Abstract
QT/QTc interval prolongation reflects delayed cardiac repolarization which can lead to Torsade de Pointes and sudden death. Many antimalarial drugs prolong QT/QTc interval. However, due to confounding factors in patients with malaria, the precise extent of this effect has been found to be highly variable among studies. We compared the effects of dihydroartemisinin-piperaquine phosphate (DHA-PQP) and artemether-lumefantrine (A-L) on QT interval duration in healthy volunteers. In this randomized, parallel groups, active moxifloxacin- and placebo-controlled study, prolongation of the QT/QTc interval following treatment with DHA-PQP in fasted and fed condition and A-L in fed state was investigated in healthy subjects (n = 287; Clinicaltrials.gov: NCT01103830). DHA-PQP resulted in significant mean (95% confidence interval (CI)) maximum increases in QTc Fridericia (QTcF) of 21.0 ms (15.7, 26.4) for DHA-PQP fasted, 35.9 ms (31.1, 40.6) for DHA-PQP high-fat/low-caloric and 46.0 ms (39.6, 52.3) for DHA-PQP high-fat/high-caloric breakfast. For A-L, the largest difference from baseline relative to placebo was 9.9 ms (95% CI: 6.8, 12.9). Increases in QTcF related to maximum plasma concentrations of piperaquine. Moxifloxacin demonstrated assay sensitivity. Increases in QTcF following DHA-PQP and A-L were clinically relevant. Food increased piperaquine exposure and QTcF interval prolongation emphasizing the need to administer DHA-PQP in the fasting state.
Similar content being viewed by others
Introduction
Malaria is a disease that although preventable and tractable still caused approximately 425,000 mortalities in 2016, with most deaths occurring in Africa1. The World Health Organization recommends artemisinin-based combination therapies for the treatment of Plasmodium falciparum malaria2. Many antimalarial drugs including artemisinin-based combination therapies have been associated with prolongation of the corrected QT interval (QTc), which reflects a delay in ventricular repolarization during the cardiac cycle3. Delayed cardiac repolarization can lead to the development of ventricular tachyarrhythmias, most notably Torsade de Pointes, which can be self-terminating but can also degenerate into ventricular fibrillation leading to sudden death.
QT interval is highly influenced by heart rate, physiologically. Assessment of possible QT-prolonging effects of antimalarial drugs in patients is often hampered by the symptoms of malaria most notably fever3. In the acute phase of the disease, and in addition to fever, stress, anxiety and discomfort may lead to an increase in heart rate. In contrast, during the recovery phase and after starting treatment, heart rate decreases and QT interval lengthens. Therefore since the clinical condition of patients with malaria may induce changes in the QT interval, the effects of antimalarial treatments on cardiac repolarization are ideally studied in healthy subjects.
The fixed-dose artemisinin-based combination therapy of dihydroartemisinin (DHA) and piperaquine phosphate (PQP) is approved in the European Union for the treatment of uncomplicated P. falciparum malaria. Preclinical experiments showed that despite significant blockade of the human ether-a-go-go related channel (hERG), which plays a critical role in cardiac repolarization, DHA-PQP did not appear to induce effects characteristic of Torsade de Pointes, affect hERG trafficking or block sodium channels although it blocked slow-potassium ion currents4. However, in patients with malaria, treatment with DHA-PQP resulted in prolongation of the Fridericia corrected QT interval (QTcF) ranging from 7 to 45 ms5,6,7,8. Marked QTcF prolongations were also observed for artemisinin-based combination therapies (artemether-lumefantrine [A-L]: 22 ms9; artesunate + mefloquine: 18 ms10; artesunate + amodiaquine: 33 ms9.). In these studies, electrocardiograms (ECGs) were recorded at various single-time points when plasma drug concentrations were at trough or around presumed peak levels; therefore, the treatment effect on QT interval may have been under- or overestimated.
The present study was conducted in healthy subjects to carefully investigate prolongation of the QT/QTc interval following treatment with DHA-PQP and A-L at the maximum plasma concentrations of their respective active compounds and over the 24 hrs following the last day of drug administrations. The methodology employed was based on that used in thorough QTc studies, which are designed to detect small changes in QTcF11.
