Abstract
Stimulated by strong demand for thermal expansion control from advanced modern industries, various giant negative thermal expansion (NTE) materials have been developed during the last decade. Nevertheless, most such materials exhibit anisotropic thermal expansion in the crystal lattice. Therefore, strains and cracks induced during repeated thermal cycling degrade their performance as thermal-expansion compensators. Here we achieved giant isotropic NTE with volume change exceeding 3%, up to 4.1%, via control of the electronic configuration in Sm atoms of SmS, (4 f)6 or (4 f)5(5d)1, by partial replacement of Sm with Y. Contrary to NTE originating from cooperative phenomena such as magnetism, the present NTE attributable to the intra-atomic phenomenon avoids the size effect of NTE and therefore provides us with fine-grained thermal-expansion compensators, which are strongly desired to control thermal expansion of microregions such as underfill of a three-dimensional integrated circuit. Volume control of lanthanide monosulfides via tuning of the 4 f electronic configuration presents avenues for novel mechanical functions of a material, such as a volume-change driven actuator by an electrical field, which has a different drive principle from those of conventional strain-driven actuators such as piezostrictive or magnetostrictive materials.
Similar content being viewed by others
Introduction
Successive discoveries of giant negative thermal expansion (NTE) materials during the last decade are causing a paradigm shift in the field of thermal expansion control1,2. Approaches using phase transitions3,4,5,6,7,8,9,10,11 achieved negative coefficients of linear thermal expansion (negative α) that are several times to ten times as large as those of conventional materials12,13,14,15,16, in which NTE originates from a characteristic of the crystal structure. Recently, the largest total volume change related to NTE, ΔV/V = 8.5%, was reported for Pb0.76La0.04Bi0.20VO311. Extremely high NTE of α = −115 × 10−6 K−1 and total volume change ΔV/V = 6.7% are also realized in layered ruthenium oxides by the microstructural effects of a sintered body17.
These giant NTE materials enable us to compensate the thermal expansion of plastics18,19,20,21, which has been difficult to date. The technology of thermal expansion control is now being innovated. However, these “phase-transition-type” giant NTE materials are mostly anisotropic. Therefore, strains and defects are induced in the materials during repeated thermal cycling, thereby degrading the reproducibility of NTE functions and mechanical strength. To avoid these difficulties, isotropic NTE materials are strongly desired. A promising candidate is samarium monosulfide SmS, which exhibits large volume change exceeding 7% according to the 4 f electronic configuration in the Sm atom22. Two electronic configurations of Sm in SmS, (4 f)6 and (4 f)5(5d)1, compete energetically. At ambient pressure, the former configuration is more stable and the system is in the larger volume insulating state in black color (black phase). However, at the low pressure of 6 kbar, the latter configuration becomes more stable and the system switches to the smaller volume metallic state with golden color (golden phase)23,24. The cubic rock salt structure is preserved in this phase transition. Actually, this phase transition can be induced also by partial replacement of Sm by other elements. The smaller-volume golden phase appears at the higher temperature (T) side25,26,27,28,29,30. Results of an earlier x-ray diffraction (XRD) study suggest that volume contraction on heating reaches 3% in the case of Sm1−xYxS25.
Samarium monosulfide has a long history of basic physics research related to its valence fluctuation23,24,25,26,27,28,29,30,31. Recently it has attracted great attention from the viewpoint of an excitonic insulator32 and a heavy-fermion metamaterial33. Nevertheless, few studies have examined it as a functional material showing NTE. The potential of a thermal-expansion compensator has yet been addressed only scarcely. We explored the thermal expansion properties of single-crystalline Sm1−xYxS using XRD and dilatometry measurements. The present results revealed details of the compositional dependence of NTE in this solid-solution system and shed light on the peculiar mechanism of broadening volume change relevant to NTE. This report presents discussion of the rich potential possessed by the intra-atomic charge transfer, which is proposed as a volume-control principle for novel mechanical functions of materials.
