Abstract
The apicomplexan parasite Sarcocystis calchasi (S. calchasi) triggers pigeon protozoal encephalitis, a neurologic disease in columbids. Accipiter hawks have been identified as the final host, and Columbidae and Psittaciformes as intermediate hosts. In this study, 368 free-ranging Accipiter hawks and 647 free-ranging common woodpigeons were sampled in a country-wide study in order to identify the prevalence of S. calchasi in these populations. A semi-nested PCR specific for S. calchasi tested positive in 7.3% (4.9–10.5) of submitted samples from Accipiter hawks. Juvenile Accipiter hawks (13.7%; 7.7–22.0) had a significantly higher infection rate with S. calchasi than adult Accipiter hawks (5.8%; 2.7–9.3). The prevalence of S. calchasi in common woodpigeons was 3.3% (5.4–9.7). Positive pigeons were identified in 14/16 federal states, and a region-dependency was detected, with higher rates of infection in the eastern parts of Germany. The results of this study suggest that the common woodpigeon is a natural reservoir for S. calchasi. In a study of one region for four consecutive years, an increase in prevalence was not detected. Findings indicate that the parasite is not newly introduced to Germany, but rather long established. The prevalence suggests that there is a substantial risk of S. calchasi infections in other free-ranging as well as captive host species.
Similar content being viewed by others
Introduction
The apicomplexan parasite Sarcocystis calchasi (S. calchasi) is the causative agent of pigeon protozoal encephalitis (PPE)1. Like all Sarcocystis species, S. calchasi has an obligate two-host life cycle2,3. While the final host is infected by feeding on tissue containing mature sarcocysts, the intermediate host ingests infectious sporocysts via the faecal-oral route4. Outbreaks of PPE were initially reported in racing pigeons (Columba livia f. dom.) in Berlin, Germany1. Subsequently, S. calchasi was detected in diseased columbids in the U.S.A. and Japan5,6,7 and psittacines were reported to be further intermediate hosts8,9. Recently, S. calchasi DNA was detected in the European green woodpecker (Picus viridus) and the Great spotted woodpecker (Dendrocopos major), but so far, sarcocysts have not been detected in these species10. The natural intermediate host reservoir of the parasite has not yet been identified. However, free-ranging columbid species are predicted to be intermediate hosts3, but this has not been confirmed to date.
The common woodpigeon (Columba palumbus palumbus) is the most common free-ranging pigeon species in Germany, with approximately 9–17 million birds11. Common woodpigeon territories are spread in urban and rural areas as well as woodlands throughout the country, with the highest density in North Rhine-Westphalia (NRW)12,13. The woodpigeon population is stable to slowly rising. Woodpigeons breeding in Germany show only short migration habits, depending on the season and weather13,14. In regions with high amounts of snow during the winter (e.g. black forest), the birds migrate into areas where food resources are available. In regions with a yearlong food supply, the migration distance is often less than 30 kilometres15,16,17. The high density of woodpigeons supports the hypothesis that the population may serve as main natural reservoir of S. calchasi9,18.
So far, the Northern goshawk (Accipiter gentilis gentilis, hereafter goshawk) and the Eurasian sparrowhawk (Accipiter nisus nisus, hereafter sparrowhawk) have been identified as final hosts of S. calchasi1,18. Goshawks are distributed almost completely throughout the Holarctic. Their spread is limited to the north by the forest boundary in the Palaearctic (79° N)19 and the southern limit is around 40 °N20. In Germany, the number of breeding pairs is estimated to be between 11,500 and 16,50013. The population is described as stable with <10% fluctuations13,21. Density is low on the coastline, the northeaster part of Germany, sparsely wooded areas in Bavaria and the Alpine foothills. In contrast, the population in NRW and Berlin is high13,22,23,24. Goshawks show a strong migratory behaviour at a juvenile age, whereas adult birds are usually bound to their territories25. During the last decades, goshawks have adapted to be a more synanthropic bird, with territories and breeding sites in urban park areas26. The goshawk population is considered almost stable, with a small decrease over the past years13.
