Abstract
Biosorption is a cost-effective and simple technique for removing heavy metals and rare earth elements from aqueous solution. Here, metals were recovered from aqueous solutions using phosphorylated dry baker’s yeast cells. The cells were phosphorylated using cyclo-triphosphate, Na3P3O9. The total P content of the phosphorylated cells was ~1.0 mmol/g dry cell weight (DCW). The zeta potential of the phosphorylated cells was −45 mV, two times higher than for the non-phosphorylated cells. The strong negative charges of the phosphorylated cells allowed the cells to adsorb heavy metal ions such as Cd2+, Cu2+, Pb2+, and Zn2+, the adsorption capacities of which reached ~1.0 mmol/g DCW. This adsorption capacity was the highest level found in the previous studies using yeast dead biomass. The adsorbed metal ions were easily desorbed in 0.1 M HCl. The phosphorylated cells also adsorbed rare earth ions including Ce3+, Dy3+, Gd3+, La3+, Nd3+, Y3+, and Yb3+ with high efficiency. Furthermore, the phosphorylated yeast cells selectively adsorbed the rare earth ions (Nd3+ and Yb3+) from a solution containing heavy metals and rare earth ions because trivalent positively charged ions were adsorbed preferentially over divalent ions. Thus, phosphorylated yeast cells therefore have great potential for use as novel bioadsorbents. It is also expected that this technique can be applied to many microbial materials as well as yeast.
Similar content being viewed by others
Introduction
Biosorption is a cost-effective and simple technique for removing heavy metals or rare earth elements from effluent. Biosorption relies on the ability of living and/or non-living biomass to rapidly adsorb and concentrate (through physicochemical pathways) heavy metal or rare earth ions from even dilute aqueous solutions1,2,3.
Many biomaterials (e.g., algae, bacteria, by-products of animal origin, food industry and agricultural waste, fungi, plants, and yeasts) have been used to biosorb metals2,3,4,5,6,7,8,9,10. Saccharomyces cerevisiae has received a great deal of attention because of its unique characteristics. S. cerevisiae in various forms (e.g., food industry waste, immobilised yeast, commercial baker’s yeast, laboratory-cultivated baker’s yeast, other laboratory-cultivated yeasts, and magnetically, chemically or thermally modified yeasts) can remove toxic metals (e.g., Cd, Hg, Pb, and Zn), radionuclides (e.g., Ce, Cs, Sr, and U), precious metals (e.g., Ag, Au, Pd, and Pt), and light metals (e.g., Al) from aqueous solutions11. Even now, yeast cells have been intensively studied from the aspect of increasing the adsorbent capacity and new application for biomineralization12,13,14,15,16. Yeast cells can be obtained as a by-product of the fermentation industry, and are therefore an accessible form of biomass for use as a bioadsorbent11,12,17.
The biosorption of metals is a complex process that is affected by the adsorbent, the types and concentrations of metals in the solution to be treated, and other environmental factors. Developments in molecular biology have made it possible to use molecular tools to engineer living microorganisms. Valuable microbial functions have been biochemically and genetically characterised, and microorganisms have been engineered to perform these functions13,18. In the microbial cell surface display technique, a heterologous protein/peptide of interest is expressed fused with various cell-surface proteins or fragments1. Using this technique, a target metal-binding protein/peptide can be expressed and displayed on the cell surfaces fused with an anchor protein to enhance the metal adsorption15,16,17,18. Although these approaches remarkably improved the metal adsorption of yeast cells, adsorption capacities are still much lower than those of inorganic adsorbent including ion-exchange resin (0.6~3 mmol/g). Furthermore, these bioadsorbents should be used under mild condition to avoid the damage of displayed protein/peptide or cell death.