Results
Subject Disposition and Demographics
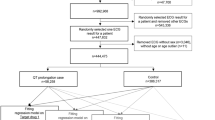
A total of 287 healthy Caucasian subjects were randomized and their disposition in the study is presented in Fig. 1. Five subjects were withdrawn from the study between Day –1 and Day 1. Two hundred and eighty-two subjects (174 men and 108 women) received at least one dose of study medication and 279 completed the study. Subjects providing a non-evaluable QT interval were replaced to maintain the pre-established sample size in each group. The mean [range] age and body mass index of the study population were 29.6 [18–50] years and 22.6 [18.0–28.2] kg/m2, respectively. Demographic variables (including age, gender, weight and height; data not shown) were similar across groups.
ECG Analysis
Fridericia’s method was the most appropriate to correct the QT interval for changes in heart rate (data not shown) and was used throughout this study. In Group 3 treatment with moxifloxacin caused a prolongation in QTcF interval (Table 1); the maximum time-matched difference from placebo was 15.5 ms (90% confidence interval [CI]: 12.5, 18.4) at 4 h post-dose. In Group 6 moxifloxacin caused a QTcF prolongation of similar magnitude: 17.4 ms (90% CI: 13.3, 21.5) at 6 h post-dose.
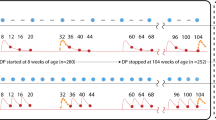
QTcF increased with DHA-PQP. Mean QTcF prolongation with DHA-PQP was >10 ms for at least 24 h post-dose regardless of food intake (Fig. 2). QTcF prolongation with DHA-PQP significantly increased after a high-fat/low-caloric breakfast compared with the fasted state. For A-L, the largest difference from baseline relative to placebo (Group 3, Day 3) was 9.9 ms (95% CI: 6.8, 12.9; p < 0.0001) and this was significantly smaller than the QT prolonging effect of DHA-PQP high-fat/low-caloric (i.e. the primary hypothesis was rejected). Other comparisons are shown in Table 1. Prolongation of QTcF was 33.8 ms for DHA-PQP and 20.4 ms for A-L, before allowing for placebo adjustment. Body weight did not significantly influence QTcF. In contrast, gender had a statistically significant effect on QTcF in all treatment groups except DHA-PQP fasted, with longer QTcF interval prolongations noted in women. QTcF change from baseline was linearly related to the Cmax and AUC of PQ (supplementary file).
Difference from placebo (Day –1 of respective treatment) in mean change from baseline in QTcF on Day 3. Data are presented as mean and 90% confidence interval. The dotted horizontal line corresponds to the 10 ms threshold. DHA-PQP = dihydroartemisinin-piperaquine phosphate. Square = DHA-PQP low caloric breakfast; triangle = DHA-PQP high caloric breakfast; filled circle = DHA-PQP fasted; open circle = artemether-lumefantrine.
Categorical analysis showed that two subjects (3.1%) in the A-L group and four subjects (10%) in the DHA-PQP fasted group had abnormal maximum absolute QTcF values in the range 450–480 ms. There were no subjects from either group with maximum absolute QTcF values ≥480 ms. No subjects in the DHA-PQP fasted and A-L groups experienced maximum time-matched changes from baseline for QTcF > 60 ms.
Pharmacokinetics
Maximum plasma concentration (Cmax) of DHA was achieved rapidly with median time to Cmax (tmax) values within 1–2 h (Table 2). Piperaquine was absorbed more slowly with median tmax values 3–4 h. When DHA-PQP was administered concomitantly with a high-fat/low-caloric breakfast, plasma PQ Cmax increased approximately 2-fold compared with the fasted state. Concomitant administration of DHA-PQP with a high-fat/high-caloric breakfast resulted in a 3-fold increase in Cmax with PQ reaching supra-therapeutic exposure. Lumefantrine was absorbed slowly (median tmax: approximately 6 h), with subsequent slow formation of its desbuthyl metabolite (median tmax: 7 h). Artemether plasma concentrations were below the limit of detection of the assay at all time-points in nine subjects. The median tmax value for its metabolite, DHA, was similar (median tmax: 2 h) to that described after DHA-PQP administration, although Cmax was lower.
