Only 12 studies12,16,17,18,19,26,36,37,40,44,49,56 were included for further analysis. However, two studies36,49 originated from the National Cancer Database (NCDB). Finally, the study by Kantor49 was excluded because of irrelevant data. In total, 11 studies12,16,17,18,19,26,36,37,40,44,56 with 4829 patients (MIDP: 1076, ODP: 3753) were included in the meta-analysis. The study characteristics are shown in Table 1. The studies originated from the United States (4 studies)18,26,36,44, China (2 studies)17,56, Korea37, the UK16, France40, Italy19, and Europe12. The tumor size, which was reported in 10 studies, was larger in patients who underwent ODP than in those who underwent MIDP (WMD: −0.45, 95% CI: −0.85, −0.05; P = 0.03). All included studies were retrospective comparative studies. The risk of bias in included studies was evaluated by the ROBINS-I tool (Table 2). Based on the ROBINS-I assessment, two studies12,37 were considered to have low risk, four studies17,18,26,40 were considered to have moderate risk, and five studies16,19,36,44,56 were considered to have serious risk of bias. Perioperative and tumor outcomes are shown in Tables 3 and 4. The results of the meta-analysis are shown in Table 5 and the Supplementary Materials.
Results of the meta-analysis
Primary outcomes
The OS was reported in ten studies12,16,17,18,19,26,37,40,44,56. However, the OS could not be extracted from the survival curve in a study by Staffer19. Finally, nine studies reported 3-year OS, and four studies reported 5-year OS. No statistically significant differences were observed in 3-year OS (HR: 1.03, 95% CI: 0.89, 1.21; P = 0.66) or 5-year OS (HR: 0.91, 95% CI: 0.65, 1.28; P = 0.59) between the two groups.
Secondary outcomes
A positive surgical margin was reported in all included studies. The pooled analysis suggested that MIDP was associated with a lower rate of positive surgical margins (OR: 0.71, 95% CI: 0.56, 0.89; P = 0.003). According to the different definitions, the MIDP group also had a lower rate of positive surgical margins for a margin to tumor distance <1 mm12,16,17 (OR: 0.66, 95% CI: 0.49, 0.89; P = 0.006). However, no significant difference was observed for a margin to tumor distance <0 mm18,19 (OR: 0.49, 95% CI: 0.20, 1.20; P = 0.12) between the two groups. Only two studies12,17 reported perineural and lymphovascular invasion; the MIDP group had less perineural and lymphovascular invasion (OR: 0.59, 95% CI: 0.44, 0.79; P = 0.0005) than the ODP group. Positive lymph nodes were reported in four studies16,26,36,56; however, no significant difference was found in this outcome between the two groups (OR: 0.95, 95% CI: 0.69, 1.31; P = 0.76). The rate of the recurrence was not significantly different (OR: 0.74, 95% CI: 0.47, 1.18; P = 0.21) between groups according to the pooled results of 4 studies17,26,37,59. Unfortunately, only one study reported multivisceral resection, and no difference (ODP: −32.1% vs MIDP: −38.6%, P = 0.66) was found between the two groups. MIDP was associated with less intraoperative blood loss (WMD: −250.03, 95% CI: −359.68, −140.39; P < 0.00001), shorter hospital stay (WMD: −2.76, 95% CI: −3.73, −1.78; P < 0.00001), and lower morbidity (OR: 0.57, 95% CI: 0.46, 0.71; P < 0.00001) and mortality (OR: 0.50; 95% CI: 0.31, 0.81; P = 0.005) than ODP. However, no significant difference was observed in operative time (WMD: 5.98, 95% CI: −13.15, 25.11; P = 0.54), POPF occurrence (OR: 1.10; 95% CI: 0.82, 1.47; P = 0.54), number of lymph nodes harvested (WMD: 0.40, 95% CI: −2.36, 3.16; P = 0.78), recurrence (OR: 0.74, 95% CI: 0.47, 1.18; P = 0.21) and adjuvant therapy (OR: 0.94; 95% CI: 0.75, 1.18; P = 0.59) between the two groups.