In contrast, non-living microbial biomass offers advantages over living microorganisms when biosorption is performed. Metal adsorption is possible not only on the cell surface but also inside the cells as there are no penetration barriers associated with the cell membrane. Non-living microbes do not require nutrients and are not affected by toxic heavy metals. In addition, non-living biomass can be stored for long periods19,20. Physical and chemical biomass pretreatment methods can improve the adsorption qualities of the biomass14. Among them, phosphorylated biomass is expected to be an excellent bioadsorbent of cationic metal ions because of the strong negative charges on the phosphate groups21. In particular, phosphorylating using inorganic sodium cyclo-triphosphate, Na3P3O9 (P3m), is a safe and efficient technique and phosphorylated cellulose has been used to adsorb metal ions22. It is also well summarized that P3m is a very useful agent for phosphorylating alcohols, amines, amino acids, and sugars in aqueous solutions23. From this point of view, phosphorylation of non-living microbial biomass is a promising method to develop a novel biosorbent because such biomass is complex, and constructed by organic substances such as amines, amino acids, and sugars.
In this study, dry baker’s yeast cells were phosphorylated using P3m. The phosphorylation efficiency and surface electric charges on the non-living phosphorylated yeast cells were determined. The phosphorylated yeast cells were then used in metal adsorption experiments. The amounts of heavy metal and rare earth ions adsorbed by the phosphorylated yeast cells were determined. Furthermore, desorption of copper ions adsorbed to the phosphorylated yeast cells was examined. Finally, the selective adsorption of rare earth ions from a mixture of ions was performed using the phosphorylated yeast cells. This is the first report endowing the yeast cells with negative charge by installing the anionic functional group for biosorption.
Results and Discussion
Phosphorylation of yeast cells and the properties of the phosphorylated cells
Yeast cells were phosphorylated using P3m following a method previously used to phosphorylate cellulose22. Microscopy images of the phospho (+) and phospho (−) cells are shown in Fig. 1. The sizes or shapes of phospho (+) and phospho (−) cells were not markedly different. The yeast cells clearly retained their normal shapes when they were phosphorylated, suggesting that phosphorylation did not destroy the cell structures.
The degree to which the yeast cells were phosphorylated by P3m was evaluated by performing elemental analysis using the vanadomolybdate method. The P content was normalised to the DCW of the yeast cells. As shown in Table 1, the P content of the phospho (−) cells produced in the first test was 0.07 mmol/g DCW, and the P content of the phospho (+) cells was approximately 10 times higher, 0.91 mmol/g DCW. The P contents of the phospho (−) and phospho (+) cells produced in the second test were 0.06 and 1.09 mmol/g DCW, respectively. The elemental analysis results indicate that the yeast cells were successfully phosphorylated using P3m and that the phosphorylation process was reproducible.
Adding PO42− groups may increase the negative charges on yeast cells; therefore, the zeta potentials of rehydrated phospho (+) and phospho (−) cells were compared. The zeta potential of the phospho (−) cells was −26 mV (Table 1), which was comparable to the zeta potentials of normal yeast cells found in a previous study24. The zeta potential of the phospho (+) cells was −45 mV, approximately two times higher than the zeta potential of the phospho (−) cells. We therefore concluded that the phospho (+) cells were markedly more negatively charged than the non-phosphorylated cells. The increase in the zeta potential of the cells through phosphorylation supported our conclusion that the yeast cells were successfully phosphorylated.
Adsorption of heavy metal ions to phosphorylated yeast cells
It has previously been found that phosphorylated cellulose paper adsorbed appreciable amounts of metal ions, which became bound to the PO42− groups24. We investigated the adsorption of metal ions to phosphorylated yeast cells. First, Cu2+ adsorption time courses for phospho (+) and phospho (−) cells were compared (Fig. 2). These experiments were performed using a Cu2+ concentration of 100 ppm and a yeast cell concentration of 0.5 mg/mL. The phospho (−) cells removed 4% of the Cu2+ ions in 5 min and ~5% in 10 min, but did not remove more as the time increased further. The phospho (+) cells removed ~28% of the Cu2+ ions in 5 min and ~30% in 10 min, but did not remove more as the time increased further (~29% of the Cu2+ ions had been removed in 60 min). These results indicated that the adsorption rate was very high and that adsorption equilibrium was reached within 10 min. An adsorption period of 10 min was therefore used in subsequent experiments. Photographs of the precipitated phospho (+) and phospho (−) cells after they had been used to adsorb Cu2+ ions are shown in Fig. 2B. The phospho (+) cells were much more visibly blue than the phospho (−) cells because more Cu2+ was adsorbed to the phospho (+) cells than to the phospho (−) cells.