Discussion
In this study, clinically significant increases in QTcF were associated with DHA-PQP and A-L. In ICH E14 guideline an increase in QTcF is considered clinically relevant when the upper limit of the 90% CI of the maximum time-matched treatment difference from placebo in QTcF change from baseline exceeds 10 ms11. For all 3 DHA-PQP groups, even the lower limit of the 90% CI was well above this 10-ms limit whereas the effect of A-L was modest with only the upper limit of the 90% CI (12.0 ms) above 10 ms. Moxifloxacin was included in the present study as a positive control and administration of a single 400 mg dose resulted in an increase in QTcF of 15.5 and 17.4 ms. The increases in QTcF in the current study are in the high range among those reported for moxifloxacin in thorough QTc studies (10.2–21.0 ms)12, indicating that the subjects participating in the current study were sensitive to drug-induced QT prolongation.
Increases in QTcF observed following administration of DHA-PQP and A-L were clinically relevant. Treatment differences compared with placebo for DHA-PQP under fasted conditions showed increases similar to those of moxifloxacin. The QTcF prolongation observed for the DHA-PQP fasted group when the effect of placebo was not subtracted was higher than that determined in previous studies (33.8 ms for DHA-PQP in the current study vs. 19 to 23 ms for DHA-PQP in previous studies)6,8,13. This variability among studies emphasizes the need for a placebo control to accurately assess drug-induced QTc interval prolongation although it should be recognized that it is not feasible in patients with acute malaria.
Although several articles report on the effects of DHA-PQP on ventricular repolarization5,6,14,15,16, studies using high-standard of ECG recording and measurements over time for the specific evaluation of QTc prolongation after DHA-PQP are lacking. One study evaluated QTc prolongation after a full course of treatment with DHA-PQP in 56 patients with malaria5. No significant changes in mean QTcF occurred 4 h after the first administration of DHA-PQP whereas an increase in QTcF was detected 4 h after the last administration (mean difference: 29 ms [95% CI: 22, 38]; p < 0.001). These results are consistent with those obtained in the present study for the DHA-PQP fasted group.
The high-fat/high-caloric meal only had a small effect on the pharmacokinetics of DHA but PQ Cmax increased up to 3-fold, consistently with previous report under different study conditions17,18. In contrast to published results that demonstrated a small or no effect of light food intake on the pharmacokinetics of PQP19, our data show that PQ concentrations increased following a high-fat/low-caloric meal compared with the fasted state. This apparent discrepancy may be explained by differences in the composition of the meals provided in each study. The observed QTc prolongation by DHA-PQP and A-L was likely due to PQ and lumefantrine and its desbuthyl metabolite, respectively, whereas DHA and artemether do not appear to be implicated.
Piperaquine4 and lumefantrine4,20 are known to block the hERG channel which mediates the repolarizing IKr current which is critical for cardiac repolarization. The observed increase in QTc interval after administration of these drugs was consistent with this blocking effect. However, the present study was not designed to address the clinical relevance of this QTc prolongation. It is well recognized that hERG blockade is a strong predictor of QT prolongation but not of Torsade de Pointes21. Indeed, retrospective analyses of the risk of Torsade de Pointes associated with drugs known to exert hERG blockade demonstrated that there is no direct correlation between the QT prolonging effect exerted by hERG blockade and Torsade de Pointes22,23.
Preclinical studies were performed to investigate the potential for DHA-PQP to elicit Torsade de Pointes4. Rabbit ventricular wedge preparations are considered a sensitive and specific experimental model for human pathological conditions characterized by a substantial reduction in repolarization reserve, a well-recognized risk factor for the potential of QT prolonging agents to trigger Torsade de Pointes. Different antimalarial drugs known to cause QT prolongation were compared using this model. Neither DHA-PQP nor A-L showed any effect on Torsade de Pointes risk score; chloroquine showed a mild risk. Dofetilide, a class III antiarrhythmic drug used as positive control, showed, as expected, a potent torsadogenic potential. This experiment also included an evaluation of early afterdepolarization. It is known that Torsade de Pointes is initiated by early afterdepolarization-dependent R-on-T extrasystoles; therefore, the absence of an induction of early afterdepolarization significantly reduces the probability of false positive results in terms of no arrhythmogenic effects of the tested drugs. DHA-PQP and A-L did not induce any early afterdepolarization.