Results
Substitution of Y for Sm induces the phase transition from a larger-volume black phase to a smaller-volume golden phase at ambient pressure25,26,27,28,29,30. This transition was confirmed also from results of the present study. Figure 1 portrays the XRD pattern of Sm1−xYxS measured at room temperature (295 K) using Cu Kα radiation. For all Y concentrations, no peaks attributable to impurities are detected. All the observed peaks can be indexed based on its cubic rock salt structure with Fm\(\bar{3}\)m symmetry. The inset is an enlarged figure of the 200 peak for x = 0 and 0.22. The peak widths are almost identical in doped and non-doped SmS, indicating that Sm and Y atoms are dispersed homogeneously. From x = 0.20 to x = 0.22, peak positions shift rapidly to higher angles, suggesting that the system undergoes the phase transition to the smaller-volume golden phase at 295 K and ambient pressure. The phase boundary is almost identical to the results described above.
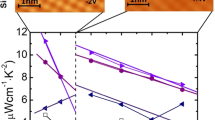
Figure 2a displays linear thermal expansion ΔL(T)/L of single-crystalline Sm1−xYxS. Negative thermal expansion corresponds to the transition from black to golden phase. Solid and open circles respectively represent data collected for warming and cooling processes. In general, hysteresis is not remarkable, although it is about ΔT = 10 K at maximum for x = 0.22. Below the onset temperature Ton = 290 K, giant NTE of α = −60 × 10−6 K−1 appears for x = 0.22. The total volume change related to NTE, ΔV/V, reaches 3.4% at 100−290 K, which is comparable to the lattice-parameter change exceeding 1% between 140 K and 375 K estimated from the previous XRD analysis for x = 0.2525. Among isotropic NTE materials, the present volume change is the largest: much greater than the former record of 2.7% in ZrW2O812 (Table 1). Additionally, it is noteworthy that the operating-temperature window is exceptionally wide (ΔT >190 K) for phase-transition-type NTE materials. Reports of earlier studies have described that the system becomes metallic immediately by Y doping30. The present resistivity ρ(T) data shown in the inset of Fig. 2a are consistent with data found in earlier works.
Thermal expansion properties of Sm1−xYxS. (a) Linear thermal expansion ΔL/L of Sm1−xYxS single crystals (reference temperature: 500 K). Data were collected on warming (solid circles) and cooling (open circles) processes using a laser interference dilatometer. Inset: Temperature dependence of resistivity ρ of Sm1−xYxS. (b) “Dilatometric” lattice parameters, aD, estimated from the room-temperature x-ray diffraction (Fig. 1) and dilatometry (a) measurements, assuming that the cubic crystal structure is preserved in the whole temperature range.
We analyzed the crystal structures of Sm0.78Y0.22S using synchrotron x-ray diffractometry to ascertain how the crystal lattice changes related to the giant NTE (Fig. 3a). The structural parameters are refined using the Rietveld method by RIETAN-FP34 (Supplementary Fig. S1). At T = 250−500 K, the system preserved the single phase of cubic rock salt structure with Fm\(\bar{3}\)m symmetry. However, at temperatures below 225 K, it is apparently separated into two peaks. Therefore, we conducted a Rietveld analysis assuming two phases with the same cubic crystal structure but a different lattice constant, i.e., the larger-volume L phase and the smaller-volume S phase. The higher-T diffraction data above 250 K are continuous with the S phase. Each phase preserves the same cubic structure of Fm\(\bar{3}\)m symmetry. Figure 3b displays the respective T-dependent fractions of the L and S phases obtained by Rietveld analysis: vL and vS. At temperatures higher than 250 K, Rietveld analysis assuming a single phase was conducted (vL = 0 and vS = 1) because no significant difference was found between analysis assuming two-phase coexistence and that assuming a single phase. At temperatures lower than 225 K, vL increases concomitantly with decreasing T. Figure 3c displays the respective