Cu2+ adsorption onto phospho (+) and phospho (−) yeast cells. (A) Time course for Cu2+ removal. Cu2+ concentration was 100 ppm and the yeast cell concentration was 0.5 mg/mL. (B) Photographs of the phospho (+) and phospho (−) yeast cells after they had been used to adsorb Cu2+. The scale bar indicates 10 mm.
The percentages of various heavy metal ions adsorbed by the phospho (+) and phospho (−) cells are shown in Fig. 3. In experiments using yeast cells at a concentration of 0.5 mg/mL, the phospho (+) cells and phospho (−) cells removed ~34% and ~7%, respectively, of the Cu2+ ions. Much larger proportions of the metal ions were adsorbed by the phospho (+) cells than by the phospho (−) cells. In experiments using yeast cells at a concentration of 2.5 mg/mL, the phospho (+) cells removed 97% of the Cu2+ ions. These results suggest that phosphorylated yeast cells are excellent bioadsorbents of metal ions. Cd2+, Pb2+, and Zn2+ were also adsorbed more efficiently by the phospho (+) cells than by the phospho (−) cells at a cell concentration of 0.5 mg/mL. The phospho (−) cells removed ~27% of the Pb2+ ions. This was because the molar concentration of Pb2+ ions was relatively low because the same mass concentration (100 ppm) was used as was used for the other metal ions even though the atomic weight of Pb (207.2) is much higher than the atomic weights of the other metals.
Adsorption of heavy metal ions onto the phospho (+) and phospho (−) yeast cells. The percentages of Cd2+, Cu2+, Pb2+, and Zn2+ ions removed in 10 min. The metal ion concentration was 100 ppm (Cd2+ 0.89 mM, Cu2+ 1.57 mM, Pb2+ 0.48 mM, and Zn2+ 1.53 mM), and the yeast cell concentration was 0.5 or 2.5 mg/mL.
The adsorption capacities of the phospho (+) and phospho (−) cells for the different metal ions that were used are summarised in Table 2. The Cu2+ adsorption capacity of the phospho (−) cells was 0.21 ± 0.10 mmol/g DCW, comparable to the adsorption capacity of dead yeast cells found in a previous study25. The Cu2+ adsorption capacity of the phospho (+) cells was 1.08 ± 0.18 mmol/g DCW, markedly higher than the adsorption capacities of the phospho (−) cells. The Cd2+, Pb2+, and Zn2+ adsorption capacities of the phospho (+) cells were all ~1.0 mmol/g DCW. The high adsorption efficiency of the phospho (+) cells could be explained by the treatment of the dead phospho (+) cells with ethanol making the cell membranes permeable. Thus, P3m could diffuse into the cells and phosphorylate the inner as well as the outer cell walls, allowing the whole structures (including the inner cell walls) of the phosphorylated cells to adsorb metal ions. Phosphorylating the yeast cells therefore strongly enhanced metal ion adsorption by the cells.
Table 3 shows a summary of the adsorption capacities of metal ions using dead biomass of S. cerevisiae. The maximum adsorption capacities of Pb2+ and Cd2+ in the literatures were 1.31 and 0.77 mmol/g DCW, respectively26,27. These values were comparable with the values in this study. However, it seems that this adsorption occurred with specific combinations between the type of metal ion and specifically treated cells. In contrast, whereas the maximum Cu2+ and Zn2+ adsorption capacities in the previous studies were approximately 0.2 mmol/g DCW, the values in this study were significantly higher (~1.0 mmol/g DCW), which suggests that the phospho (+) cells proposed in this study have broad utility for various types of metal ions.