Sensitivity analysis
A sensitivity analysis was conducted by changing the type of effects model or excluding individual studies from the outcome analysis. The results in operation time were different in the fixed and random effects models. Although high heterogeneity was found for blood loss, this result was presented in both the fixed and random effects models. The heterogeneity was large for hospital stay; however, the heterogeneity was zero when two studies (Sulipice40 and Bauman59 were excluded.
Publication bias
Funnel plots based on the 3-year OS, 5-year OS and positive surgical margins are shown in Fig. 2. No study was outside the limits of the 95% CI; therefore, no evidence of publication bias was present.
Discussion
MIDP has been performed for more than a decade, and the technical feasibility, safety and clinical benefit have been well documented by many studies comparing it to ODP34,60,61. However, some centers still favor adopting a conventional, open approach for PDAC62,63.This systematic review and meta-analysis suggested that the OS and oncologic outcomes are not significantly different between the MIDP and ODP groups. Although the pooled results were mainly based on nonrandomized data, the results suggested that MIDP did not adversely affect long-term survival in PDAC patients. Furthermore, the MIDP group also had a lower rate of positive surgical margins and less perineural and lymphovascular invasion. Therefore, MIDP might be feasible and safe regarding long-term survival and oncological outcomes in PDAC patients.
Currently, the rate of long-term survival after distal resection for PDAC patients remains poor. Despite the significant improvement in operative skill for MIDP, the long-term survival is still not different between the two groups of PDAC patients. Radical surgical resection remains the only potentially curative treatment for patients with resectable PDAC64. A recent review from a large randomized trial involving resection of PDAC showed positive margin rates ranging from 0% to 83%65. The finding in the current report is consistent with those in the published literature30. Importantly, the MIDP results in fewer resections with positive margins than ODP. This advantage might be due to the clearer view and more elaborate procedure of MIDP. However, the rate of positive margin resection in present study should be interpreted with caution because margin status was influenced by the use of different definitions and pathologic assessment methods. Furthermore, conclusions may vary by pathologists and institutions due to the absence of standardized pathology assessment and reporting. Moreover, surgeons determine which method is more suitable for the PDAC patients before surgery. Therefore, patients with less extensive cancer may undergo MIDP, and patients with more extensive cancer would undergo ODP16,36.
Regarding other oncological outcomes, no significant differences were found for recurrence, lymph nodes harvested and positive lymph nodes. The extent of the lymphadenectomy determined the number of retrieved lymph nodes. According to one report, at least 12 lymph nodes should be evaluated histologically to determine metastatic disease and adequately stage PDAC patients66. Moreover, according to the consensus of the International Study Group on Pancreatic Surgery (ISGPS), a standard lymphadenectomy with resection of lymph node stations 10, 11, and 18 is recommended for cancer of the body and tail of the pancreas67. In most included studies, the mean number of harvested lymph nodes was greater than 12. In present study, no significant difference was observed in the number of harvested lymph nodes between the two groups. Based on this finding, MIDP is a reasonable procedure for PDAC patients.
No difference was observed in some clinical outcomes. However, MIDP is associated with a steep learning curve. This observation potentially affected several outcomes, including operative time, blood loss and the length of hospital stay16. Therefore, the hospital stay and blood loss were decreased in the MIDP group. Moreover, MIDP was associated with significantly lower morbidity and mortality rates than ODP. Our results are consistent with those of many studies. The technical feasibility, safety and clinical benefit have been well confirmed by various matched comparison studies34,60,61. Thus, MIDP might be safer with regard to oncological outcomes in PDAC patients.
However, our study has some limitations. First, all of the included studies were retrospective, which could lead to inevitable selection bias toward resection of larger or locally advanced tumors via the open approach, especially in the earlier years of the study period. Second, the follow-up time differed and was relatively short in some studies. Therefore, the long-term survival was difficult to accurately estimate. Third, the definitions of some outcomes were different among studies.
