Abstract
Short anterior chamber depth (ACD) is considered a risk factor of endothelial-cell loss after phacoemulsification. However, whether it is an independent risk factor or not remains controversial. We investigated the relationship between ascorbic acid (AA) concentrations in the aqueous humour (AqH) and ACD. We analysed 165 AqH samples of 97 patients (42 men and 55 women) who underwent small incision cataract surgery. AqH and plasma AA concentrations were measured using a high-performance liquid chromatography - electrochemical detection method. Patient characteristics were compared between and within the sexes. As a result, age and ACD were significantly correlated with AqH AA concentrations (r = −0.206, P = 0.045; r = 0.339, P < 0.001) only in women. Moreover, plasma AA concentrations were significantly correlated with AqH AA concentrations (r = 0.420, P < 0.001; r = 0.316, P = 0.002) both in men and women. After adjusting for confounding factors (age and plasma AA concentrations), ACD was significantly and positively correlated with AqH AA concentrations (partial.r = 0.275, P = 0.009) only in women. In conclusion, AqH AA concentrations were reduced in women with smaller ACD. This may suggest that women with short ACD could be more susceptible to oxidative damage.
Similar content being viewed by others
Introduction
Ascorbic acid (AA) concentration in the human aqueous humour (AqH) is more than 20-fold higher than in the plasma1,2,3. There are various theories regarding the reasons why AqH AA concentration is high. First, it has been suggested that AA acts as an ultraviolet filter for internal eye structures because diurnal mammals have higher AqH AA concentrations than nocturnal mammals4. AA absorbs ultraviolet light of 310 nm or less and reduces fluorescence emission of ultraviolet A of 320 to 400 nm5,6. Second, AA controls the metabolism of the extracellular matrix of tissues that are in contact with the AqH because AA regulates the synthesis of various extracellular-matrix molecules such as collagen and elastin7,8. In addition, AA is considered a radical scavenger in the eye. Free radical species in vivo reacts with stable molecules such as nucleic acids, proteins, sugars, and lipids and promotes oxidisation, which results in various disease states. AA has strong reducing action and protects the cornea, crystalline lens, and other intraocular tissues from oxidative damage9,10,11,12.
Ultrasonic phacoemulsification for cataract surgery results in the formation of free radical species and causes injury in corneal endothelial cells13,14. In contrast, adding the antioxidant AA to the irrigation solution significantly reduces corneal endothelial-cell damage10,12. The risk of endothelial-cell loss after phacoemulsification depends on several preoperative and intraoperative parameters (high nucleus grade, advanced age, long phaco time, high ultrasound energy, short axial length, and surgical skill)15,16,17,18. Especially, Walkow et al.15 and Storr-Paulsen et al.16 reported that eyes with shorter axial length (AL) had significantly increased risk for endothelial-cell loss. Although the direct relationship between anterior chamber depth (ACD) and endothelial-cell loss remains unclear, strong positive correlations between ACD and AL have been frequently reported19,20,21. Some researchers have considered that short ACD might be a risk factor for corneal endothelial-cell damage15,22,23. Short ACD leads to phacoemulsification being performed closer to the corneal endothelial cells and may therefore be associated with an increased risk of corneal endothelial-cell loss. However, to the best of our knowledge, no report has considered the association between ACD and AA concentrations in the AqH. We hypothesised that short ACD would be more susceptible to oxidative damage and that it would be associated with decrease in AqH AA concentration. Therefore, in this study, we examined the relationship between ACD and AqH AA concentrations in patients with cataract and examined whether the association between these two factors is affected by patient characteristics.
Results
Patient characteristics
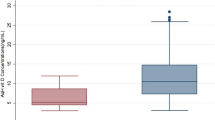
Patient characteristics are presented in Table 1. Seventy eyes of 42 men and 95 eyes of 55 women were included. ACD and AL were significantly shorter in women (3.34 ± 0.37 mm vs. 3.09 ± 0.42 mm; P < 0.001, 24.0 ± 1.3 mm vs. 23.5 ± 1.5 mm; P = 0.042, respectively). AqH AA concentrations were significantly lower in men (1535 ± 326 μmol/L vs. 1733 ± 355 μmol/L, respectively; P < 0.001), whereas there was no significant difference in plasma AA concentrations between the sexes (48.4 ± 18.1 μmol/L vs. 52.7 ± 15.9 μmol/L, respectively; P = 0.105).