Desorption of metal ions from the phosphorylated yeast cells
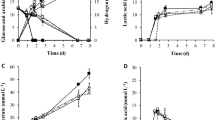
The desorption of metal ions from the phosphorylated yeast cells is important in terms of recovering metals removed from solution by the cells. It has previously been found that the adsorption of metal ions onto phosphorylated cellulose paper was strongly influenced by the pH of the solution and that almost no adsorption occurred when the paper was treated with 0.1 M HCl22. We therefore attempted to desorb Cu2+ ions from the phosphorylated yeast cells using 0.1 M HCl. Photographs of the phospho (+) cells before and after adsorption of Cu2+ ions and after the ions had been desorbed using HCl are shown in Fig. 4A. The Cu2+ ion concentration in the test solution was 100 ppm, and the yeast cell concentration was 2.5 mg/mL. The phospho (+) cells were white before being exposed to Cu2+ ions and light blue when Cu2+ ions had been adsorbed. The cells with adsorbed Cu2+ ions turned white again when treated with HCl, suggesting that the Cu2+ ions were successfully desorbed into the 0.1 M HCl. The adsorption and desorption of Cu2+ ions were quantified by performing inductively coupled plasma (ICP) analyses. As shown in Fig. 4B, approximately 95% of the Cu2+ ions in solution became adsorbed to the phospho (+) cells, and approximately 98% of the Cu2+ ions adsorbed to the cells became desorbed when the cells were treated with 0.1 M HCl. We concluded that 98% of the Cu2+ ions adsorbed to phospho (+) cells could be recovered simply by treating the cells with HCl.
Desorption of adsorbed Cu2+ under acidic conditions. (A) Photographs of the phospho (+) yeast cells before and after Cu2+ had been adsorbed and after the Cu2+ had been desorbed by exposing the cells to 0.1 M HCl (each scale bar indicates 2 mm). The metal ion concentration was 100 ppm and the yeast cell concentration was 2.5 mg/mL. (B) (left) Cu2+ removal by phospho (+) yeast cells and (right) Cu2+ recovery in 0.1 M HCl.
Adsorption of rare earth ions onto the phosphorylated yeast cells
The results presented in the previous section indicate that the phosphorylated yeast cells could adsorb heavy metals. However, only a few reports have described the biosorption of rare earth ions using S. cerevisiae biomass28,29. Therefore, we tested the ability of the phosphorylated yeast cells to adsorb seven rare earth ions (Ce3+, Dy3+, Gd3+, La3+, Nd3+, Y3+, and Yb3+). As shown in Fig. 5, the phospho (+) cells removed 50–70% of most of the rare earth ions from solution, whereas the phospho (−) cells removed <10%, which indicates that the phosphorylated yeast cells could be used to adsorb rare earth ions from solution. However, only ~30% of the Y3+ ions were removed by the phospho (+) cells, because Y has an atomic weight of 88.91, about half the atomic weights of the other rare earth elements (138.9–173.04). The phospho (+) and phospho (−) cell adsorption capacities for the rare earth ions were calculated. As shown in Table 4, the phospho (+) cell adsorption capacities were 0.7–0.8 mmol/g DCW, which were much higher than the phospho (−) cell adsorption capacities.
Percentages of Ce3+, Dy3+, Gd3+, La3+, Nd3+, Y3+, and Yb3+ removed from solution by the phospho (+) and phospho (−) yeast cells. The metal ion concentration was 100 ppm (Ce3+ 0.71 mM, Dy3+ 0.62 mM, Gd3+ 0.64 mM, La3+ 0.72 mM Nd3+ 0.69 mM, Y3+ 1.12 mM, and Yb3+ 0.58 mM), and the yeast cell concentration was 0.5 mg/mL.