Sparrowhawks are even more common than goshawks, with over 18,000 breeding pairs in Germany13,27 and up to 450,000 breeding pairs across Europe11. The distribution area of sparrowhawks extends from the Palaearctic to the Mediterranean sub region28. The east-west extension reaches from the Canaries to Japan19. The sparrowhawk is commonly seen in urban areas and rural lowlands29,30. In Germany, a particularly high density is seen in the Münsterland lowland, and a reduced density is documented in the north-east German lowland13. Sparrowhawk migration is mostly age- and season-dependent, with reaches into central and southern Europe and North Africa27,31.
Both sexes of goshawks prey on adult woodpigeons, mostly during the winter months and while breeding25,32,33,34,35. Female sparrowhawks prey on birds as large as Eurasian jays (Garrulus glandarius) and woodpigeons. Males do not prey on adult woodpigeons, but they are nest predators36. The overlapping distributions of goshawks, sparrowhawks and woodpigeons results in a close predator-prey relationship37,38,39,40.
This close contact between the known final host of S. calchasi and the suspected intermediate host reservoir led to the hypothesis that S. calchasi’s life cycle is maintained in these species. The present study was designed in order to determine the potential risk to domestic and captive held species, such as racing pigeons and psittacines, as well as to free-ranging intermediate hosts arising from S. calchasi-infected free-ranging Accipiter hawks and wood pigeons. Additionally, a longitudinal study was designed in order to investigate a potential on-going expansion of S. calchasi and to assess if the parasite was either recently introduced into Germany, still spreading or if it was already present with a long-term stable occurrence. In order to investigate the dynamics of a possible current expansion of S. calchasi, the woodpigeon was again identified as the host species to be examined, as this species is easily accessible and prevalence increases should be clearly visible.
Results
Accipiter hawks
Sample collection
In the period of 2012–2016, 368 samples of free-ranging Accipiter hawks were collected across Germany. Of these, 119 (32.3%) samples were from goshawks; these consisted of 82 organ and 37 faecal samples. Samples were submitted in every month of the year, but most samples (n = 22) were collected in February. In total, 249 (67.7%) samples originated from sparrowhawks, consisting of 220 organ and 29 faecal samples. Samples were submitted year-round, but most samples (n = 41) were again collected in February.
Semi-nested polymerase chain reaction specific to S. calchasi
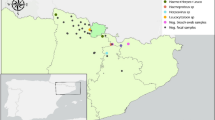
Sarcocystis calchasi DNA was detected in 27/368 (7.3%; 95% C.I. = 4.9–10.5%) samples from Accipiter hawks (Fig. 1).
Statistical correlations
The age of the Accipiter hawks demonstrated a significant dependency on the prevalence of S. calchasi (p = 0.022; Odds Ratio = 2.60): Juvenile Accipiter hawks were significantly more commonly infected than adult Accipiter spp. (Table 1).
Goshawks were more often infected with S. calchasi than sparrowhawks (Odds Ratio = 1.75), although the difference between these species was not statistically significant (p = 0.17) (Table 2). Due to the similar size of female sparrowhawks and male goshawks41,42, they share a similar prey spectrum and therefore, were grouped into a single group for the calculation of species-dependency. However, a significant dependency was again not seen (p = 0.65; Odds ratio = 0.71) (Table 3). Neither region, month, quarter or year of submission, type of sample, weight nor sex (within the species) had an effect on S. calchasi infection (see Supplementary Table S1). A dependency between the result of the semi-nested PCR and the recorded variables via multi-step logistic regression was not detected. Further steps did not lead to additional significant dependency. Age and species had an independent influence. The entire list of data collected on samples from Accipiter hawks can be found as Supplementary Table S2.
Common woodpigeons
In the period of 2012–2016, 647 free-ranging woodpigeons were collected and sampled. Forty-five woodpigeons were harvested in the border region between Bavaria and Saxony, therefore, those two federal states were considered as one state in the statistical analysis.
Prevalence of S. calchasi
DNA of S. calchasi was detected in 48 out of 647 (7.4%) sampled woodpigeons. These positive samples originated from 11 federal states. The federal state based prevalence varied from 0% (95% C.I. = 0–6.4%) in Bavaria/Saxony to 25.0% (95% C.I. = 8.7–49.1%) in Baden-Wuerttemberg (Table 4, Fig. 2). There was a highly significant difference between the infection rates of woodpigeons with S. calchasi across federal states (p < 0.0001). The weighted average prevalence of S. calchasi in Germany within the woodpigeon population was calculated on the basis of hunting bags as weight variables. A hunting bag of a hunting season includes all animals of a species which were hunted within a year and reported to authorities. The hunting authority in each federal state publishes the numbers after every season. The prevalence of S. calchasi for Germany is 3.3% (95% C.I. = 5.4–9.7%)(Table 4).
