Abstract
Dracunculus medinensis, or human Guinea worm (GW), causes a painful and debilitating infection. The global Guinea Worm Eradication Program (GWEP) has successfully reduced human GW cases from 3.5 million in 21 countries in 1986 to only 30 cases in three remaining countries in 2017. Since 2012, an increase in GW infections in domestic dogs, cats and baboons has been reported. Because these infections have not followed classical GW epidemiological patterns resulting from water-borne transmission, it has been hypothesized that transmission occurs via a paratenic host. Thus, we investigated the potential of aquatic animals to serve as paratenic hosts for D. medinensis in Chad, Africa. During three rainy and two dry season trips we detected no GW larvae in 234 fish, two reptiles and two turtles; however, seven GW larvae were recovered from 4 (1.4%) of 276 adult frogs. These data suggest GW infections may occur from ingestion of frogs but the importance of this route is unknown. Additional studies are needed, especially for other possible routes (e.g., ingestion of fish intestines that were recently shown to be a risk). Significantly, 150 years after the life cycle of D. medinensis was described, our data highlights important gaps in the knowledge of GW ecology.
Similar content being viewed by others
Introduction
Guinea Worm Disease (GWD), caused by the parasitic nematode Dracunculus medinensis, is a painful and debilitating disease of people1,2. The global campaign to eradicate GWD has been an international success and national Guinea Worm Eradication programs (GWEPs) have reduced the number of human cases from 3.5 million annually in 21 countries to only 30 cases in three remaining countries in 20172. In Chad, infections in dogs, and more recently, cats, have been recorded2. Notably, these infections do not typically follow the classic epidemiology of GWD in humans wherein infection occurs from drinking water containing infected copepods. The result of such transmission typically results in a clustering of cases nearby a single water source, which has not been observed in dog or cat cases. While it is still possible that animals may become infected via ingestion of infected copepods in water, an alternate route was hypothesized; fish or frogs may be playing a role in transmission of D. medinensis3. This hypothesis is further supported by the dearth of human cases in many areas where a high incidence of dog infections occur3. Furthermore, previous experimental work confirmed that frogs can serve as paratenic hosts for D. medinensis, that fish may act as short-term transport hosts, and that both routes (frog and fish) can result in infection of a definitive host4,5. In 2016, for the first time, a natural infection of an amphibian host from the Sarh region of Chad was reported providing field evidence that amphibians may be involved in transmission of D. medinensis6.
The purpose of this study was to better understand the possible role of aquatic animals (amphibians, fish, and reptiles) as paratenic hosts for D. medinensis in Chad, Africa. These data are critical for the development of new interventions aimed at interrupting Guinea worm transmission, especially now since the numbers of infections in dogs and cats have increased annually since 20122. Our primary objective was to determine the prevalence of D. medinensis third-stage (L3) infectious larvae in aquatic animals, with particular attention paid to those species that are likely consumed by humans, those fed to dogs or cats by people, or those that reside in ponds in close proximity to villages where a dog infection or human case has been reported. We also attempted to collect information on which frog species were consumed by humans, the season in which frogs were harvested, and whether or not frogs or fish were fed to dogs and cats.
Materials and Methods
Study location and animal acquisition
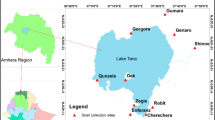
Surveys for D. medinensis L3s in aquatic hosts from Chad occurred during five trips during 2016–2018. Regions of Chad reporting human cases and dog infections were prioritized for sampling and included Sarh, Bousso, Guelengdeng, and N′Djamena (Fig. 1). Amphibians were captured by local villagers either by hand or through the use of submersible nets baited with fish tissue. Fish were typically purchased from local fishermen either at the pond or river locale being sampled, or from nearby outdoor markets. Reptiles included in the sampling were provided by local villagers.