Selective adsorption of rare earth ions from mixtures of rare earth ions and heavy metal ions
As mentioned above, Cu2+ ions adsorbed to the phosphorylated yeast cells were desorbed by 0.1 M HCl. We therefore acquired profiles for the adsorption of heavy metal and rare earth ions onto the phospho (+) cells at various HCl concentration. The adsorption profiles for four heavy metal ions (Cd2+, Cu2+, Pb2+, and Zn2+) and two rare earth ions (Nd3+ and Yb3+) are shown in Fig. 6 A. The percentages of the heavy metals adsorbed decreased dramatically as the HCl concentration increased from 0.0001 to 0.001 M. The amount of Cu2+ ions removed at a HCl concentration of 0.001 M was approximately 40% of the amount removed at a HCl concentration of 0.0001 M. The amount of Cu2+ ions removed gradually decreased as the HCl concentration increased further, and no Cu2+ ions were removed at a HCl concentration of 0.1 M. The amounts of Cd2+ and Zn2+ ions removed followed similar trends to the amount of Cu2+ ions removed, decreasing markedly as the HCl concentration increased from 0.0001 to 0.001 M solution and approaching zero at a HCl concentration of 0.1 M. The amount of Pb2+ ions removed followed a different trend, not decreasing dramatically as the HCl concentration increased from 0.0001 to 0.001 M HCl and remaining relatively high compared with the amounts of the other heavy metal ions removed. The amounts of rare earth ions removed followed different trends to the amounts of heavy metals removed. Between 70% and 90% of the rare earth ions were removed at a HCl concentration of 0.001 M, and the percentage removed decreased slightly as the HCl concentration was increased from 0.001 to 0.01 M, but remained in the range 50%–60%. Rare earth ions were therefore adsorbed more strongly than heavy metal ions by the phosphorylated yeast cells, which would have been because the rare earth ions are trivalent whereas the heavy metal ions are divalent.
These results led us to adjust the HCl concentration to selectively adsorb rare earth ions from a mixture of rare earth ions and heavy metal ions. We exposed the phosphorylated yeast cells to solutions containing four types of heavy metal ions and one type of rare earth ion. The results of adsorption tests using solutions containing four heavy metal ions (Cd2+, Cu2+, Pb2+, and Zn2+) and Nd3+ or Yb3+ in 0.01 M HCl are shown in Fig. 6B. Each metal ion was used at a concentration of 10 ppm, and the phospho (+) cell concentration was 0.4 mg/mL. Interestingly, ~10% of each heavy metal ion was removed but ~70% of the Nd3+ was removed, which indicates that the Nd3+ was selectively adsorbed by the phosphorylated yeast cells. A relatively high percentage of the Pb2+ ions was adsorbed in 0.01 M HCl (Fig. 6A), but Nd3+ was still selectively adsorbed from mixtures including Pb2+. The adsorption of Yb3+ from a solution also containing heavy metal ions followed a similar trend, with ~70% of the Yb3+ being removed from the solution. This was a much higher percentage than the percentages of the heavy metal ions that were removed. These results suggest that various rare earth ions could be selectively adsorbed by the phospho (+) cells. These results demonstrate that the phosphorylated yeast cells preferentially adsorbed rare earth ions from a mixture of rare earth ions and heavy metals, with means that phosphorylated yeast cells may find uses in the metal recycling field.
Selective adsorption of rare earth ions from solutions containing heavy metal and rare earth ions. (A) Influence of the HCl concentration on metal ion removal by the phospho (+) yeast cells. The metal ion concentration was 10 ppm and the yeast cell concentration was 0.2 mg/mL. (B) Simultaneous adsorption of Nd3+ or Yb3+ and the heavy metal ions Cd2+, Cu2+, Pb2+, and Zn2+. Each metal ion concentration was 10 ppm and the yeast cell concentration was 0.4 mg/mL.