Longitudinal study
From 2012 to 2015, the required number of woodpigeons (n = 45) were collected each year in the same area of NRW. Four-year sampling in NRW revealed no expansion of S. calchasi in the woodpigeon population (p = 1.00) (Table 5).
Discussion
Regionally limited outbreaks of pigeon protozoal encephalitis have been reported in Germany, the United States and Japan1,5,7. The evidence presented here strongly suggests a widespread distribution of the parasite in the populations of final and intermediate hosts in Germany, and identifies the woodpigeon as a natural intermediate host reservoir of S. calchasi. The woodpigeon population demonstrated a low prevalence of S. calchasi. However, the estimated prevalence of certain individual federal states was considerably higher than the weighted average prevalence for Germany. The low average prevalence is mainly influenced by the extraordinary large population size and hunting bag in North Rhine-Westphalia. Therefore, the individual verified prevalence of each federal state should be considered in the risk assessment for each individual region.
The transmission of parasites within their natural host reservoir depends on different relations between predator and prey43. Clinical disease increases prey vulnerability significantly, simplifying predator success40,44. Prey vulnerability increases after an infection with S. calchasi, when neurological symptoms are present. Thus, it seems possible that animals with clinical signs of PPE may be more frequently removed by predators and may be lacking in the samples collected in this study.
Our longitudinal study failed to detect a spread of S. calchasi in the woodpigeon population over a four-year period. Thus, it can be assumed that S. calchasi had previously established its life cycle within the population. In 2006, S. calchasi was detected in racing pigeons in Berlin; the absence of reports prior to this might be a result of a lack of regular histopathological investigations of muscular tissue from birds with central nervous disorders. The chronic phase of PPE is characterised by encephalitis that is usually not associated with protozoan cysts in the brain but in the skeletal muscles4. As skeletal muscles may not be examined routinely in cases of neurologic disease, the presence of S. calchasi may have been undetected in previous cases of PPE in Germany.
Sarcocystis calchasi was detected by PCR in 7.3% of all samples from Accipiter hawks. This contrasts with the findings of a previous study: in a small Accipiter hawk population located in the Berlin area, 31 of 50 (62.0%) goshawks and 14 of 20 (71.4%) sparrowhawks were positive for S. calchasi DNA according to PCR18. The higher infection rates noted in the literature may indicate local and/or temporal variances or may be a result of small sample sizes. Fluctuations in the incidence of parasitic diseases are commonly seen45,46. The Berlin area may be an endemic hotspot for S. calchasi since its prevalence in the woodpigeon population is well above the average of the surrounding federal states (Brandenburg, Mecklenburg-Western Pomerania, Saxony-Anhalt) (Fig. 2).
This study confirms the previously reported18 occurrence of S. calchasi in both species of Accipiter hawks across Germany. Both Accipiter hawks show a distinct sexual dimorphism in body size47,48, resulting in different prey preferences33,49. While both female and male goshawks prey on pigeons, male sparrowhawks do not often prey on columbids, due to their small body size41,50,51,52,53. As a result, a higher prevalence of S. calchasi had been suspected in goshawks and in female sparrowhawks, compared to male sparrowhawks. Indeed, a higher infection rate was detected in goshawks (Table 2), but sex of the Accipiter hawks did not significantly influence prevalence of S. calchasi, similarly to a previous study18. Re-grouping into three groups of the same body size with similar prey preferences did not have an impact on prevalence either (Table 3). Therefore, intermediate host species other than columbids can be assumed. Even though most Sarcocystis spp. are described as having a narrow host spectrum, some species profit from either multiple intermediate or final hosts (e.g. S. neurona, S. falcatula and S. riley)54,55,56,57,58,59,60,61,62,63. Besides columbids, several psittacine species have already been confirmed as intermediate hosts of S. calchasi8,9 and recently, DNA of S. calchasi was detected in two Picidae species in Germany10. As a result, other avian prey species, such as small songbirds (Passeri) or woodpeckers (Picidae), may serve as additional reservoirs for S. calchasi.