Necropsy and sample collection
Necropsies were performed and gastrointestinal (GI) tracts were removed because L3s should only be present in non-GI tissues and muscles4,5. Small tissue samples (<1 cm) were taken from every animal and preserved in 70% ETOH for molecular species identification (as described below). For animals <10 cm in length, blunt dissections in petri dishes were performed. Skin was removed and muscle tissue was teased apart using forceps and needle tools. The tissue was allowed to soak in water for at least four hours to allow migration of larvae out of tissue. After four hours, water in petri dishes was observed under a dissecting microscope (30x magnification; StereoZoom 4; Optek, USA) for characteristic sinusoidal movement of larvae. For animals >10 cm in length, after GI and skin removal, samples were minced using a metallic mincer that would render muscle into <0.5 cm diameter pieces (LEM Products, West Chester, Ohio). This tissue was then placed into a mesh screen inside a Baermann funnel with enough water so that all tissue was submerged, and sedimentation of potential nematode larvae was allowed to occur for at least four hours (Fig. 2). At four hours, 5 ml of fluid from the funnel was collected into a petri dish and examined as described above. If the first 5 ml were negative for suspect nematode larvae, two subsequent draws of 5 ml were examined. Any larvae that were morphologically similar to Dracunculus were preserved in 70% ETOH for subsequent molecular analyses. Morphological characteristics which constituted subsequent molecular analyses were as follows: an overall length of approximately 0.5–0.7 mm, sinusoidal movement, striated cuticle, and a blunt, trifid tail6.
Molecular identification of host and suspect Dracunculus larvae
All suspect samples were observed under a compound microscope for defining characteristics of D. medinensis L3s, such as a trilobed tail, striated cuticle, and approximate length of 0.581–0.643 mm7. Morphologically-compatible larvae and tissue samples were placed in a 0.5 ml microcentrifuge tube and any residual ethanol allowed to evaporate for 12 hours. DNA was extracted using a commercial DNA extraction kit (DNeasy, QIAGEN, Valencia, California) following manufacturer’s instructions for tissue. However, larvae were allowed to incubate at 56 °C with proteinase K and Buffer ATL for six-eight hours to ensure full digestion. For species identification of animal samples, the 16 s rRNA gene target was amplified for fish8 and a portion of the cytochrome-c oxidase I (COI) gene was amplified for amphibians4. To identify larvae, a partial (COI) gene target was amplified using a cocktail of six M13-tagged primers9. Amplicons were purified from a 0.8% agarose gel stained with gel red (Biotium Inc., Hayward, California, USA) using a commercial gel-purification kit (QIAGEN). Bi-directional Sanger sequencing was conducted on amplicons at the University of Georgia Genomics Facility (Athens, Georgia) or Genwiz (South Plainfield, New Jersey). Chromatograms were analyzed in Geneious R7 (Auckland, New Zealand) and consensus sequences were compared to sequences in the GenBank database.
Identification of frog use by villagers
Where possible, local villagers were queried regarding use and consumption of amphibians as food sources. These questions were as follows: (1) Do you catch and eat frogs?; (2) If you catch frogs, during what season and how?; (3) Can you describe the frogs you catch?; (4) Are frogs cooked or eaten raw?; (5) Do you own a cat or dog, and if so, do you feed them frogs? If individuals stated that they captured frogs, they were shown photos of Hoplobatrachus occipitalis (African Crowned bullfrog locally referred to as black-backed, white-bellied river frog) and Pyxicephalus edulis (Giant Bullfrog locally referred to as black-backed, yellow-bellied frog) and asked to identify which frogs they capture and during which time of the year (Fig. 3).
Ethical approval and informed consent
All animal procedures were reviewed and approved by the University of Georgia’s Institutional Animal Care and Use Committee (A2016 07–024). In addition, all methods were performed in accordance with the relevant guidelines and regulations within the aforementioned University of Georgia’s Institutional Animal Care and Use Committee (A2016 07–024).
Results
During the five trips, we sampled 234 individual fish representing 18 different species, 276 individual amphibians representing six species, two African helmeted turtles (Pelomedusa sp.), and two Nile Monitor lizards (Varanus niloticus) (Table 1). No D. medinensis L3s were detected in the tissues of any fish, turtles, or Nile monitor lizards. Of particular note, when skinning the monitor lizards, we found large subcutaneous nematodes on the ventral surface extending along the intercostal muscles and into the thoracic cavity. Previous work had suggested that Varanus spp. could be hosts for adult D. medinensis10; however, the morphology of these nematodes clearly indicated they were not Dracunculus and molecular characterization using the same primers as used for larvae showed these nematodes were most similar to Ochoterenella spp. (Onchocercidae).