In conclusion, P3m was used to phosphorylate yeast cells. The P content of the phosphorylated yeast cells was ~1 mmol/g DCW, and the zeta potential was −45 mV, twice as high as the zeta potential of the non-phosphorylated yeast cells. The phosphorylated yeast cells adsorbed heavy metal ions, giving maximum heavy metal contents of ~1 mmol/g DCW, comparable with the highest contents found in previous studies using yeast biomass. The adsorbed metal ions were easily desorbed by HCl. The phosphorylated yeast cells preferentially adsorbed trivalent rare earth ions (Nd3+ and Yb3+) from mixtures of heavy metal and rare earth ions. The phosphorylated yeast cells have great potential for use as bioadsorbents.
Methods
Phosphorylation of yeast cells
Commercial dry baker’s yeast (Nisshin Seifun Group, Tokyo, Japan) was used throughout the study. Yeast cells were first washed five times with pure water and then fixed with 70% (v/v) ethanol for 2 h. The yeast cells were then phosphorylated using sodium cyclo-triphosphate hexahydrate, Na3P3O9·6H2O, following a method previously used to phosphorylate cellulose22. The yeast cells were phosphorylated using a 20% P3m solution at 50 °C for 5 d. The solution pH gradually decreased as the reaction progressed; therefore 6 M sodium hydroxide(aq) was added (with stirring) to keep the solution at pH 12. After the reaction, the phosphorylated yeast cells were washed with distilled water and lyophilised. The phosphorylated cells are called phospho (+) cells. Negative controls (called phospho (−) cells) were prepared following the same procedure but without adding P3m.
Characteristics of the phosphorylated yeast cells
The P contents of the phosphorylated yeast cells were determined using the vanadomolybdate method at the Center for Organic Elemental Microanalysis at Kyoto University. The zeta potentials of the phosphorylated and non-phosphorylated dry baker’s yeast cells, suspended in pure water to give an optical density at 600 nm of 0.5, were determined using a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK).
Bioadsorption of heavy metal and rare earth ions by the phosphorylated yeast cells
Stock solutions of the metals of interest were prepared using 1,000 mg/L (1,000 ppm) of Cd2+, Ce3+, Cu2+, Dy3+, Gd3+, La3+, Nd3+, Pb2+, Y3+, Yb3+, and Zn2+. The stock solutions were used to prepare standards at the different concentrations required. Standards at different concentrations were analysed using a Varian Vista MPX simultaneous ICP optical emission spectrometer (Agilent Technologies, Santa Clara, CA, USA).
Each adsorption test involved suspending yeast cells at a concentration of between 0.2 and 2.5 mg DCW/mL in 4 or 10 mL of a metal ion solution at a concentration of 10 or 100 ppm (prepared by diluting the appropriate stock solution with pure water) in a test tube. The test tube was shaken on a reciprocal shaker at 140 rpm at 30 °C for a specified time; the mixture was then centrifuged at 3,000 g for 10 min and then at 17,800 g for 5 min to remove the cells and debris. The adsorption tests using rare earth ions were performed using 0.0001 M HCl to allow the effects of phosphorylation to be clearly identified. The supernatant solutions after centrifugation were analysed by ICP optical emission spectrometry.
Desorption of copper ions adsorbed to the phosphorylated yeast cells
Metal ions adsorbed to the phosphorylated yeast cells were desorbed by adding 0.1 M HCl and allowing the mixture to stand for 3 h. The supernatant was analyzed by ICP optical emission spectrometry and the copper ion recovery efficiency was calculated.
Selective adsorption of rare earth ions from a mixture of heavy metal and rare earth ions
The heavy metal and rare earth ion adsorption profiles for the phospho (+) cells at various HCl concentration were determined in preliminary experiments. In these experiments, the metal ions were used at a concentration of 10 ppm and the yeast cells at a concentration of 0.2 mg/mL. The HCl concentrations were 0.00001–0.3 M. Selective adsorption of rare earth ions was studied by performing adsorption experiments using mixtures of four heavy metal ions (Cd2+, Cu2+, Pb2+, and Zn2+) and either Nd3+ or Yb3+ in 0.01 M HCl. In these experiments, the metal ions were used at a concentration of 10 ppm and the yeast cells at a concentration of 0.4 mg/mL. The test tubes containing the cells and test solutions were shaken on a reciprocal shaker at 140 rpm at 30 °C for 10 min. The mixtures were then centrifuged and the supernatants were analysed by ICP optical emission spectrometry.