In conclusion, both DHA-PQP and A-L produced significant prolongation of the QTc interval, which is a risk factor for Torsades de Pointes. However, current preclinical4 and clinical8,24,25,26,27 data do not suggest an increased risk of cardiac toxicity. The risk of drug-induced proarrhythmia due to QTc prolongation should further be addressed by epidemiological studies or meta-analyses. The magnitude of prolongation of the QTc interval observed with DHA-PQP was dependent on how it was administered; the smallest QTc prolongation occurred when the drug was administered in the fasted state whereas the largest QTc prolongation occurred when DHA-PQP was administered following a high-fat/high-caloric meal. Because the pharmacokinetic data obtained in the DHA-PQP fasted group were similar to those determined in a study of patients taking DHA-PQP 3 h after food intake28, the QTcF interval prolongation that can be experienced by patients in conditions of standard clinical care will be similar to that observed in the subjects from the DHA-PQP fasted group. Although the risk for Torsades de Pointes for DHA-PQP appears to be low, caution is still advised and DHA-PQP should be given to both adults and children on an empty stomach pending future research. A post-marketing observational study conducted in 10,000 patients with P. falciparum malaria of whom 10% (1,000) had a QTc monitoring did not find a clinical cardiac safety signal8. This was also the case in a recent large-scale phase III trial25.
Methods
Study Population
Healthy men and women aged 18–50 years with a body mass index 18–27 kg/m2 were included between February and August 2010. Key exclusion criteria included a history of risk factors for Torsade de Pointes (e.g., heart failure, hypokalemia, family history of Long QT Syndrome), the concomitant use of any other medication (except paracetamol) and any condition that might have interfered with study results. Subjects were allowed to smoke up to 5 cigarettes or equivalent per day but had to refrain from smoking while confined in the clinical center. The regular drinking of alcohol of up to 21 units (1 unit = 4 cL spirits or equivalent) for males or 14 units for females per week was allowed.
Study Design
This was a randomized, parallel group, active- and placebo-controlled study stratified to ensure that ≥37.5% of subjects in each group were female (Clinicaltrials.gov: NCT01103830, posted 15 April 2010). Group 1 (n = 64) received placebo on Day –1 and DHA-PQP once daily for 3 days after a high-fat/low-caloric breakfast; Group 2 (n = 64) received placebo on Day –2 and A-L twice daily for 3 days from Day –1 (afternoon) to Day 3 (morning) after a high-fat/low-caloric breakfast and dinner; Group 3 (n = 40) received placebo from Day –1 to Day 3, and a single dose of 400 mg moxifloxacin on Day 4 after a high-fat/low-caloric breakfast; Group 4 (n = 40) received placebo on Day –1 and DHA-PQP once daily for 3 days after a high-fat/high-caloric breakfast. An overview of the different treatments received in each group is presented in Table 3.
The study was performed under double blind conditions for groups 1, 3 (up to Day 4) as well as 5 and 6 (up to Day 4) morning, and in open condition for group 2 and 4. All ECG manual readings performed by Cardiabase were under blinded conditions. It is usual not to blind moxifloxacin administration in order to avoid encapsulating the tablets. Also, it was not possible to blind Groups 2 and 5 due to different dosing scheme or meal composition.
Pharmacokinetic data collected in the high fat/high caloric DHA-PQP group suggested that exposure to piperaquine as free base (PQ) was three-fold higher than previously observed in patients treated with DHA-PQP29. It was postulated that food intake might have increased the absorption of PQP. Therefore, two additional groups of subjects were enrolled: Group 5 (n = 40) received placebo on Day –1 and DHA-PQP once daily for 3 days in the fasted state; Group 6 (n = 20) received placebo on Day –1 to Day 3 in the fasted state, then 400 mg of moxifloxacin on Day 4 after intake of a high-fat/low-caloric breakfast. The study was double blinded for Groups 1, 3, 5 and 6 up to the morning of Day 4 and single blinded (i.e. subjects did not know whether they had received placebo or active drug) for Group 4. Group 2 received the study medication without any blinding.