lattice parameters of the L and S phases: aL and aS. aL increases concomitantly with decreasing T (i.e., aL shows NTE). It elongates to 5.7832(2) Å at 180 K, which is 1.6% elongation compared with the lattice parameter at 300 K (just above Ton = 290 K), 5.6949(2) Å. However, aS decreases continuously, despite the phase separation, with decreasing T down to 200 K. Impressively, it turns upward with further cooling, increasing from 5.6909(2) Å at 200 K to 5.6965(2) Å at 180 K (i.e., aS shows NTE at T = 180−200 K). Figure 3c displays aL, aS, and their averaged sum, aav = vLaL + vSaS. At 200 K, for example, aL = 5.6909(2) Å, aS = 5.7770(3) Å, vL = 0.831, and vS = 0.169. As a result, aav is calculated to be 5.705 Å. For comparison, it also shows the “dilatometric” lattice parameter aD (Fig. 2b), estimated based on dilatometry data and the room-temperature (295 K) lattice parameter, assuming that the cubic structure is preserved over the whole T region. Here aav = aS at T = 250−500 K because vL is zero in this region. aav is almost identical to aD, implying that NTE of the bulk crystal originates from NTE of crystallographic unit cells. It differs from the giant NTE in Ca2RuO4 ceramics, in which microstructural effects in a sintered body play an important role17,21. We also conducted Rietveld analysis by assuming three phases (Supplementary Fig. S2). The reliability factor improves from Rwp = 2.76%, Rp = 2.22% and S = 1.25 for two phase analysis to Rwp = 2.60%, Rp = 2.09% and S = 1.17 by assuming three phases, but it is natural because the fitting parameters increase. Rather, probably two-phase analysis is fairly good as long as the difference is of this order of magnitude. In the three-phase analysis, the coincidence between aav and aD improves. In this case aav is defined to be Σviai (vi and ai being respectively the fraction and the lattice parameter of the phase i obtained by Rietveld analysis and Σvi = 1). Therefore, the core part of the arguments – anomaly in the crystallographic unit-cell volume is relevant to NTE – is not change even if we adopt a three-phase analysis.
Temperature dependence of the crystallographic parameters. The parameters are ascertained from Rietveld analysis results (see Supplementary Fig. S1) (a) Temperature dependence of the 200 peak in the x-ray diffraction measurements for Sm0.78Y0.22S using synchrotron radiation (λ = 0.6521 Å). (b) Fraction of the larger-volume L and smaller-volume S phases: vL and vS. (c) Lattice parameter of the L and S phases: aL and aS. The averaged lattice parameter aav = vLaL + vSaS, and the “dilatometric” lattice parameter aD, presented in Fig. 2b, are also shown.
Phase-transition-type NTE materials are broadly divisible into two categories: “phase-separation” type and “second-order-transition” type. In the former category, which includes bismuth-nickel oxides9,19, the system separates into the larger-volume L phase and the smaller-volume S phase, while preserving the first-order phase transition. NTE is induced because the fraction of the L phase increases concomitantly with decreasing T. In that case, it is important that no phase exhibit NTE. This class of phase-transition-type NTE materials is, so to speak, in a state to be described as “homocomposite”, in the sense that it is divided into domains with different physical properties such as lattice volume though chemically homogeneous. In the latter category, which includes magnetic NTE materials such as antiperovskite manganese nitrides3, the phase transition changes from first-order-like to second-order-like. The volume increases gradually with decreasing T without phase separation. It is noteworthy that Sm1−xYxS has both characters. Although the large NTE of Sm1−xYxS is dominated by phase separation between the L and S phases, each phase shows NTE below a certain temperature. The smaller hysteresis in Sm1−xYxS than in the bismuth-nickel oxides19 is partly explainable by this duality. The peculiarity of the phase-separation behavior is later discussed.