We recovered a total of 863 larvae from 276 amphibians, 90 (10%) of which met morphological criteria warranting molecular identification. Of those, seven (1%) were confirmed as D. medinensis with sequences 100% similar to the previously accessioned cytochrome c oxidase subunit I, D. medinensis HQ216219.1 (Genbank). The first trip was conducted June-July 2016 in Marabe (Moyen Chari region, Kyabe district), and a single individual larvae was found in an African puddle frog (Phrynobatrachus francisci) as previously reported6. On two separate trips (Jan. 2017 and April 2018), we recovered six D. medinensis L3’s; two of the six were found in a single H. occipitalis bullfrog during Jan. 2017 from the village Sidi-1in the Bousso area (Fig. 1) while the remaining four were detected in April 2018 from Tarangara village in the Sarh area (three larvae from a H. occipitalis and one larva from another H. occipitalis) (Fig. 1). Additional nematodes were obtained from the amphibian samples, although we did not enumerate or identify all specimens. Of those identified, we found Raillietnema spp., Cosmocercoides spp., and Spirurida species.
When collecting samples in villages (Fig. 1), numerous villagers answered the questionnaire about frog hunting and consumption. Villagers stated that frogs were captured and consumed; however, some villagers did not eat frogs for various reasons. Two species of frogs (H. occipitalis and P. edulis) were the targets of frog catchers. When interviewed, frog catchers were able to describe both aforementioned species prior to seeing photos, then accurately picked photos of each species when shown. No one who admitted to eating frogs said that they consumed frogs raw, all stated that they cook frogs very well (Fig. 4), similar to fish, and they do not feed frogs to dogs for fear that the dogs would become sick.
Discussion
Annual increases of GW infections since 2012 among domestic dogs and more recently cats, and the absence of classical water-borne outbreaks of GWD among humans led to the hypothesis that transmission was not occurring via the classical route of ingesting water containing copepods infected with L3s. Instead, it was posited that a paratenic host may be involved and that domestic animals were being infected via consumption of undercooked flesh or viscera of aquatic animals3. Our previous experimental studies showed that various amphibian species are susceptible to infections; however, in order to be important in the natural transmission cycle of D. medinensis, natural infections must occur. Thus, the goal of this study was to investigate what aquatic animals may be infected with D. medinensis L3s (as a result of ingestion/consumption of copepods containing L3s). Our intensive surveys in areas of concern in Chad identified several infected frog species including two species that are known to be eaten by people. No D. medinensis larvae were found in any of the sampled fish, Varanus, or turtles, although only a limited number of the latter two groups were sampled.
Many Chadian villages rely heavily on fish protein during off-seasons of agricultural production (July-November). Villages in close proximity to the Chari river that identify as fishing villages are at highest risk for D. medinensis transmission based on biological, epidemiological, and environmental investigations by the Centers for Disease Control and Prevention and The Carter Center Guinea Worm Eradication Program2. The absence of detection of D. medinensis L3s from any of the fish samples obtained from villages actively fishing the same species from local ponds and river tributaries suggests a limited role of fish as paratenic hosts for D. medinensis. Our detection method (using Baermann funnels and sedimentation) has been widely used in detection of larvae specimens from muscular tissues of hosts11,12. However, this method likely lacks sensitivity as it requires larvae to migrate out of tissues. When considering the role of fish, data from experimental studies indicate fish have been challenging to infect with D. insignis (commonly found in North American raccoons (Procyon lotor)), and D. medinensis infection in fish has not occurred in Chad4,13.
We did not recover any larvae from fish; however the importance of fish to the transmission of D. medinensis in Chad remains uncertain. Although it appears unlikely that fish act as a paratenic host in the wild, a recent study using fish species found in Chad showed that fish may serve as short term transport hosts5. In this situation, a transport host would be a smaller fish that feeds on copepods infected with D. medinensis L3s and is subsequently consumed by a definitive host prior to complete digestion or passage of parasites thereby “transporting” potential infection to definitive hosts5. Larger fish may also ingest copepods so allowing dogs or cats to ingest any fish intestines could pose a risk5. The methodology for larval recovery is different than that of paratenic hosts, and L3s would need to be recovered from the gastrointestinal tract. The role of fish as transport hosts in natural settings has yet to be determined and warrants investigation, especially considering increasing incidence of dog and cat infections in Chad along the Chari river corridor where there is significant fishing pressure2.