Study drugs were administered as tablets containing DHA-PQP 40 mg/320 mg (Eurartesim®, Sigma-Tau s.p.a Industrie Farmaceutiche Riunite, Rome, Italy) and A-L 20 mg/120 mg (Riamet®, Novartis, Basel, Switzerland). The dose of DHA-PQP and A-L was selected based on subject’s body weight according to current recommendations.
Additional information on meals composition, blood sampling procesures, drug assays, and recommended drug dosages are shown in the supplementary file.
ECG Assessments
Twelve-lead ECGs were recorded using a MAC5500 GE Cardiograph® (GE Healthcare, Freiburg, Germany). Printouts of ECGs were taken regularly throughout the study and analysed locally to safeguard the subjects’ safety. Holter monitoring was performed on Days –1, 1, 3, and 4. Time-matched triplicate ECGs with at least 1-minute intervals were extracted at the following time-points using the expected dosing time on Day –1 and the actual dosing times on Days 1, 3 and 4: pre-dose, hourly up to 13 h and 24 h post-dose on Day –2 (Group 2), Day –1 (all other groups) and Day 3 (all groups). In addition, triplicate ECGs were recorded at 1-h intervals up to 6 h post-dose on Day 1 (Groups 1, 4 and 5) and 1, 2, 3, 4, 6, 8, 12, and 24 h post-dose on Days 1 and 4 (Groups 3 and 6). All ECG evaluations were performed without knowledge of treatment assignment.
Digitally recorded ECGs were transmitted electronically to a core ECG laboratory for computer-based, manually verified, digital caliper measurement of HR and RR, PR, QRS complex and QT intervals using the tangent method30,31. The core ECG laboratory personnel were blinded to treatment and all ECGs from each subject were read by the same person. Whenever possible, measurements were performed on three consecutive ECGs from a single lead, preferably lead II, and the same lead was used throughout the study for the same subject. For each subject, triplicate values at each time-point were averaged. Three different correction methods were used: Bazett, Fridericia32 and a population method based on a correction factor obtained by assessing the relationship between QT and RR intervals using a power model QT = αRRβ where α is QTc and α and β are regression parameters33.
Pharmacokinetic Assessments
On Day 3 (all groups) and Day 4 (Groups 3 and 6), blood samples for the measurement of study drug concentrations were taken pre-dose, hourly up to 13 h, and 24 h post-dose. Only samples taken on Day 4 were analyzed for moxifloxacin in Group 3 and Group 6. Blood samples from Day 3 were taken to maintain blinded conditions relative to Groups 1, 4, and 5. Plasma concentrations were determined by validated reverse-phase liquid chromatography with tandem mass spectrometric detection methods. Pharmacokinetic parameters were derived from the time-concentration data by standard non-compartmental analysis using WinNonlin Professional Version 5.2 (Pharsight Corporation, Mountain View, CA, USA).
Sample Size and Statistical Evaluations
The primary objective of this study was to demonstrate non-superiority of DHA-PQP (high-fat/low-caloric breakfast – Group 1) versus A-L (Group 2). Non-superiority was defined as an upper limit of 10 ms for the two-sided 95% confidence interval of QTcF.
Sample size calculations were based on a power of 80% and a two-sided type I error risk of 5%. For Groups 1 and 2, 64 subjects per group were considered sufficient to demonstrate non-superiority assuming a standard deviation (SD) for maximum time-matched changes from baseline in QTc of 8 ms and an expected difference of 6 ms. For Group 3, 40 subjects were considered sufficient to demonstrate a moxifloxacin effect on QTc prolongation ≥5 ms assuming an SD of 8–9 ms for time-matched changes from baseline and an expected difference of 10 ms. Forty subjects in Group 5 were considered sufficient to detect a difference of 10 ms compared with Group 1, assuming an SD of 15 ms (α = 0.05, β = 0.10). No formal statistical analysis was performed to determine the sample sizes in Groups 5 and 6 because of the large QTc prolongation seen in groups 1 and 4.
The moxifloxacin vs. placebo analysis was performed by comparing the mean effect in Group 3 of time-matched changes on Day 4 minus Day 1 with the mean effect in the same group of time-matched changes on Day 3 minus Day –1 using an analysis of variance (ANOVA) with terms for treatment, time and gender, including a random effect for subject. This comparison was performed within 2–8 h post-dose. Assay sensitivity was demonstrated if the lower bound of the two-sided 90% confidence interval for the difference between moxifloxacin and placebo was >5 ms; the estimated mean difference was 10 ms and the pattern of the time-effect curve of moxifloxacin was as expected.