Conclusion
In summary, the meta-analysis demonstrated that MIDP might be safer with regard to the oncological outcomes of PDAC patients. However, these results need to be confirmed in a future prospective randomized trial.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 67, 7–30, https://doi.org/10.3322/caac.21387 (2017).
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research 74, 2913–2921 (2014).
Gao, J. J. et al. Standardization of perioperative management on hepato-biliary-pancreatic surgery. Drug Discov Ther 6, 108–111 (2012).
Soper, N. J., Brunt, L. M., Dunnegan, D. L. & Meininger, T. A. Laparoscopic distal pancreatectomy in the porcine model. Surgical Endoscopy 8, 57–60 (1994).
Kim, S. C. et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surgical Endoscopy and Other Interventional Techniques 22, 2261–2268 (2008).
Mehta, S. S., Doumane, G., Mura, T., Nocca, D. & Fabre, J. M. Laparoscopic versus open distal pancreatectomy: a single-institution case-control study. Surgical Endoscopy 26, 402–407 (2012).
Melotti, G. et al. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Annals of Surgery 246, 77–82 (2007).
Rooij, T. D. et al. Pan-European survey on the implementation of minimally invasive pancreatic surgery with emphasis on cancer. Hpb the Official Journal of the International Hepato Pancreato Biliary Association 18, 170 (2016).
Van, H. J. et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. Hpb the Official Journal of the International Hepato Pancreato Biliary Association 19, 190 (2017).
Mabrut, J. Y. et al. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery 137, 597–605 (2005).
Riviere, D. et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database of Systematic Reviews 4, CD011391 (2016).
Van, H. J. et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Annals of Surgery 17, S20 (2017).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj 355, i4919 (2016).
Xiong, J. J. et al. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Br J Surg 101, 1196–1208 (2014).
Bassi, C. et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138, 8–13 (2005).
Rehman, S. et al. Oncological Feasibility of Laparoscopic Distal Pancreatectomy for Adenocarcinoma: A Single-Institution Comparative Study. World Journal of Surgery 38, 476–483 (2014).
Zhang, M. et al. LDP vs ODP for pancreatic adenocarcinoma: a case matched study from a single-institution. Bmc Gastroenterology 15, 1–8 (2015).
Kooby, D. A. et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 210, 779–785 (2010).
Stauffer, J. A., Coppola, A., Mody, K. & Asbun, H. J. Laparoscopic Versus Open Distal Pancreatectomy for Pancreatic Adenocarcinoma. World Journal of Surgery 40, 1477–1484 (2016).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 23, 2815–2834 (2004).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560 (2003).
Dersimonian, R. & Laird, N. Meta-analysis in clinical trials. Controlled Clinical Trials 7, 177 (1986).
Sterne, J. A. C., Egger, M. & Smith, G. D. Investigating and dealing with publication and other biases in meta-analysis. BMJ 323, 101–105, https://doi.org/10.1136/bmj.323.7304.101 (2001).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Medicine 3, e123–130 (2009).
Vijan, S. S. et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg 145, 616–621 (2010).
Magge, D. et al. Comparative Effectiveness of Minimally Invasive and Open Distal Pancreatectomy for Ductal Adenocarcinoma. JAMA Surgery 148, 525–531 (2013).
Duran, H. et al. Does robotic distal pancreatectomy surgery offer similar results as laparoscopic and open approach? A comparative study from a single medical center. International Journal of Medical Robotics + Computer Assisted Surgery Mrcas 10, 280 (2014).
Hu, M. et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surgical Endoscopy 28, 2584–2591 (2014).
Zhang, Y., Chen, X. & Sun, D. Laparoscopic versus open distal pancreatectomy: a single-institution comparative study. World Journal of Surgical Oncology 12, 327–327 (2014).
Adam, M. A. et al. Minimally Invasive Distal Pancreatectomy for Cancer: Short-Term Oncologic Outcomes in 1,733 Patients. World Journal of Surgery 39, 2564–2572 (2015).
Braga, M. et al. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost-benefit analysis, and quality of life assessment. Surgical Endoscopy 29, 1871–1878 (2015).
De, R. T. et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. Journal of the American College of Surgeons 220, 263–270 (2015).