DNA of S. calchasi was detected in 13.7% of the juvenile and 5.8% of the adult Accipiter hawks submitted. The higher rate of infection in juvenile birds may be explained by their less developed immune system, making them less resistant to parasitic infections64,65,66,67. In addition, juvenile Accipiter hawks are less effective hunters than older birds of the same species32,68,69,70; they may feed more frequently on carrion, seriously ill or dying prey. Therefore, they may more frequently consume intermediate hosts infected with S. calchasi or hosts infected with higher doses of S. calchasi.
This large-scale cross-sectional study demonstrates that Sarcocystis calchasi is prevalent throughout Germany in the final host population, as well as in the free-ranging intermediate host. It can be assumed that the common woodpigeon serves as a natural intermediate host reservoir for the parasite. Therefore, there is a risk of outbreaks of S. calchasi-induced PPE in domestic pigeons and captive psittacines across Germany.
Methods
Accipiter hawks – Sample collection and statistical calculation
Sample collection
Within a period of four years (2012–2016), 368 samples from Accipiter hawks were submitted by rescue centres, veterinary clinics, taxidermists, forestry admissions and veterinary diagnostic laboratories. Samples consisted of whole carcasses, intestine samples from wildlife casualties, and faecal samples (see Supplementary Table S2); the latter were collected during the first three days of captivity prior to any antiparasitic treatment. Portions of 200 mg faecal samples were stored for DNA extraction. Carcasses and organ samples were submitted fresh, cooled or frozen. Fresh and cooled samples were investigated directly. Frozen submissions were defrosted at room temperature. A necropsy of the carcasses was performed and tissue samples were taken from two locations of the small intestine and one location of the large intestine and either quick-frozen or immediately submitted for DNA extraction. Sex, age, weight, month and year of collection were recorded in a questionnaire. The questionnaire was completed either during necropsy or, in the case of submission of faecal samples and tissue, by the dispatcher.
Statistical calculation
The sample size for Accipiter hawks was calculated to gain data for an individual-based prevalence. Calculation was based on the prevalence detected in Berlin, Germany (60%)18 and conducted with BiAS for Windows (Version 9.05–02/2010)71. To acquire a 95% confidence interval (C.I.) with an accepted deviation of ± 5%, a sample size of 368 samples was calculated. A sampling plan based on region was not necessary due to the large distribution range of Accipiter and the high migration rate of the species13. All samples collected over the length of the study were included. Statistical data analysis was based on a multiple stepwise logistic regression, with the following variables: ‘species’, ‘location’, ‘type of sample’, ‘age’, ‘sex’, ‘prey spectrum’, ‘weight’, ‘month of collection’, ‘calendar quarter of collection’ and ‘year of collection’. The analysis was carried out with the statistical program package BMDP (BMDP Statistical Software, Inc.72).
Common woodpigeon – Sample collection and statistical calculation
Sample collection
In Hesse and North-Rhine Westphalia, samples were taken at a hunting spot, while woodpigeons from Rhineland-Palatine and Lower Saxony were submitted cooled and sampled at the Clinic for Birds, Reptiles, Amphibians and Fish, Justus Liebig University Giessen, Germany (hereafter clinic). Woodpigeons collected from Mecklenburg-Vorpommern, Schleswig-Holstein, Bavaria, Baden-Württemberg, Saxony-Anhalt, Thuringia and Saarland were submitted frozen to the clinic. They were defrosted at room temperature prior to having samples taken. Biopsies of 2 × 2 × 2 cm were taken from the muscular tissue of the upper and lower breast muscle and were either quick-frozen or immediately submitted for DNA extraction.