The DHA-PQP high-fat/low-caloric meal vs. A-L (non-superiority) analysis was performed by comparing the mean maximum effect in Group 1 of time-matched changes on Day 3 minus Day –1 with the mean maximum effect in Group 2 of time-matched changes on Day 3 minus Day –2, using ANOVA with a term for treatment. The objective of this analysis was to evaluate whether the upper bound of the two-sided 95% confidence interval for the difference between treatments was <10 ms.
A comparison of QTcF maximum time-matched changes from baseline for DHA-PQP after a high-fat/low-caloric meal (Group 1) and under fasting conditions (Group 5) was conducted to demonstrate inferiority of DHA-PQP under fasting conditions and was performed using ANOVA with a term for treatment.
All other comparisons among the six groups were performed for QTcF in terms of 95% CI and statistical testing as described above. The effects of gender and body weight were investigated by including these terms and their interaction with treatment in the ANOVA model.
Linear regression was used to assess the relation between Cmax of PQ and the change in QTcF from baseline using data from all DHA-PQP groups (group 1, 4, and 5).
Ethical standards
The independent Ethics Committee of Ile de France VIII, Boulogne-Billancourt, France, and the French Health Products Safety Agency, Saint-Denis, France approved the study protocol. The study was conducted in accordance with Good Clinical Practice and the principles of the Declaration of Helsinki. All subjects provided written informed consent prior to their inclusion into the study.
References
WHO. World Malaria Report 2017 (accessed 30 September May 2018). http://www.who.int/malaria/publications/world-malaria-report-2017/en/.
WHO. Guidelines for the treatment of malaria. Third edition. (accessed 30 September 2018). http://www.who.int/malaria/publications/atoz/9789241549127/en/.
White, N. J. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 7, 549–58 (2007).
Borsini, F. et al. In vitro cardiovascular effects of dihydroartemisin-piperaquine combination compared with other antimalarials. Antimicrob Agents Chemother 56, 3261–70 (2012).
Mytton, O. T. et al. Electrocardiographic safety evaluation of dihydroartemisinin piperaquine in the treatment of uncomplicated falciparum malaria. Am J Trop Med Hyg 77, 447–50 (2007).
Valecha, N. et al. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 5, e11880 (2010).
Hanboonkunupakarn, B. et al. Open-label crossover study of primaquine and dihydroartemisinin-piperaquine pharmacokinetics in healthy adult thai subjects. Antimicrob Agents Chemother 58, 7340–6 (2014).
Baiden, R. et al. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim(R) (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J 14, 160 (2015).
Ndiaye, J. L. et al. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J 10, 237 (2011).
Krudsood, S. et al. Effect of artesunate and mefloquine in combination on the Fridericia corrected QT intervals in Plasmodium falciparum infected adults from Thailand. Trop. Med. Int. Health 16, 458–65 (2011).
ICH. E14 The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. 2005 (accessed 30 September 2018). http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/the-clinical-evaluation-of-qtqtc-interval-prolongation-and-proarrhythmic-potential-for-non-antiarrh.html.
Fosser, C., Duczynski, G., Agin, M., Wicker, P. & Darpo, B. Comparison of manual and automated measurements of the QT interval in healthy volunteers: an analysis of five thorough QT studies. Clin. Pharmacol. Ther. 86, 503–6 (2009).
Kabanywanyi, A. M. et al. Multi-Country Evaluation of Safety of Dihydroartemisinin/Piperaquine Post-Licensure in African Public Hospitals with Electrocardiograms. PLoS One 11, e0164851 (2016).
Manning, J. et al. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother 58, 6056–67 (2014).
Wisniowska, B., Tylutki, Z., Wyszogrodzka, G. & Polak, S. Drug-drug interactions and QT prolongation as a commonly assessed cardiac effect - comprehensive overview of clinical trials. BMC Pharmacol Toxicol 17, 12 (2016).
Karunajeewa, H. et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br. J. Clin. Pharmacol. 57, 93–9 (2004).