Statistical analysis
Each result is presented as the mean ± the standard deviation for more than three independent experiments except in Fig. 6A, for which n was 2.
References
Li, P. S. & Tao, H. C. Cell surface engineering of microorganisms towards adsorption of heavy metals. Crit. Rev. Microbiol. 41, 140–149 (2015).
Das, N. Recovery of precious metals through biosorption — A review. Hydrometallurgy 103, 180–189 (2010).
Das, N. & Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earth. 31, 933–943 (2013).
Abbaas, S. H., Ismail, I. M., Mostafa, T. M. & Sulaymon, A. H. Biosorption of heavy metals: a review. J. Chem. Sci. Technol. 3, 74–102 (2014).
Huang, M. et al. Chitosan-rectorite nanospheres embedded aminated polyacrylonitrile nanofibers via shoulder-to-shoulder electrospinning and electrospraying for enhanced heavy metal removal. Appl. Surf. Sci. 437, 294–303 (2018).
Tu, H. et al. Chitosan-rectorite nanospheres immobilized on polystyrene fibrous mats via alternate electrospinning/electrospraying techniques for copper ions adsorption. Appl. Surf. Sci. 426, 545–553 (2017).
Luk, C. H. J. et al. Biosorption performance of encapsulated Candida krusei for the removal of copper(II). Sci. Rep. 7, 2159 (2017).
Vijayaraghavan, K., Teo, T. T., Balasubramanian, R. & Joshi, U. M. Application of Sargassum biomass to remove heavy metal ions from synthetic multi-metal solutions and urban storm water runoff. J. Hazard. Mater. 164, 1019–1023 (2009).
Vimala, R., Charumathi, D. & Das, N. Packed bed column studies on Cd(II) removal from industrial wastewater by macrofungus Pleurotus platypus. Desalination 275, 291–296 (2011).
Jiang, Z. et al. Comparison on the surface structure properties along with Fe(II) and Mn(II) removal characteristics of rice husk ash, inactive Saccharomyces cerevisiae powder, and rice husk. BioMed. Research. International. 2016, 9 (2016).
Wang, J. & Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 24, 427–451 (2006).
Xin, S. et al. Recyclable Saccharomyces cerevisiae loaded nanofibrous mats with sandwich structure constructing via bio-electrospraying for heavy metal removal. J. Hazard. Mater. 324, 365–372 (2017).
Geva, P., Kahta, R., Nakonechny, F., Aronov, S. & Nisnevitch, M. Increased copper bioremediation ability of new transgenic and adapted Saccharomyces cerevisiae strains. Environ. Sci. Pollut. Res. Int. 23, 19613–19625 (2016).
De Rossi, A. et al. Chromium (VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ. Sci. Pollut. Res. Int. 25, 19179–19186 (2018).
Qiu, L., Feng, J., Dai, Y. & Chang, S. Biosorption of the strontium ion by irradiated Saccharomyces cerevisiae under culture conditions. J. Environ. Radioact. 172, 52–62 (2017).
Shen, Y., Zheng, X., Wang, X. & Wang, T. The biomineralization process of uranium(VI) by Saccharomyces cerevisiae - transformation from amorphous U(VI) to crystalline chernikovite. Appl. Microbiol. Biotechnol. 102, 4217–4229 (2018).
Park, J. K., Lee, J. W. & Jung, J. Y. Cadmium uptake capacity of two strains of Saccharomyces cerevisiae cells. Enzyme Microb. Technol. 33, 371–378 (2003).
Kuroda, K. & Ueda, M. Engineering of microorganisms towards recovery of rare metal ions. Appl. Microbiol. Biotechnol. 87, 53–60 (2010).