Correlation between AqH AA concentrations and patient characteristics
The sex-specific correlation coefficients between ACD and the characteristics of the patients are shown in Table 2. The AqH AA concentrations were significantly correlated with age (r = −0.206, P = 0.045), ACD (r = 0.339, P < 0.001) and plasma AA concentrations (r = 0.316, P = 0.002) in women. The AqH AA concentrations were significantly correlated with plasma AA concentrations (r = 0.420, P < 0.001) in men. AqH AA was not significantly associated with nuclear sclerosis (NS), endothelial-cell density (ECD), AL, or central corneal thickness (CCT).
Correlations between AL and AqH AA concentrations
Table 3 shows the sex-specific correlation coefficients between AL and AqH AA concentrations. There were no significant correlations between AL and the AqH AA concentrations in either men or women.
Correlation between ACD and AqH AA concentrations
Table 4 shows the sex-specific correlation coefficients between ACD and AqH AA concentrations. In women, ACD was positively correlated with AqH AA concentrations (partial.r = 0.275, P = 0.009) after adjustment. There were no significant correlations in men (partial.r = 0.049, P = 0.700).
Discussion
The present study showed that AqH AA concentrations were significantly and positively correlated with ACD in women after adjusting for confounding factors (age and plasma AA concentrations).
The AA concentration in the corneal epithelium is the highest among all known tissue concentrations in the eye4,24,25. Since blood vessels are not distributed on the cornea, the cornea receives AA from the tears and the AqH11,24,25. In the present study, AA concentrations in the AqH were 1535 ± 326 μmol/L in men and 1733 ± 355 μmol/L in women, at levels similar to those reported by a US study with patients with cataract (1410 ± 550 μmol/L in men and 1640 ± 580 μmol/L in women)3. In contrast, Senthilkumari et al.1 reported that AqH AA concentrations were 1010 ± 469 μmol/L in men and 1138 ± 613 μmol/L in women with poor nutritional status in India. In this study, the plasma and AqH AA concentrations tended to be lower in men. Some studies have shown a similar trend1,3. Even considering the influence of dietary intake and preferences between the sexes, renal excretion is higher in men than in women26. Therefore, we speculate that the results were influenced by sex differences in excretion, absorption, and retention of AA. AA is considered to be actively transported from the blood through the ciliary body into the AqH27,28. However, the mean concentration ratio between AqH AA and plasma AA varied from 18 to 71 in previous studies1,2,3. Our study showed that the ratio was 32 in men and 33 in women. AqH AA concentrations reportedly have a positive correlation with plasma AA concentrations1,2,3. There was a positive correlation between plasma and AqH AA concentrations both in men (r = 0.420, P < 0.001) and in women (r = 0.316, P = 0.002) in this study, in support of past reports. In addition, Čanadanović et al.29 reported that AqH AA concentration decreases with age. Therefore, in order to investigate the correlation between ACD and AqH AA concentrations, we adjusted for two confounding factors (age and plasma AA concentrations).
Several in vitro and in vivo studies have shown that AA scavenges free radicals in phacoemulsification and reduces corneal endothelial-cell damage12,13. This protective effect on corneal endothelial cells is attributable to AA directly eliminating free radicals generated in the AqH. Therefore, as phacoemulsification always replaces the anterior chamber with irrigating solutions, AqH AA concentrations before surgery may have little involvement in radical scavenging during surgery. However, since corneal endothelial cells have the ability to absorb AA30, AA may act as a protective factor against oxidative stress even intracellularly31. Yue et al.32 and Reddy et al.33 reported that AA might be an important factor in endothelial-cell healing, migration, and regeneration. Moreover, Biaggi et al.34 reported that after phacoemulsification in dogs, AqH AA concentrations were reduced until 15 days postoperatively. Consequently, in patients with low AA concentrations in the AqH, corneal endothelial cells may be affected by oxidative damage from early postoperatively and extending to the long term. Because this was a cross-sectional study, we could not demonstrate the accelerated reduction of corneal endothelial cells in relation to AqH AA concentrations; there is a need for a prospective longitudinal study on the effects of AqH AA concentrations on long-term corneal endothelial-cell loss after phacoemulsification.
In this study, no correlation was found between AL and AqH AA concentrations (partial.r = 0.032, P = 0.760), but in women there was a positive correlation between ACD and AqH AA concentrations (partial.r = 0.275, P = 0.009), which remained even after adjusting for age and plasma AA concentrations. Therefore, the fact that AqH AA concentrations are lower in women with short ACD may suggest that corneal endothelial cells are more susceptible to postoperative oxidative damage.