We recovered D. medinensis L3s from several amphibians, but the prevalence and burden of infection per individual frog was low (1–2 larvae/infected individual); however, our sampling may have missed infections due to low sensitivity of our recovery method or because infections in amphibians may be temporally and/or spatially clustered. For example, had we sampled tadpoles or frogs soon after they emerged from a water body known to have been contaminated with D. medinensis, the prevalence or worm burdens may have been higher. In North America, D. insignis has been used as a model parasite to evaluate potential transmission routes for D. medinensis. Additionally, field-based studies on D. insignis transmission have been conducted and we recently detected D. insignis L3s in several southern leopard frogs (Lithobates sphenocephala) at a site where prevalence of D. insignis is very high in raccoons (Procyon lotor) and one adult frog contained 45 larvae in its musculature (Cleveland, C.A. unpublished data). This finding of a single, heavily infected paratenic host could support the hypothesis that paratenic hosts, if ingested, could lead to infection with numerous adult nematodes in definitive hosts.
In Chad, there have been dog infections in which >50 worms emerge, but the average infection per dog is 2 worms with a range of 1–79 (The Carter Center)14. For those dogs with high worm burdens, a large number of L3s must be ingested, either from a fish acting as a transport host, a number of frogs acting as paratenic hosts, ingestion of water containing infected copepods, or a combination of these possible routes. Certain individual dogs may also exhibit behaviors that put them at higher risk for infection, such as preferentially predating and consuming amphibians or living in households that feed large numbers of raw fish or fish entrails to their animals. Although there are now several hypothesized alternative transmission routes, classical transmission by ingestion of water with infected copepods remains a possibility. A recent experimental study shows that dogs are capable of ingesting copepods during a normal drinking event; however, low numbers were ingested per drinking event and in cases where larger number of copepods were ingested, the density of copepods was typically higher than what is normally seen in Chadian water-bodies15. Instances of cat infections in Chad further complicate identifying the type of transmission occurring, and studying the diets and movement of cats that have been infected or live in villages with dog infections could be informative.
Integration of the potential transmission routes may be advanced using mathematical modeling. For example, a combination of transmission routes in avian influenza16 and Ebola17 help to explain phenomena including pathogen invasion and persistence. Uncertainty in model structure, including the existence of certain transmission routes, as well as parametric uncertainty, especially the rates and probabilities associated with transmission routes, can be explored using mathematical models in conjunction with sensitivity analyses18. These approaches allow an assessment of which features of transmission are most likely to interrupt the overall transmission cycle19. In the case of D. medinensis in Chad, transmission via transport and paratenic hosts may be combined with waterborne transmission in a model to both explore the likely impact of reducing or blocking one of these routes on overall transmission, and to estimate key parameters by fitting such models to data.
Additional data on the role of paratenic and transport hosts as food sources could also be used to strengthen model development as well as in the design and implementation of interventions in Chad. For example, H. occipitalis (Fig. 3), which was infected with D. medinensis L3s, was confirmed as a human food source through questionnaires conducted in numerous villages among various local ethnicities. Additionally, P. edulis (Fig. 3), was also identified as a human food source, and although we did not detect D. medinensis in this species, only three individuals of this species have been sampled to date so additional testing is needed. It is important to note that people consume frogs commonly and consistently report cooking them (Fig. 4), which would kill any parasites present. They also alleged they did not purposely feed frogs to dogs, but bringing the frogs into the village could provide a scavenging opportunity for dogs and Carter Center researchers have observed children feeding tadpoles to dogs (unpublished data). However, ingestion of both paratenic and transport hosts could occur independently of human activities during foraging and scavenging events by animals.
While we have provided evidence supporting the possibility of an alternative method of transmission for Guinea worm, our results should be considered a conservative estimate of the number of amphibians infected with Guinea worm L3s. Two of the surveys occurred outside of the peak transmission season (January-June), and may not be representative of the prevalence in amphibians or possible host diversity. An additional important consideration is that detection of D. medinensis L3s via our methods is likely insensitive, and it is possible that larvae were missed during the process of necropsy and microscopy. Also, the application of Abate® (Temephos) to control copepod populations post potential contamination of water-bodies with Guinea worm could affect the number of L3s recovered from an amphibian paratenic host or from fish that may be transport hosts.