Figure 2b presents the T and Y-concentration dependence of aD for Sm1−xYxS. This result is reminiscent of past findings related to Y-concentration dependence of the boundary temperature T* between the black and golden phases in Sm1−xYxS26. In that study, the golden phase suddenly appears at around x = 0.15; the T*(x) curve is almost vertical there. Actually, T*(x), which is highest (600 K) at around x = 0.15, instantly decreases to 250 K by further Y doping, and subsequently decreases slowly, concomitantly with increasing x. It becomes about 200 K for x = 0.3. Systems are, respectively, in the golden and black phases above and below T*. Past x-ray absorption studies have indicated that this black-to-golden phase transition results from intra-atomic charge transfer from 4 f to 5d orbitals in Sm atom29. In the present experiments, the onset temperature of NTE, Ton, exceeds 500 K for x = 0.20, decreases rapidly to 290 K for x = 0.22, and decreases slowly for additional Y doping. Present correlations between T* and Ton imply that the giant NTE observed in this study originates from the lattice volume change because of the intra-atomic charge transfer in Sm atoms.
Discussion
The unit-cell volume of SmS is related closely to the number of 4 f electrons in a Sm atom. Yttrium is a useful dopant for tuning the Sm 4 f electronic states in SmS. However, a Y atom in Sm1−xYxS donates one electron to the d band. In addition, the 4 f electronic state is apparently quite sensitive to Y.
Additional electrons donated by Y dopants might prevent us from realizing the full charge transfer related to NTE in SmS. In the case of pressure-induced golden phase, the charge transfer in Sm is almost one [(4 f)6 → (4 f)5]24. This charge transfer induces large volume change up to 7%. However, the intra-atomic charge transfer caused by temperature in Sm1−xYxS is much smaller. An earlier x-ray absorption study revealed that the charge transfer from 10 K to 300 K as 0.13 [(4 f)5.65 → (4 f)5.52] for Sm0.67Y0.33S29. This might be true presumably because the electronic state near the Fermi level is altered by the electrons donated by Y.
The excessive sensitivity of Y produces a wide blank region between x = 0.20 and x = 0.22 (Fig. 2b). As Y doping proceeds, the bottom of the 5d band lowers. Around this concentration, it fully overlaps with the 4 f level and the charge transfer from 4 f to 5d levels occurs intensely25. For Sm0.80Y0.20S, in which Ton exceeds 500 K, the black phase is yet stable. The volume does not contract so much, even at high temperatures, resulting in weak NTE (α = −12 × 10−6 K−1 at 230−500 K). However, a slight amount of additional Y dopants drastically alters the electronic state and the golden phase is stabilized. As a result, for x = 0.22, Ton decreases to 290 K. The volume does not expand greatly, even at low T, compared to x = 0.20.
In addition, the excessive sensitivity induces phase separation in the critical composition x = 0.22. In general, inhomogeneity in atomic scale is intrinsically unavoidable in a solid solution no matter how high the quality to which we form it. The Sm atom in Sm1−xYxS has twelve nearest-neighbor Sm(Y) atoms and these twelve sites are randomly occupied by Sm with a probability of 0.78 and by Y with a probability of 0.22 for x = 0.22, for example. It is important that in the present system such an atomic fluctuation may produce a large difference in lattice parameter because of the sensitivity. The phase separation in the present XRD analysis reflects this peculiarity of Sm1−xYxS, which might be qualitatively different from the usual chemical phase separation. This unique phase separation phenomenon is a difficulty that is related directly to improvement of NTE properties. This study shed light on important subjects to pursue as possibilities of this monosulfide for use as a thermal-expansion compensator. The phase separation phenomena should be explored in detail in future studies.