There has been no report so far on the association between ACD and AqH AA concentrations. AqH AA concentrations could be low in women with short ACD due to low transportation capacity of AA into the AqH. Recently, Ma et al.35 reported that in the human ciliary epithelium sodium-dependent AA transporter (SVCT) 2 is expressed only in the pigmented epithelium, and glucose transporter (GLUT) 1 is predominately expressed in the nonpigmented epithelium. This may explain why SVCT2 and GLUT1 are involved in the maintenance of higher AqH AA concentrations in humans. Further, Senthilkumari et al.1 reported that polymorphisms in the SVCT genes encoding SVCT1 and SVCT2 influenced AqH AA concentrations. Although the relationship between the ACD and SVCT genes needs to be confirmed in future studies, women with short ACD may have a genotype that lowers AqH AA concentrations.
A strength of this study was that AA was evaluated using high-performance liquid chromatography (HPLC)-electrochemical detection systems. HPLC-electrochemical detection systems have high sensitivity and specificity for AA analysis36. In humans, few studies on AqH AA concentrations have employed HPLC-electrochemical detection systems.
This study had some limitations. We did not measure dietary AA intake in the present study. Variation in dietary intake and the time interval between the collection of blood and AqH samples may have introduced bias. The time interval between the collection of blood and AqH samples was approximately 1 month or less. However, in both cases, we collected samples after the patients had fasted for at least 5 h in order to ensure uniformity in the collection conditions. Hence, dietary variation may have been negligible. Moreover, in this report, both AqH and plasma AA concentrations were at levels comparable to those reported by previous studies in Japanese, American, and European populations37,38,39. Further, patients receiving dialysis or with eating disorders, dementia, and systemic inflammatory diseases were excluded from this study; therefore, we did not include patients with conditions causing markedly-poor nutritional status. Another limitation of this study is that we could not determine whether short ACD directly caused decrease in AqH AA concentrations. The ACD is affected by other factors such as lens vault, zonular weakness, iris curvature, and iris thickness in addition to the AL19,20,21,40,41,42,43. Changes in the lens and the morphology of the iris may also affect AqH AA concentrations. Therefore, we plan to further investigate the relationship between lens vault, iris curvature, iris thickness, intraocular pressure (IOP), and AqH AA concentrations in the future.
In conclusion, there was a positive correlation between ACD and AqH AA concentrations in women, and AqH AA concentrations were lower in women with short ACD. This may suggest that women with short ACD have low reducing power in the AqH and could be more susceptible to oxidative damage.
Methods
This cross-sectional consecutive study was performed in accordance with the Declaration of Helsinki. It was approved by the institutional ethics review board of Dokkyo University Hospital (I-15-51). Informed consent was obtained from all participants.
Patients
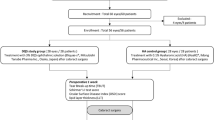
A total of 223 consecutive patients who visited Dokkyo Medical Hospital and other associated hospitals to undergo small incision cataract surgery from April 2017 to January 2018 were recruited. Patients with inherited cataract or trauma-related cataract, prior intraocular surgeries, prior laser treatment, congenital eye disease, corneal disease, acute infection, uveitis, acute angle closure glaucoma, primary angle closure glaucoma, primary open angle glaucoma, retinal disease, exfoliation syndrome, renal failure, eating disorders, dementia, and inflammatory systemic diseases were excluded from the study, as were those from whom we could not obtain more than 50 μL of AqH due to a shallow anterior chamber. Ultimately, 165 eyes of 97 patients were included. The included patients had normal IOP (defined as lower than 21 mm Hg) and were not using any topical or internal intraocular tension depressors. Moreover, we did not use capsule stabilisation devices or intraocular lens scleral suture fixation in the patients. The patient selection procedure and distribution of the study population are shown in Fig. 1.
Patients with inherited cataract or trauma-related cataract, prior intraocular surgeries, prior laser treatment, congenital eye disease, corneal disease, acute infection, uveitis, acute angle closure glaucoma, primary angle closure glaucoma, primary open angle glaucoma, retinal disease, exfoliation syndrome, renal failure, eating disorders, dementia, and inflammatory systemic diseases were excluded from the study, as were those from whom we could not obtain more than 50 μL of AqH due to a shallow anterior chamber. Ultimately, 165 eyes of 97 patients were included.