The role of amphibians is not yet fully defined, but there are additional challenges based on life-stages of amphibians. For example, tadpoles are very likely the key life-stage wherein ingestion of infected copepods occur and if tadpoles consume large numbers of infected copepods, they could be acting as a sink for infection if tadpole/amphibian ingestion by dogs or people is ultimately shown to be low. Also, if the tadpoles consuming the copepods are from a fully aquatic amphibian species, such as Xenopus, then they are less likely to be scavenged or hunted by a cat or dog, despite having potential infective L3s in their tissue. Conversely, the possibility for amphibians to act as long-term paratenic hosts must be considered. Data from an experimental study showed that motile larvae of D. insignis and D. medinensis were recovered from frog tissues (Xenopus spp.) for up to 8 months and 2 months, respectively (Cleveland and Yabsley, unpublished data).
Conclusions and management implications
Stopping transmission of Guinea worm and achieving eradication of D. medinensis is the goal of the global Guinea Worm Eradication Program. Alternative transmission routes for D. medinensis to humans and animals are a concern for eradication. While we have identified D. medinensis infection in frogs, we have not received reports nor collected data on dogs or cats consuming frogs. Consumption of frogs by peri-domestic animals may occur, and further studies are needed to identify the location and frequency of these behaviors. Furthermore, while our data do not provide evidence that fish are paratenic hosts, there has been no research yet conducted on the presence of D. medinensis larvae in the intestines of fish in Chad; however, large numbers of copepods have been observed in the digestive tracts of small fish (Cleveland, unpublished data). A recent case-control study conducted by The Centers for Disease Control and Prevention personnel in Chad investigating risk-factors contributing to infection with D. medinensis reported a statistically significant association between provisioning of fish entrails to dogs and infections among dogs (S. Roy, The Centers for Disease Control and Prevention, personal communication).
Understanding how transmission is occurring among dogs and cats would allow for implementation of timely and more effective preventative measures to be devised. In order to ensure compliance among village residents, the intervention(s) must be simple and straightforward. The owners of peri-domestic animals in Chad must understand the importance of limiting the ability of dogs and cats to scavenge or consume frog flesh and fish entrails. It should also be noted that occurrence of D. medinensis transmission in Chad primarily occurs along the Chari river and tributaries, but, there are few human cases and animal infections from communities closer to the Logone river (Fig. 1), a major tributary of the Chari river (H. Zirimwabagabo, The Carter Center-Chad, personal communication). Furthermore, dog, cat and baboon (Papio anubis) infections have been documented in Ethiopia and dog infections in Mali, suggesting that potential animal behaviors and transmission route(s) may differ20. Therefore, careful field investigations in each region will be essential during, what is hoped to be, the tail end of the Guinea worm eradication program.
Data Availability Statement
Any data discussed herein will be made available upon written request.
References
Hopkins, D. R., Ruiz-Tiben, E., Eberhard, M. L. & Roy, S. L. Progress toward global eradication of dracunculiasis, January 2014–June 2015. MMWR Morb. Mortal. Wkly. Rep. 64, 1161–1165 (2015).
Hopkins, D. R., Ruiz-Tiben, E., Eberhard, M. L., Roy, S. L. & Weiss, A. J. Progress toward global eradication of dracunculiasis, January 2016–June 2017. MMWR Morb. Mortal. Wkly. Rep. 66, 1327–1331 (2017).
Eberhard, M. L. et al. The peculiar epidemiology of dracunculiasis in Chad. Am. J. Trop. Med. Hyg. 90, 61–70 (2014).
Eberhard, M. L. et al. Possible role of fish and frogs as paratenic hosts of Dracunculus medinensis, Chad. Emerg. Infect. Dis. 22(8), 1428–1430 (2016).
Cleveland, C. A. et al. Possible role of fish as transport hosts for Dracunculus spp. larvae. Emerg. Infect. Dis. 23(9), 1590–1592 (2017).