We have experimentally obtained results predicting that optimization of tuning of the Sm 4 f electronic state engenders higher performance of the NTE function. In the critical composition x = 0.22, sample dependence was observed in the dilatometry result. A crystal showing a larger total volume change than the one discussed above was also found (Fig. 4a). In this crystal, giant NTE of α = −65 × 10−6 K−1 appears below Ton = 315 K and ΔV/V reaches 4.1%, which is among the largest of all the NTE materials reported to date. Although the XRD pattern of this crystal measured at 295 K using Cu Kα radiation is similar to that of #1 presented in Fig. 1, the pattern obtained from high-resolution synchrotron x-ray diffractometry clearly indicates the presence of multiple divided peaks (Supplementary Fig. S3). The XRD pattern can be refined roughly to obtain the averaged lattice parameter aav by assuming multiple cubic phases with the same Fm\(\bar{3}\)m symmetry and different lattice parameters obtained by Rietveld analysis (aav = Σviai). Considering that the data reproducibility is good for x = 0.27 (Fig. 4b), the present result might be ascribed to sensitivity of the electronic state to Y in the critical composition rather than to a different degree of the chemical inhomogeneity.
Sample dependence of linear thermal expansion for Sm1−xYxS. (a) x = 0.22 and (b) x = 0.27 (reference temperature: 500 K). For each composition, the data labeled as #1 are also presented in Fig. 2a. There exists sample dependence in the thermal expansion data for the critical composition x = 0.22, although the data are reproducible for x = 0.27. We found the largest total volume change related to negative thermal expansion, ΔV/V ~ 4.1%, for the crystal labeled as #2 for x = 0.22, but x-ray diffraction peaks of this crystal are multiply splitting at low temperatures (see Supplementary Fig. S3), which suggests that the lattice parameter is highly fluctuating within the crystal.
Although fine tuning of the Sm 4 f electronic state might be difficult to achieve solely by Y doping, the samarium monosulfides have many routes to control the electronic states. Elements other than Y have also been regarded as dopants. To date, as a single dopant, Y is superior in terms of the operating temperature and the total volume change related to NTE ΔV/V24,26, but simultaneous doping of Y and other elements might solve this sensitivity difficulty. Another important avenue is greater use of the S site35. High-T crystal growth of SmS in principle might induce nonstoichiometry of sulfur. An earlier study we conducted using the same grade of the present crystals30,31,32,33 revealed that no fluctuation exists in the sulfur content that affects the systematic characteristics of the data. However, sulfur content and substitution of sulfur sites are important in the future tuning of NTE properties. Optimization of these parameters might fill in the blank region between x = 0.20 and x = 0.22 and provide a giant NTE with larger volume change and higher Ton. The present experiment suggests that large volume change ΔV/V of 4.8% (equivalent to Δa/a = 1.6%) is possible if the L phase can be purified (see the temperature dependence of aL depicted in Fig. 3c).
Because it is an atomic phenomenon related to the 4 f electronic configuration of the Sm atom, NTE of the samarium monosulfides presents potential advantages over NTE of existing materials. First, isotropy can be an important property for a thermal-expansion compensator in the sense that NTE function is reproducible without defects or strains against repeated thermal cycling. At present, for the critical concentration around at x = 0.22 showing large NTE, some crystals are broken during repeated thermal cycling, which indicates that internal strain exists because of the effect of phase separation. The potential advantage of isotropy has not been realized yet. This tendency is not observed for the Y concentrations deviating from x = 0.22. Therefore, this difficulty originates from the sensitivity of the Y dopant. It should be solved by optimizing the 4 f electronic state using simultaneous doping of Y and other elements. Next, NTE originating from atomic phenomena is independent of the grain size. For that reason, the difficulty that NTE function depends strongly on the grain size, characteristics of manganese nitrides36, can be avoided. Recently, fine-grained thermal-expansion compensators with submicrometer grain size are sought for use in many fields of industry, including electronics37, to control the thermal expansion of microregions. Finally, the wide operating-temperature window of Sm1−xYxS might be attributable to a characteristic of the atomic phenomena. For cooperative phenomena such as magnetic and charge orderings, it is difficult to make, artificially, the gradual phase transition, which is the cause of the narrow operating-temperature window of the phase-transition-type NTE materials. By contrast, the phase transition related to atomic phenomena is expected to be susceptible to external perturbation. Thereby, the operating-temperature window would be expanded.