Clinical examination
All subjects underwent a thorough ophthalmic evaluation before cataract surgery. Uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were tested with Landolt C charts. IOP was measured with a non-contact tonometer (TONOREFII; Nidek Corp, Gamagori, Japan). Lens NS was graded with a slit lamp using the Emery-Little classification with scores ranging from 1 to 5. Corneal ECD was measured using specular microscopy (Nonconrobo FA-3509; Konan Medical, Hyogo, Japan). AL, ACD, and CCT were obtained using partial optical coherence interferometry (IOLMaster; Carl Zeiss Meditec AG, Jena, Germany).
AqH and blood samples
The sampling of AqH was performed by the surgeon who performed the cataract surgery. Patients undergoing surgery in both eyes may select a 5- or 7-day interval between the operations. The AqH was obtained under sterile conditions at the beginning of surgery after topical anaesthesia. First, the AqH was obtained by directly puncturing the corneal limbus with a 30-gauge needle attached to a disposable tuberculin syringe without touching the iris, lens, or corneal endothelium. An AqH sample of at least 50 μL was obtained from the periphery of the anterior chamber. Immediately after collection, the AqH was frozen at −20 °C using a cooling system (Corning® CoolBox™ M30 System; Corning, NY, USA) and transferred to the laboratory. The AqH samples were added to cold 10% metaphosphoric acid (MPA) and centrifuged at 15000 rpm for 15 min at 4 °C. The 50-μL samples were collected and stored at −80 °C until AA could be measured. The blood samples were drawn into collection tubes (Terumo Corporation, Tokyo, Japan) containing ethylenediaminetetraacetic acid (EDTA)−2Na as anticoagulant and centrifuged at 3000 rpm for 10 min at 4 °C. After centrifugation, 500-μL plasma was added at the exact same volume of cold 10% MPA and centrifuged at 15000 rpm for 15 min at 4 °C. Then, the 500-μL supernatant was stored at −80 °C until use. Both AqH and blood samples were collected at least 5 h after the last meal.
Determination of AA
AA was analysed using an HPLC-electrochemical detection method. The samples were treated as previously reported36. Detection was performed with a Waters 2695 separations module coupled with a Waters 2465 electrochemical detector (Nihon Waters, Tokyo, Japan). After thawing, the samples were reduced with 35 mM tris (2-carboxyethyl) phosphine hydrochloride for 2 h on ice. After reduction, the reaction mixture was analysed for total AA with an HPLC-electrochemical detection method. Separation was performed on an Atlantis dC18 5-μm column (4.6 × 150 mm) combined with an Atlantis dC18 5-μm guard column (4.6 × 20 mm) (Nihon Waters, Tokyo, Japan). The mobile phase comprised 50 mM phosphate buffer (pH 2.8), 540 μM EDTA, and 2% methanol. The flow rate was 1.3 mL/min, and electrical signals were recorded using an electrochemical detector with a glassy carbon electrode at +0.6 V. Representative HPLC-electrochemical detection chromatograms are shown in Fig. 2.
Representative high-performance liquid chromatography -electrochemical detection chromatograms of AA, metaphosphoric acid (MPA)/ethylenediaminetetraacetic acid (EDTA), and tris (2-carboxyethyl) phosphine hydrochloride (TCEP). (A) AA standard solution (5.7 μM) in 5% MPA/EDTA reduced by 35mM TCEP. (B) 5% MPA/EDTA. (C) Aqueous humour sample in 5% MPA/EDTA reduced by 35 mM TCEP for 2 h on ice.
Statistical analyses
Statistical analyses were performed using software (SPSS 24.0; IBM Corp., Armonk, NY, USA). The data are expressed as mean ± SD. A P value less than 0.05 was considered statistically significant. The normality of data distribution was tested with histograms and the Shapiro-Wilk test. Categorical data were assessed using the Mann-Whitney U test, and continuous variables were assessed using independent Student’s t-tests. Pearson’s correlation coefficient was calculated for normally-distributed data. If the data distribution was not normal, Spearman correlation analyses were used. To examine the relationship between ACD and AqH AA concentration, partial correlation coefficients were calculated for statistical adjustment of covariates (age and plasma AA concentration).
Data Availability Statement
The corresponding author had full access to all the data in the study and all authors shared final responsibility for the decision to submit for publication.
References
Senthilkumari, S. et al. Polymorphisms in sodium-dependent vitamin C transporter genes and plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp. Eye Res 124, 24–30 (2014).
Huang, W., Koralewska-mak, A. & Bauer, B. Extracellular glutathione peroxidase and ascorbic acid in aqueous humor and serum of patients operated on for cataract. Clin. Chim. Acta. 261, 117–130 (1997).