Khaled, Y. S. et al. A Case-matched Comparative Study of Laparoscopic Versus Open Distal Pancreatectomy. Surgical Laparoscopy Endoscopy & Percutaneous Techniques 25, 363–367 (2015).
Lee, S. Y. et al. Distal pancreatectomy: a single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg 220, 18–27 (2015).
Ricci, C. et al. Laparoscopic Distal Pancreatectomy in Benign or Premalignant Pancreatic Lesions: Is It Really More Cost-Effective than Open Approach? Journal of Gastrointestinal Surgery Official Journal of the Society for Surgery of the Alimentary Tract 19, 1415 (2015).
Sharpe, S. M. et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. American Journal of Surgery 209, 557–563 (2015).
Shin, S. H. et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. Journal of the American College of Surgeons 220, 177 (2015).
De, R. T. et al. Outcomes of Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma in the Netherlands: A Nationwide Retrospective Analysis. Annals of Surgical Oncology 23, 585–591 (2016).
Iype, S. et al. Short-term and long-term outcomes of distal pancreatectomy. Hpb 18 (2016).
Sulpice, L. et al. Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: Time for a Randomized Controlled Trial? Results of an All-inclusive National Observational Study. Annals of Surgery 262, 868 (2016).
Zhang, Y. H., Zhang, C. W., Hu, Z. M. & Hong, D. F. Pancreatic cancer: Open or minimally invasive surgery? World journal of gastroenterology 22, 7301 (2016).
Anderson, K. L., Adam, M. A., Thomas, S., Roman, S. A. & Sosa, J. A. Impact of minimally invasive vs. open distal pancreatectomy on use of adjuvant chemoradiation for pancreatic adenocarcinoma. American Journal of Surgery 213, 601–605 (2017).
Aprea, G. et al. Laparoscopic distal pancreatectomy in elderly patients: is it safe? Aging Clinical & Experimental Research 29, 41–45 (2017).
Bauman, M. D. et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective. Surgical Endoscopy & Other Interventional Techniques 32, 1–9 (2017).
Conlon, K. C. et al. Minimally invasive pancreatic resections: cost and value perspectives. HPB 19 (2017).
De Rooij, T. et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): study protocol for a randomized controlled trial. Trials 18, 166 (2017).
Gabriel, E., Thirunavukarasu, P., Attwood, K. & Nurkin, S. J. National disparities in minimally invasive surgery for pancreatic tumors. Surgical Endoscopy and Other Interventional Techniques 31, 398–409, https://doi.org/10.1007/s00464-016-4987-6 (2017).
Joliat, G.-R., Demartines, N., Halkic, N., Petermann, D. & Schaefer, M. Short-term outcomes after distal pancreatectomy: Laparotomy vs. laparoscopy - A single-center series. Annals of Medicine and Surgery 13, 1–5, https://doi.org/10.1016/j.amsu.2016.12.001 (2017).
Kantor, O. et al. Laparoscopic Distal Pancreatectomy for Cancer Provides Oncologic Outcomes and Overall Survival Identical to Open Distal Pancreatectomy. Journal of Gastrointestinal Surgery 21, 1620–1625 (2017).
Nassour, I. et al. Conversion of Minimally Invasive Distal Pancreatectomy: Predictors and Outcomes. Annals of Surgical Oncology 24, 3725–3731, https://doi.org/10.1245/s10434-017-6062-5 (2017).
Ome, Y. et al. Laparoscopic radical antegrade modular pancreatosplenectomy for left-sided pancreatic cancer using the ligament of Treitz approach. Surgical Endoscopy 31, 1–2 (2017).
Plotkin, A. et al. Reduced morbidity with minimally invasive distal pancreatectomy for pancreatic adenocarcinoma. Hpb the Official Journal of the International Hepato Pancreato Biliary Association 19, 279 (2017).
Rosok, B. I. et al. Minimally invasive distal pancreatectomy. Hpb 19, 205–214, https://doi.org/10.1016/j.hpb.2017.01.009 (2017).