Volume control of lanthanide monosulfides via tuning of the 4 f electronic configuration (intra-atomic charge transfer) opens avenues towards novel mechanical functions of a material. Although similar concepts have been suggested to date for Sm2.75C6038 and YbGaGe39, the monosulfides are absolutely attractive in terms of variation in materials and in terms of their physical properties22. For example, an electrical field might induce the metallic phase because the insulating black phase and the metallic golden phase compete energetically40. This field-induced transition might be accompanied by large volume change at largest 7%. These are useful as a volume-change driven actuator, which has a different drive principle from conventional strain-driven actuators such as piezostrictive or magnetostrictive materials.
Methods
Sample preparation
Single crystals of Sm1−xYxS were grown using Bridgeman method31. Powders of Sm, Y, and S (99.9% or higher purity) weighed at appropriate molar ratios were mixed in a glove box and were sealed in a quartz tube under vacuum (<10−3 Pa). The quartz ampoule was heated at a temperature of T = 873 K for 6 h and was then cooled to 573 K for 24 h. The obtained powder was reground and was then reheated in the same condition. Finally, the obtained powder was reground and was sealed in a tungsten crucible (15 mm diameter, 75 mm long) under vacuum (<10−3 Pa) using an electron beam welding system. The sealed tungsten crucible was heated with an induction heating furnace up to 2453 K for 20 h, held for 2 h, then cooled to 2173 K for 24 h and cooled to 1073 K. Subsequently, the furnace was switched off. We analyzed the compositional ratio between samarium and yttrium using inductively coupled plasma (ICP) method. The obtained crystals were identified as the monosulfide from x-ray powder diffraction measurements at room temperature with Cu Kα radiation (Rint2000; Rigaku Corp.).
Measurements of physical properties
Detailed crystallographic analyses were conducted using synchrotron x-ray powder diffractomety at 180−500 K at Aichi Synchrotron Radiation Center with synchrotron radiation of λ = 0.6521 Å. Linear thermal expansion ΔL(T)/L was measured using a rectangular crystal (typically 5 × 5 × 12 mm3) by means of a laser-interference dilatometer (LIX-2; Ulvac, Inc.) at T = 100−500 K. Several specimens broke into pieces during repeated thermal cycling because of the large volume change. In such cases, we used pressed powder without sintering to measure the linear thermal expansion. For some compositions, we confirmed that the data obtained for the pressed samples are equivalent to those obtained for the single crystals.
Data Availability
Data supporting the findings of this study are available from the corresponding author on request.
References
Takenaka, K. Negative thermal expansion materials: Technological key for control of thermal expansion. Sci. Technol. Adv. Mater. 13, 013001 (2012).
Chen, J., Hu, L., Deng, J. X. & Xing, X. R. Negative thermal expansion in functional materials: Controllable thermal expansion by chemical modifications. Chem. Soc. Rev. 44, 3522–3567 (2015).
Takenaka, K. et al. Magnetovolume effects in manganese nitrides with antiperovskite structure. Sci. Technol. Adv. Mater. 15, 015009 (2014).
Fujita, A., Fujieda, S., Fukamichi, K., Mitamura, H. & Goto, T. Itinerant-electron metamagnetic transition and large magnetovolume effects in La(FexSi1−x)13 compounds. Phys. Rev. B 65, 014410 (2001).
Wang, C. et al. Tuning the range, magnitude, and sign of the thermal expansion in intermetallic Mn3(Zn, M)xN (M=Ag, Ge). Phys. Rev. B 85, 220103R (2012).
Huang, R. J. et al. Giant negative thermal expansion in NaZn13-type La(Fe, Si, Co)13 compounds. J. Am. Chem. Soc. 135, 11469–11472 (2013).
Zhao, Y. Y. et al. Giant negative thermal expansion in bonded MnCoGe-based compounds with Ni2In-type hexagonal structure. J. Am. Chem. Soc. 137, 1746–1749 (2015).