Taylor, A. et al. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr. Eye Res. 16, 857–64 (1997).
Reiss, G. R., Werness, P. G., Zollman, P. E. & Brubaker, R. F. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch. Ophthalmol. (Chicago, Ill. 1960) 104, 753–5 (1986).
Ringvold, A. Aqueous humour and ultraviolet radiation. Acta Ophthalmol. 58, 69–82 (1980).
Ringvold, A. The significance of ascorbate in the aqueous humour protection against UV-A and UV-B. Exp. Eye Res. 62, 261–4 (1996).
Yue, B. Y., Higginbotham, E. J. & Chang, I. L. Ascorbic acid modulates the production of fibronectin and laminin by cells from an eye tissue-trabecular meshwork. Exp. Cell Res. 187, 65–8 (1990).
Higginbotham, E., Yue, B. Y., Crean, E. & Peace, J. Effects of ascorbic acid on trabecular meshwork cells in culture. Exp. Eye Res. 46, 507–16 (1988).
Reddy, V. N., Giblin, F. J., Lin, L. R. & Chakrapani, G. The effect of aqueous humor ascorbate on ultraviolet B induced DNA damage in lens epithelium. Invest. Ophthalmol. Vis. Sci. 39, 344–350 (1998).
Rubowitz, A., Assia, E. I., Rosner, M. & Topaz, M. Antioxidant Protection against Corneal Damage by Free Radicals during Phacoemulsification. Invest. Ophthalmol. Vis. Sci. 44, 1866–1870 (2003).
Talluri, R. S., Katragadda, S., Pal, D. & Mitra, A. K. Mechanism of L-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr. Eye Res. 31, 481–9 (2006).
Nemet, A. Y., Assia, E. I., Meyerstein, D. & Meyerstein, N. Protective effect of free-radical scavengers on corneal endothelial damage in phacoemulsification. J. Cataract Refract. Surg. 33, 310–315 (2007).
Geffen, N., Topaz, M., Kredy-farhan, L. & Barequet, I. S. Phacoemulsification-induced injury in corneal endothelial cells mediated by apoptosis: In vitro model. J. Cataract Refract. Surg. 34, 2146–2152 (2008).
Takahashi, H. et al. Free radicals in phacoemulsification and aspiration procedures. Arch. Ophthalmol. (Chicago, Ill. 1960) 120, 1348–52 (2002).
Walkow, T., Anders, N. & Klebe, S. Endothelial cell loss after phacoemulsification: Relation to preoperative and intraoperative parameters. J. Cataract Refract. Surg. 26, 727–732 (2000).
Storr-Paulsen, A., Norregaard, J. C., Ahmed, S., Storr-Paulsen, T. & Pedersen, T. H. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J. Cataract Refract. Surg. 34, 996–1000 (2008).
Hayashi, K., Hayashi, H. & Nakao, F. Risk factors for corneal endothelial injury during phacoemulsification. J. Cataract Refract. Surg. 22, 1079–1084 (1996).
Dick, H. B., Kohnen, T., Jacobi, F. K. & Jacobi, K. W. Long-term endothelial cell loss following phacoemulsification through a temporal clear corneal incision. J. Cataract Refract. Surg. 22, 63–71 (1996).
Hosny, M., Alio, J. L., Claramonte, P., Attia, W. H. & Perez-Santonja, J. J. Relationship between anterior chamber depth, refractive state, corneal diameter, and axial length. J. Refract. Surg. 16, 336–40 (2000).
Fernández-Vigo, J. I. et al. Determinants of anterior chamber depth in a large Caucasian population and agreement between intra-ocular lens Master and Pentacam measurements of this variable. Acta Ophthalmol. 94, 150–155 (2016).
Jonas, J. B. et al. Anterior chamber depth and its associations with ocular and general parameters in adults. Clin. Experiment. Ophthalmol. 40, 550–556 (2012).
Cho, Y. K., Chang, H. S. & Kim, M. S. Risk Factors for Endothelial Cell Loss after Phacoemulsification: Comparison in Different Anterior Chamber Depth Groups. Korean J Ophthalmol 24, 10–15 (2010).
Reuschel, A., Bogatsch, H., Oertel, N. & Wiedemann, R. Influence of anterior chamber depth, anterior chamber volume, axial length, and lens density on postoperative endothelial cell loss. Graefes Arch Clin Exp Ophthalmol 253, 745–752 (2015).