Statistical calculation
The prevalence of S. calchasi in woodpigeons is presently unknown. Initial studies failed to detect the parasite in this species3. Therefore, the prevalence was predicted to be rather low (5%). To identify the prevalence of S. calchasi in the woodpigeon population, a cross sectional study was designed. The population of woodpigeons is not evenly spread in Germany, but varies between the federal states13. The spatial distribution of S. calchasi, therefore, can only be estimated via stratified sampling. In the present study, stratification was done by federal states. All federal states were included in the study, even if the hunting bag in some federal states is very low (Table 4). City-states were attributed to the surrounding states. The hunting bags served as a basis for calculating the minimum sample size per federal state, because of the correlation between bag size and the regional population of free-ranging pigeons. The requested specific confidence interval probability was defined as 95%, with a tolerable deviation of \(\pm \) 2%. Using BiAS for Windows (Version 9.05–02/2010)71, a sample size of 455 samples was calculated. As the estimated prevalence is considered inaccurate, and an even lower true prevalence cannot be ruled out, the sample size was raised to n ≥500. As a premise, we defined the probability of detection of at least one positive bird in each federal state to be at least 0.9, resulting in the formula: P(X \(\ge 1\) | Prevalence = 5%) ≥0.90. Cannon and Roe’s charts identified a necessary sample size of 45 samples in each federal state73. If the collection was not feasible due to a small harvest in the period from 2012 until spring 2015, woodpigeon populations were sampled during the winter hunting season of 2015 within the migration distance of 25 kilometres at the borders of any federal state which had yet acquired enough samples. With 13 federal states in total, this led to a required sample size of 585 samples (13 × 45).
In order to assess the on-going expansion of S. calchasi, a longitudinal study was conducted in North Rhine-Westphalia, which harbours the largest woodpigeon population13 and hunting bags in Germany. Sample collection of woodpigeons was extended to at least three consecutive years in NRW. The sample size was aligned to the total sample size per federal state of 45 samples per year. Comparisons of the prevalence between the sampled years was done using Fisher’s exact test74.
In the subsequent evaluation, extrapolation for a nationwide prevalence was based on the hunting bags for the period of sample collection in each federal state. Comparisons of the prevalence between federal states was done using Fisher’s exact test74. Additionally, the 95% C.I. of the prevalence and the odds ratio (OR) were calculated.
Semi-nested polymerase chain reaction specific to Sarcocystis calchasi
DNA was extracted (DNeasy Blood & Tissue Kit; Qiagen, Hilden, Germany) from intestinal samples of the Accipiter hawks and muscular tissues of the woodpigeons. Faecal samples were treated according to the protocol for the Stool Extraction Kit (Qiagen, Hilden, Germany). Afterwards, DNA concentration was measured (260–280 nm) (NanoDrop 2000c Spectrophotometer; Thermo Fisher Scientific, Wilmington, DE, USA) and, if necessary, diluted to < 5 ng/μl according to manufacturer instructions. A semi-nested PCR was performed according to a previously described protocol75. Sarcocystis calchasi DNA extracted from the Berlin strain18 was used in a 10-fold serial dilution as a positive control, and DNAse-free water was included as a non-template control.
Statement of ethical approval
The samples of the current study were obtained from animals that had died or were killed for reasons that are not related with the purpose of this study. Samples were collected from wood pigeons which had been killed for hunting purposes. Carcasses and organ samples from Accipiter hawks were provided by clinics, rescue centres or other institutions, where these animals had died or had been humanely killed due to their fatal clinical condition. Faecal samples from living Accipiter hawks were collected during regular cage cleaning and did not involve any further handling of these birds. Therefore, this study was not subject to ethics review.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Olias, P. et al. Sarcocystis calchasi sp. nov. of the domestic pigeon (Columba livia f. domestica) and the Northern goshawk (Accipiter gentilis): light and electron microscopical characteristics. Parasitol. Res. 106, 577–585 (2009).
Dubey, J. P., Speer, C.A. & Fayer, R. Sarcocystosis of Animal and Man. (CRC Press, Inc, 1988).
Olias, P., Olias, L., Lierz, M., Mehlhorn, H. & Gruber, A. D. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet. Parasitol. 171, 7–14 (2010).
Olias, P. et al. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis. 54, 1032–1037 (2010).
Wünschmann, A., Armien, A. G., Reed, L., Gruber, A. D. & Olias, P. Sarcocystis calchasi-associated neurologic disease in a domestic pigeon in North America. Transbound. Emerg. Dis. 58, 526–530 (2011).
Hodo, C. L. et al. Histopathologic and molecular characterization of Sarcocystis calchasi encephalitis in White-winged Doves (Zenaida asiatica) and Eurasian Collard Doves (Streptopelia decaocto), East-central Texas, USA. J. Wildl. Dis. 52, 395–399 (2016).
Ushio, N. et al. Sarcocystis calchasi encephalitis in a Rock Pigeon. J. Vet. Med. Sci. 77, 1523–1526 (2015).