Long, Y. W. et al. Temperature-induced A-B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature 458, 60–63 (2009).
Azuma, M., Oka, K. & Nabetani, K. Negative thermal expansion induced by intermetallic charge transfer. Sci. Technol. Adv. Mater. 16, 034904 (2015).
Pan, Z. et al. Colossal volume contraction in strong polar perovskites of Pb(Ti, V)O3. J. Am. Chem. Soc. 139, 14865–14868 (2017).
Yamamoto, H., Imai, T., Sakai, Y. & Azuma, M. Colossal negative thermal expansion in electron-doped PbVO3 perovskites. Angwe. Chem. Int. Ed. 57, 8170–8173 (2018).
Evans, J. S. O. Negative thermal expansion materials. J. Chem. Soc., Dalton Trans., 3317–3326 (1999).
Tucker, M. G. et al. Negative thermal expansion in ZrW2O8: Mechanisms, rigid unit modes, and neutron total scattering. Phys. Rev. Lett. 95, 255501 (2005).
Phillips, A. E., Goodwin, A. L., Halder, G. J., Southon, P. D. & Kepert, C. J. Nanoporosity and exceptional negative thermal expansion in single-network cadmium cyanide. Angew. Chem. Int. Ed. 47, 1396–1399 (2008).
Attfield, J. P. A fresh twist on shrinking materials. Nature 480, 465–466 (2011).
Chen, J. et al. Tunable thermal expansion in framework materials through redox intercalation. Nat. Commun. 8, 14441, https://doi.org/10.1038/ncomms14441 (2017).
Takenaka, K., Okamoto, Y., Shinoda, T., Katayama, N. & Sakai, Y. Colossal negative thermal expansion in reduced layered ruthenate. Nat. Commun. 8, 14102, https://doi.org/10.1038/ncomms14102 (2017).
Takenaka, K. & Ichigo, M. Thermal expansion adjustable polymer matrix composites with giant negative thermal expansion filler. Compos. Sci. Technol. 104, 47–51 (2014).
Nabetani, K. et al. Suppression of temperature hysteresis in negative thermal expansion compound BiNi1−xFexO3 and zero-thermal expansion composite. Appl. Phys. Lett. 106, 061912 (2015).
Lin, J. C. et al. The GaNMn3-Epoxy composites with tunable coefficient of thermal expansion and good dielectric performance. Compos. Sci. Technol. 146, 177–182 (2017).
Takenaka, K. et al. Giant negative thermal expansion in Fe-doped layered-ruthenate ceramics. Appl. Phys. Express 10, 115501 (2017).
Cox, P. A. The Electronic Structure and Chemistry of Solids. Oxford University Press (Oxford, 1987).
Jayaraman, A., Narayanamurti, V., Bucher, E. & Maines, R. G. Continuous and discontinuous semiconductor-metal transition in samarium monochalcogenides under pressure. Phys. Rev. Lett. 25, 1430–1433 (1970).
Deen, P. P. et al. Structural and electronic transitions in the low-temperature, high-pressure phase of SmS. Phys. Rev. B 71, 245118 (2005).
Jayaraman, A., Dernier, P. & Longinotti, L. D. Study of valence transition in SmS induced by alloying, temperature, and pressure. Phys. Rev. B 11, 2783–2794 (1975).
Tao, L. J. & Holtzberg, F. Valence transition of Sm in monosulfide solid-solutions. Phys. Rev. B 11, 3842–3849 (1975).
Jayaraman, A. & Maines, R. G. Study of the valence transition in Eu-, Yb-, and Ca-substituted SmS under high pressure and some comments on other substitutions. Phys. Rev. B 19, 4154–4161 (1979).
Schefzyk, R., Steglich, F., Penny, T. & Holtzberg, F. thermal expansion of the intermediate valence system Sm1−xYxS. J. Magn. Magn. Mater. 54-57, 341–342 (1986).