Vijayaraghavan, K., Jegan, J., Palanivelu, K. & Velan, M. Biosorption of cobalt(II) and nickel(II) by seaweeds: batch and column studies. Sep. Puri. Technol. 44, 53–59 (2005).
Amini, M., Younesi, H. & Bahramifar, N. Biosorption of U(VI) from aqueous solution by Chlorella vulgaris: equilibrium, kinetic, and thermodynamic studies. J. Environ. Eng. 139, 410–421 (2013).
Illy, N. et al. Phosphorylation of bio-based compounds: the state of the art. Polym. Chem. 6, 6257–6291 (2015).
Inoue, H., Baba, Y. & Tsuhako, M. Phosphorylation of cellulose with cyclo-Triphosphate. Chem. pharm. bull. 43, 677–678 (1995).
Inoue, H., Nakayama, H. & Tsuhako, M. Phosphorylation of organic compounds by inorganic cyclo-triphosphate. Recent Res. Dev. Chem. Pharm. Sci. 2, 31–46 (2002).
Thonart, P., Custinne, M. & Paquot, M. Zeta potential of yeast cells: application in cell immobilization. Enzyme Microb. Technol. 4, 191–194 (1982).
Machado, M. D., Santos, M. S., Gouveia, C., Soares, H. M. & Soares, E. V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: the flocculation as a separation process. Bioresour. Technol. 99, 2107–2115 (2008).
Ozer, A. & Ozer, D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J. Hazard. Mater. 100, 219–229 (2003).
Vasudevan, P., Padmavathy, V. & Dhingra, S. C. Kinetics of biosorption of cadmium on Baker’s yeast. Bioresour. Technol. 89, 281–287 (2003).
Andres, Y., Thouand, G., Boualam, M. & Mergeay, M. Factors influencing the biosorption of gadolinium by micro-organisms and its mobilisation from sand. Appl. Microbiol. Biotechnol. 54, 262–267 (2000).
Palmieri, M. C., Garcia, O. & Melnikov, P. Neodymium biosorption from acidic solutions in batch system. Process Biochem. 36, 441–444 (2000).
Bustard, M. & McHale, A. P. Biosorption of heavy metals by distillery-derived biomass. Bioprocess Eng. 19, 351–353 (1998).
Stoica, L., Stanescu, A.-M., Constantin, C. & Bacioiu, G. Cadmium(II) removal from aqueous solutions by biosorption onto inactive instant dry baker’s yeast. Rev. Chim. (Bucharest) 65, 844–847 (2014).
Göksungur, Y., Uren, S. & Guvenc, U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 96, 103–109 (2005).
Acknowledgements
This study was supported by the Japan Science and Technology Agency through the target-driven R&D Adaptable & Seamless Technology Transfer Program (A-STEP). We thank Gareth Thomas, PhD, and Sara J. Mason, MSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
M.A. proposed the research concept, designed the experiments, and provided necessary tools for experiments and experimental instructions. Y.O. analyzed the data and wrote the manuscript. S.K., M.K. and N.M. conducted the experiments and analysed the data. K.I. assisted in some scientific experiments. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojima, Y., Kosako, S., Kihara, M. et al. Recovering metals from aqueous solutions by biosorption onto phosphorylated dry baker’s yeast. Sci Rep 9, 225 (2019). https://doi.org/10.1038/s41598-018-36306-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36306-2
This article is cited by
-
Biofabricated yeast: super-soldier for detoxification of heavy metals
World Journal of Microbiology and Biotechnology (2023)
-
A review of the application of fungi as an effective and attractive bio-adsorbent for biosorption of heavy metals from wastewater
Environmental Monitoring and Assessment (2023)
-
Lead removal at trace concentrations from water by inactive yeast cells
Communications Earth & Environment (2022)
-
Isolation and characterization of heavy metals and non-metallic pollutant-tolerant microorganism from wastewater of Tollygunge Canal (Kolkata) West Bengal, India
Biologia (2022)
-
Towards rare earth element recovery from wastewaters: biosorption using phototrophic organisms
Applied Microbiology and Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.