Rimoldi, G. et al. An outbreak of Sarcocystis calchasi encephalitis in multiple psittacine species within an enclosed zoological aviary. J. Vet. Diagn. Investig. 25, 775–781 (2013).
Olias, P. et al. Sarcocystis calchasi has an expanded host range and induces neurological disease in cockatiels (Nymphicus hollandicus) and North American rock pigeons (Columbia livia f. dom.). Vet. Parasitol. 200, 59–65 (2014).
Ziegler, L. et al. Investigations in causes of neurological signs and mortality, and the role of Sarcocystis calchasi in free-ranging woodpeckers in Germany. J. Zoo Wildl. Med. 49, 247–251 (2018).
Burfield, I. & Van Bommel, F. In BirdLife Conservation Series No. 12 374 (BirdLife International, 2004).
Flade, M. Die Brutvogelgemeinschaften Mittel- und Norddeutschlands. (IHW-Verlag, 1994).
Kai, G., Christoph, G., Mitschke, A. & Sudfeld, C. Atlas of German Breeding Birds. (Stiftung Vogelmonitoring Deutschland, Dachverband Deutscher Avifaunisten, 2014).
Klinz, E. Die Wildtauben Mitteleuropas. (Die neue Brehm Bücherei, 1955).
Gasow, H. Die Ringeltaube (Columba palumbus); mit Kennzeichen anderer Tauben für den Jäger. (Deutscher Jagdschutz-Verband e.V., 1970).
Alterstam, T. & Ulfstrand, S. A radar study of the autumn migration of wood pigeons Columba palumbus in southern Scandinavia. Ibis (Lond. 1859). 116, 522–542 (1974).
Dolenec, Z. & Dolenec, P. Changes in spring migration of the wood pigeon (Columba palumbus) in northwestern Croatia. Turkish J. Zool. 34, 267–269 (2010).
Olias, P., Olias, L., Krücken, J., Lierz, M. & Gruber, A. D. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet. Parasitol. 175, 230–236 (2011).
Glutz von Blotzheim, U. N. In Handbuch der Vögel Mitteleuropas (Aula-Verlag, 1989).
Ferguson-Lees, J. & Christie, D. A. In Raptors of the World 148–180 (Houghton Mifflin Harcourt, 2008).
Mammen, U. & Stubbe, M. Populationsökologie Greifvogel- und Eulenarten, Bd. 6. Popul. Greivogel- und Eulenarten 9–25 (2009).
Nicolai, B. Atlas der Brutvögel Ostdeutschlands. (Gustav Fischer Verlag, 1993).
Rheinwald, G. Atlas der Verbreitung und Häufigkeit der Brutvögel Deutschlands - Kartierung 1985. (Schriftenreihe des Dachverbandes Deutscher Avifaunisten 12, 1993).
Rödl, T., Rudolph, B.-U., Geiersberger, I., Weixler, K. & Görgen, A. Atlas der Brutvögel in Bayern - Verbreitung 2005–2009. (Verlag Eugen Ulmer, 2012).
Fischer, W. Die Habichte: Accipiter. (Spektrum Akademischer Verlag, 1995).
Rutz, C. Home range size, habitat use, activity patterns and hunting behaviour of urban-breeding Northern Goshawks Accipiter gentilis. Ardea 94, 185–202 (2006).
Ferguson-Lees, J., Christie, D. A., Franklin, K., Mead, D. & Burton, P. Raptors of the World: an Identification Guide to the Birds of Prey of the World. (Houghton, 2001).
Glutz von Blotzheim, U. N. & Bauer, K. M. Handbuch der Vögel Mitteleuropas. (Akademische Verlagsgesellschaft, 1971).
Opdam, P. Feeding Ecology and Niche Differentiation in Goshawk Accipiter gentilis L. and Sparrowhawk Accipiter nisus L. (Radboud Universiteit Nijmegen (former Katholieke Universiteit Nijmegen), 1980).
Götmark, F. & Andersson, M. Predation by sparrowhawks decreases with increased breeding density in a songbird, the great tit. Oecologia 142, 177–183 (2005).
Ortlieb, R. In Der Sperber: Accipitridae 140–144 (Die neue Brehm Bücherei, 1987).