Eberhard, M. L. et al. Guinea worm (Dracunculus medinensis) infection in a wild-caught frog, Chad. Emerg. Infect. Dis. 22(11), 1961–1962 (2016).
Cleveland, C. A. et al. The wild world of Guinea Worms: a review of the genus Dracunculus in wildlife. Int. J. Parasitol. Parasites. Wildl. 7, 289–300 (2018).
Wang, J. et al. Phylogenetic relationships of five asian schilbid genera including Clupisoma (Siluriformes: Schilbeidae). PloS one. 11(1), e0145675 (2016).
Prosser, S. W., Velarde‐Aguilar, M. G., León‐Règagnon, V. & Hebert, P. D. Advancing nematode barcoding: a primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol. Ecol. Resour. 13(6), 1108–1115 (2013).
Mirza, M. B. & Basir, M. A. A report on the guinea-worm found in Varanus sp., with a short note on Dracunculus medinensis. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 5(1), 26–32 (1937).
Ash, L. R. The occurrence of Angiostrongylus cantonensis in frogs of New Caledonia with observations on paratenic hosts of metastrongyles. J. Parasitol. 54(3), 432–436 (1968).
Holliman, R. B. & Meade, B. Native trichinosis in wild rodents in Henrico County, Virginia. J. Wildl. Dis. 16(2), 205–208 (1980).
Crichton, V. F. J. & Beverley-Burton, M. Observations on the seasonal prevalence, pathology, and transmission of Dracunculus insignis in the raccoon in Ontario. J. Wildl. Dis. 13, 273–280 (1977).
WHO Collaborating Center for Research Training and Eradication of Dracunculiasis, Guinea Worm Wrap-Up #241, June 17, 2016, Centers for Disease Control and Prevention (CGH): Atlanta (2016).
Garrett, K. B., Box, E. K., Cleveland, C. A., Majewska, A. A. & Yabsley, M. J. Dogs and the classic route of transmission: an evaluation of copepod ingestion. Manuscript submitted for publication (2018).
Rohani, P., Breban, R., Stallknecht, D. E. & Drake, J. M. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl. Acad. Sci. 106(25), 10365–10369 (2009).
Vinson, J. E., Drake, J. M., Rohani, P. & Park, A. W. The potential for sexual transmission to compromise control of Ebola virus outbreaks. Biol. Let. 12(6), 20151079 (2016).
Simpson, J. E. et al. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. Lond. [Biol.] 279(1730), 925–933 (2012).
Fraser, C., Riley, S., Anderson, R. M. & Ferguson, N. M. Factors that make an infectious disease outbreak controllable. Proc. Natl. Acad. Sci. 101(16), 6146–6151 (2004).
Molyneux, D. & Sankara, D. P. Guinea worm eradication: Progress and challenges-should we beware of the dog? PLoS Negl. Trop. Dis. 11(4), e0005495 (2017).
Acknowledgements
The Carter Center’s work to eradicate Guinea worm disease was made possible by financial and in-kind contributions from many donors. A full listing of supporters is available at http://www.cartercenter.org/donate/corporate-government-foundation-partners/index.html. We would also like to thank E. Chop for assistance with creating maps. Additional support for C. Cleveland was received from the ARCS Atlanta chapter.
Author information
Authors and Affiliations
Contributions
C.C., M.Y., M.E., H.Z., P.O., T.M. and E.R.T. planned, designed, and conducted surveys in Chad. C.C., A.T., L.S. and K.G. conducted molecular analyses. C.C. and M.Y. wrote the main manuscript text and prepared tables and figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cleveland, C.A., Eberhard, M.L., Thompson, A.T. et al. A search for tiny dragons (Dracunculus medinensis third-stage larvae) in aquatic animals in Chad, Africa. Sci Rep 9, 375 (2019). https://doi.org/10.1038/s41598-018-37567-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37567-7
This article is cited by
-
Susceptibility of anurans, lizards, and fish to infection with Dracunculus species larvae and implications for their roles as paratenic hosts
Scientific Reports (2021)
-
Dracunculiasis in a domestic dog in Brazil
Parasitology Research (2021)
-
Dogs and the classic route of Guinea Worm transmission: an evaluation of copepod ingestion
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.