Alekseev, P. A. et al. Magnetic spectral response and lattice properties in mixed-valence Sm1−xYxS solid solutions studied with X-ray diffraction, X-ray absorption spectroscopy, and inelastic neutron scattering. Phys. Rev. B 74, 035114 (2006).
Imura, K. et al. Origin of the black-golden transition in Sm1−xYxS. J. Phys.: Conf. Ser. 592, 012028 (2015).
Matsubayashi, K. et al. Effect of nominal composition on transport, optical, magnetic, and thermodynamic properties of SmS single crystals. J. Phys. Soc. Jpn. 76, 064601 (2007).
Mizuno, T. et al. Excitonic instability in the transition from the black phase to the golden phase of SmS under pressure investigated by infrared spectroscopy. J. Phys. Soc. Jpn. 77, 113704 (2008).
Gilbert Corder, S. N. et al. Near-field spectroscopic investigation of dual-band heavy fermion metamaterials. Nat. Commun 8, 2262, https://doi.org/10.1038/s41467-017-02378-3 (2017).
Izumi, F. & Ikeda, T. A Rietveld-analysis program RIETAN-98 and its applications to zeolites. Mater. Sci. Forum 321–324, 198–205 (2000).
Singhal, R. K., Chandra, U. & Garg, K. B. X-ray absorption study of valence fluctuations in alloys of SmSe with SmSb. J. Magn. Magn. Mater. 123, 311–316 (1993).
Song, X. Y. et al. Adjustable zero thermal expansion in antiperovskite manganese nitride. Adv. Mater. 23, 4690–4694 (2011).
Kino, H., Fukushima, T. & Tanaka, T. Remarkable suppression of local stress in 3D IC by manganese nitride-based filler with large negative CTE. Proceedings of 2017 IEEE 67th Electronic Components and Technology Conference, 1523–1528; https://doi.org/10.1109/ECTC.2017.209 (2017).
Arvanitidis, J., Papagelis, K., Margadonna, S., Prassides, K. & Fitch, A. N. Temperature-induced valence transition and associated lattice collapse in samarium fulleride. Nature 425, 599–602 (2003).
Sleight, A. Zero-expansion plan. Nature 425, 674–676 (2003).
Takahashi, H. et al. Electrical oscillation in SmS induced by a constant external voltage. Phys. Rev. B 89, 195103 (2014).
Acknowledgements
The authors are grateful to M. Azuma, K. Imura, and N.K. Sato for fruitful discussion. This work was financially supported by Grants-in-Aid for Scientific Research (Nos JP16K13663 and JP17H02763) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by the Tanikawa Foundation. Synchrotron powder x-ray diffraction experiments were conducted at the BL5S2 of Aichi Synchrotron Radiation Center, Aichi Science & Technology Foundation, Aichi, Japan (Proposal Nos 201702101 and 201703027). This work was supported by NIMS Joint Research Hub Program and was conducted partly at the Materials Design and Characterization Laboratory under the Visiting Researcher Program of the Institute for Solid State Physics, The University of Tokyo.
Author information
Authors and Affiliations
Contributions
K.T. proposed and organized the project and wrote the manuscript. D.A., R.K., Y.M. and Y.Y. fabricated the single crystals and performed structural analysis as well as dilatometry and transport measurements. Y.O. conducted chemical analyses of the single crystals. N.K. provided advice on the structural analysis. H.S.S. and Y.I. supervised the crystal growth. All authors discussed the results and worked on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takenaka, K., Asai, D., Kaizu, R. et al. Giant isotropic negative thermal expansion in Y-doped samarium monosulfides by intra-atomic charge transfer. Sci Rep 9, 122 (2019). https://doi.org/10.1038/s41598-018-36568-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36568-w
This article is cited by
-
Negative thermal expansion in YbMn2Ge2 induced by the dual effect of magnetism and valence transition
npj Quantum Materials (2021)
-
Negative thermal expansion: Mechanisms and materials
Frontiers of Physics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.