Wellner, U. F. et al. Laparoscopic versus open distal pancreatectomy-a propensity score-matched analysis from the German StuDoQ|Pancreas registry. International Journal of Colorectal Disease, 1–8 (2017).
Xourafas, D., Ashley, S. W. & Clancy, T. E. Comparison of Perioperative Outcomes between Open, Laparoscopic, and Robotic Distal Pancreatectomy: an Analysis of 1815 Patients from the ACS-NSQIP Procedure-Targeted Pancreatectomy Database. Journal of Gastrointestinal Surgery 21, 1442–1452 (2017).
Zhang, A., Wang, Y., Hu, C., Shen, Y. & Zheng, S. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center experience. Journal of Zhejiang University-science B 18, 532–538 (2017).
Fisher, A. V. et al. Analysis of 90-day cost for open versus minimally invasive distal pancreatectomy. Hpb (2018).
Raoof, M. et al. Propensity score-matched comparison of oncological outcomes between laparoscopic and open distal pancreatic resection. British Journal of Surgery 105, 578–586, https://doi.org/10.1002/bjs.10747 (2018).
Bauman, M. D. et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective. Surgical Endoscopy and Other Interventional Techniques 32, 53–61, https://doi.org/10.1007/s00464-017-5633-7 (2017).
Eom, B. W. et al. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surgical Endoscopy and Other Interventional Techniques 22, 1334–1338 (2008).
Soh, Y. F. et al. Perioperative outcomes of laparoscopic and open distal pancreatectomy: our institution’s 5-year experience. Asian J Surg 35, 29–36, https://doi.org/10.1016/j.asjsur.2012.04.005 (2012).
Marangos, I. P. et al. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery 151, 717–723 (2012).
Deoliveira, M. L. et al. Assessment of Complications After Pancreatic Surgery A Novel Grading System Applied to 633 Patients Undergoing Pancreaticoduodenectomy. Annals of Surgery 244, 931–939 (2006).
Wagner, M. et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. British Journal of Surgery 91, 586–594 (2004).
Butturini, G. et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Archives of Surgery 143, 75–83 (2008).
Slidell, M. B. et al. Impact of Total Lymph Node Count and Lymph Node Ratio on Staging and Survival after Pancreatectomy for Pancreatic Adenocarcinoma: A Large, Population-Based Analysis. Annals of Surgical Oncology 15, 165–174 (2008).
Tol, J. A. M. G. et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 156, 591–600 (2014).
Acknowledgements
This work is supported by the Sichuan Provincial Department of Science and Technology Supporting Project (Grant Number: 2018SZ0174, Sichuan, China). This meta-analysis was registered at https://www.crd.york.ac.uk/prospero/under registration number CRD42018106941.
Author information
Authors and Affiliations
Contributions
Du-Jiang Yang, Jun-Jie Xiong and Wei-Ming Hu designed the study. Du-Jiang Yang and Jun-Jie Xiong performed the study and wrote the paper. Hui-Min Lu and Yi Wei assessed the study and collected the data. Ling Zhang and Shan Lu analyzed the data. Wei-Ming Hu reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, DJ., Xiong, JJ., Lu, HM. et al. The oncological safety in minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Sci Rep 9, 1159 (2019). https://doi.org/10.1038/s41598-018-37617-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37617-0
This article is cited by
-
Minimally invasive distal pancreatectomy for pancreatic cancer: cranial-to-caudal approach with identification of Gerota’s fascia (with video)
Surgical Endoscopy (2023)
-
Systematic review and meta-analysis of cost-effectiveness of minimally invasive versus open pancreatic resections
Langenbeck's Archives of Surgery (2023)
-
Surgical and oncological outcomes of laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic ductal adenocarcinoma
Surgery Today (2022)
-
Laparoscopic radical antegrade modular pancreatosplenectomy: preliminary experience with 10 cases
BMC Surgery (2021)
-
Robotic-assisted versus open distal pancreatectomy for benign and low-grade malignant pancreatic tumors: a propensity score-matched study
Surgical Endoscopy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.