Brubaker, R. F., Bourne, W. M., Bachman, La & McLaren, J. W. Ascorbic acid content of human corneal epithelium. Invest. Ophthalmol. Vis. Sci. 41, 1681–3 (2000).
Ringvold, A., Anderssen, E. & Kjønniksen, I. Distribution of ascorbate in the anterior bovine eye. Investig. Ophthalmol. Vis. Sci. 41, 20–23 (2000).
Oreopoulos, D. G. et al. Renal excretion of ascorbic acid: effect of age and sex. J. Am. Coll. Nutr. 53, 77–85 (1983).
Chu, T. & Candia, O. A. Active Transport of Ascorbate Across the Isolated Rabbit Ciliary Epithelium. Invest Ophthalmol Vis Sci 29, 594–599 (1988).
Socci, R. R. & Delamere, N. A. Characteristics of ascorbate transport in the rabbit iris-ciliary body. Exp. Eye Res. 46, 853–61 (1988).
Čanadanović, V., Latinović, S., Barišić, S., Babić, N. & Jovanović, S. Age-related changes of vitamin С levels in aqueous humour. Vojnosanit. Pregl. 72, 823–826 (2015).
Bode, A. M., Vanderpool, S. S., Carlson, E. C., Meyer, D. A. & Rose, R. C. Ascorbic acid uptake and metabolism by corneal endothelium. Invest. Ophthalmol. Vis. Sci. 32, 2266–71 (1991).
Winterbourn, C. C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 (2008).
Yue, B. Y., Niedra, R. & Baum, J. L. Effects of ascorbic acid on cultured rabbit corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 19, 1471–6 (1980).
Reddy, T. S., Varnell, E. D., Beuerman, R. W., Bazan, N. G. & Kaufman, H. E. Endothelial cell damage in human and rabbit corneas stored in K-sol without antioxidants. Br. J. Ophthalmol. 73, 803–808 (1989).
De Biaggi, C. P., Barros, P. S. M., Silva, V. V., Brooks, D. E. & Barros, S. B. M. Ascorbic acid levels of aqueous humor of dogs after experimental phacoemulsification. Vet. Ophthalmol. 9, 299–302 (2006).
Ma, N. et al. Expression profiling of ascorbic acid-related transporters in human and mouse eyes. Investig. Ophthalmol. Vis. Sci. 57, 3440–3450 (2016).
Sato, Y. et al. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol. Pharm. Bull. 33, 364–369 (2010).
Saito, K. et al. A Significant Relationship between Plasma Vitamin C Concentration and Physical Performance among Japanese Elderly Women. J Gerontol A Biol Sci Med Sci. 67, 295–301 (2012).
Simon, J. A. & Hudes, E. S. Serum Ascorbic Acid and Other Correlates of Self-Reported Cataract Among Older Americans. J Clin Epidemiol 52, 1207–1211 (1999).
Woodside, J. V. et al. Factors associated with serum / plasma concentrations of vitamins A, C, E and carotenoids in older people throughout Europe: the EUREYE study. Eur J Nutr 52, 1493–1501 (2013).
Leung, C. K. et al. Comparisons of anterior segment biometry between Chinese and Caucasians using anterior segment optical coherence tomography. Br. J. Ophthalmol. 94, 1184–1189 (2010).
Sng, C. C. et al. Determinants of Anterior Chamber Depth: The Singapore Chinese Eye Study. Ophthalmology 119, 1143–1150 (2012).
Wang, D., Qi, M., He, M., Wu, L. & Lin, S. Ethnic Difference of the Anterior Chamber Area and Volume and Its Association with Angle Width. Invest Ophthalmol Vis Sci 53, 3139–3144 (2012).
Chen, Y., Bao, Y. & Pei, X. Morphologic changes in the anterior chamber in patients with cortical or nuclear age-related cataract. J. Cart. Refract. Surg. 37, 77–82 (2010).
Acknowledgements
This study was supported by Young Investigator Award No. 2016-14 from Dokkyo Medical University. The funding organisation had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
S.I. designed the project, performed experiments, and wrote the manuscript. T.S. performed statistical analyses. T.M. collected the clinical data. Y.T., Y.K. and K.M. performed experiments. G.K. performed statistical analyses. A.I. performed experiments and supervised the project. T.S. performed operations and supervised the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ito, S., Sairenchi, T., Machida, T. et al. Reduced aqueous humour ascorbic-acid concentration in women with smaller anterior chamber depth. Sci Rep 9, 372 (2019). https://doi.org/10.1038/s41598-018-36899-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36899-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.