Sim, I. K., Davis, T. M. & Ilett, K. F. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother 49, 2407–11 (2005).
Reuter, S. E. et al. Effect of food on the pharmacokinetics of piperaquine and dihydroartemisinin. Clin. Drug Investig. 35, 559–67 (2015).
Hai, T. N., Hietala, S. F., Van Huong, N. & Ashton, M. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 107, 145–9 (2008).
Traebert, M. et al. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol 484, 41–8 (2004).
Gintant, G. An evaluation of hERG current assay performance: Translating preclinical safety studies to clinical QT prolongation. Pharmacol. Ther. 129, 109–19 (2011).
Redfern, W. S. et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58, 32–45 (2003).
Crumb, W. J. Jr., Vicente, J., Johannesen, L. & Strauss, D. G. An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods 81, 251–62 (2016).
WHO Malaria Policy Advisory Committee. The cardiotoxicity of antimalarials. (accessed 30 September 2018). http://www.who.int/malaria/mpac/mar2017/en/ and http://www.who.int/entity/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf. (2017).
West African Network for Clinical Trials of Antimalarial Drugs. Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 391, 1378–90 (2018).
Millat-Martinez, P. & Bassat, Q. Reappraising the cardiosafety of dihydroartemisinin-piperaquine. Lancet Infect Dis 18, 824–6 (2018).
Chan, X. H. S. et al. Risk of sudden unexplained death after use of dihydroartemisinin-piperaquine for malaria: a systematic review and Bayesian meta-analysis. Lancet Infect Dis 18, 913–23 (2018).
European Medicines Agency. Eurartesim assessment report - EMA/739355/2011. (accessed 30 September 2018). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001199/WC500118116.pdf.
Eurartesim summary of product characteristics. (accessed 30 September 2018). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf.
Locati, E. In Non-Invasive Electrocardiography in Clinical Practice. (eds W. Zareba, P. Maison-Blanche, & EH. Locati) 71–96 (Futura Publishing Company, 2001).
Postema, P. G., De Jong, J. S., Van der Bilt, I. A. & Wilde, A. A. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm 5, 1015–8 (2008).
Funck-Brentano, C. & Jaillon, P. Rate-corrected QT interval: techniques and limitations. Am. J. Cardiol. 72, 17B–22B (1993).
Malik, M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 12, 411–20 (2001).
Acknowledgements
This study was performed at SGS Aster, Paris, France. The authors thank Paul van Giersbergen (Van Giersbergen Consulting, sponsored by Medicines for Malaria Venture) for editorial assistance.
Author information
Authors and Affiliations
Contributions
C.F.-B., A.B., G.V., S.P., S.T. and M.C. were involved in the study design, preparation and supervision of the study conduct and review of the results. D.U. and S.D. were involved in the study design and review of the results. E.E. was responsible for study conduct. P.V. was responsible for the central reading of the ECGs and M.F. for the statistical analyses. P.V. and M.F. also contributed to data interpretation C.F.-B. was the writer of the final version. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
This study was co-funded by Sigma-tau Industrie Farmaceutiche Riunite s.p.a. and the Medicines for Malaria Venture. A.B., G.V., S.P., S.T. are employees of Sigma-tau. M.C. is a former employee of Sigma-tau. S.D. is an employee of the Medicines for Malaria Venture (MMV) and D.U. is a former employee of MMV. P.V. is CEO of Cardiabase and M.F. is an employee of Phinc Development. E.E. was an employee of SGS-Aster, the contract research organization that performed the study. C.F.-B. was a consultant for Sigma-Tau and MMV.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Funck-Brentano, C., Bacchieri, A., Valentini, G. et al. Effects of Dihydroartemisinin-Piperaquine Phosphate and Artemether-Lumefantrine on QTc Interval Prolongation. Sci Rep 9, 777 (2019). https://doi.org/10.1038/s41598-018-37112-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37112-6
This article is cited by
-
Safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials
Malaria Journal (2022)
-
Longitudinal study based on a safety registry for malaria patients treated with artenimol–piperaquine in six European countries
Malaria Journal (2021)
-
Evaluation of the effects on the QT-interval of 4 artemisinin-based combination therapies with a correction-free and heart rate-free method
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.