Tornberg, R. & Colpaert, A. Survival, ranging, habitat choice and diet of the Northern Goshawk Accipiter gentilis during winter in Northern Finland. Ibis (Lond. 1859). 143, 41–50 (2001).
Penteriani, V., Rutz, C. & Kenward, R. Hunting behaviour and breeding performance of northern goshawks Accipiter gentilis, in relation to resource availability, sex, age and morphology. Naturwissenschaften 100, 935–942 (2013).
Lepom, P. & Schubert, P. In Die Vogelwelt von Brandenburg und Berlin (eds Mädlow, W. et al.) 179–182 (Die Arbeitsgemeinschaft Berlin-Brandenburgerischer Ornithologen (ABBO), 2001).
Altenkamp, R. & Herold, S. In Die Vogelwelt von Brandenburg und Berlin (eds Mädlow, W. et al.) 175–179 (Die Arbeitsgemeinschaft Berlin-Brandenburgerischer Ornithologen (ABBO), 2001).
Gotmark, F. Predation by sparrowhawks favours early breeding and small broods in great tits. Oecologia 130, 25–32 (2002).
Gotmark, F. & Post, P. Prey selection by sparrowhawks, Accipiter nisus: relative predation risk for breeding passerine birds in relation to their size, ecology and behaviour. Philos.Trans. R. Soc. Lond. 351, 1559–1577 (1996).
Geer, T. Factors affecting the delivery of prey to nestling Sparrowhawks (Accipiter nisus). J. Zool. Lond. 195, 71–80 (1981).
Millon, A., Nielsen, J. T., Bretagnolle, V. & Møller, A. P. Predator-prey relationships in a changing environment: The case of the sparrowhawk and its avian prey community in a rural area. J. Anim. Ecol. 78, 1086–1095 (2009).
Møller, A. P. Interactions between interactions: Predator-prey, parasite-host, and mutualistic interactions. Ann. N. Y. Acad. Sci. 1133, 180–186 (2008).
Newton, I. The Sparrowhawk. (T & A.D. Poyser, 1986).
Kenward, R. E. The Goshawk. (T & A.D.Poyser/A & C. Black, 2006).
Loehle, C. Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335 (1995).
Cresswell, W., Lind, J. & Quinn, J. L. Predator-hunting success and prey vulnerability: Quantifying the spatial scale over which lethal and non-lethal effects of predation occur. J. Anim. Ecol. 79, 556–562 (2010).
Duncan, A. B., Gonzalez, A. & Kaltz, O. Stochastic environmental fluctuations drive epidemiology in experimental host-parasite metapopulations. Proc. R. Soc. B Biol. Sci. 280, 20131747 (2013).
Altizer, S. et al. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484 (2006).
Kramer, V. Habicht und Sperber. (Die neue Brehm Bücherei, 1973).
Quinn, J. L. & Cresswell, W. Predator hunting behaviour and prey vulnerability. J. Anim. Ecol. 73, 143–154 (2004).
Selås, V. Selection of avian prey by breeding sparrowhawks Accipiter nisus in southern Norway: the importance of size and foraging behaviour of prey. Ornis Fenn. 70, 144–154 (1993).
Tinbergen, L. De Sperver als roofvijand van zangvogels. Ardea 34, 1–213 (1946).
Kenward, R. E., Marcström, V. & Karlbom, M. Post-nestling behaviour in goshawks, Accipiter gentilis: II. Sex differences in sociality and nest-switching. Anim. Behav. 46, 371–378 (1993).
Rutz, C. Breeding season diet of Northern Goshawks Accipiter gentilis in the city of Hamburg, Germany. Corax 19, 311–322 (2004).
Rutz, C. Predator fitness increases with selectivity for odd prey. Curr. Biol. 22, 820–824 (2012).
Box, E. D. & Smith, J. H. The intermediate host spectrum in a Sarcocystis species of birds. J. Parasitol. 68, 668–673 (1982).
Dubey, J. P. et al. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 95, 89–131 (2001).
Dubey, J. P., Benson, J. & Larson, M. A. Clinical Sarcocystis neurona encephalomyelitis in a domestic cat following routine surgery. Vet. Parasitol. 112, 261–267 (2003).
Dubey, J. P., Chapman, J. L., Rosenthal, B. M., Mense, M. & Schueler, R. L. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet. Parasitol. 137, 36–49 (2006).
Mansfield, L. S. et al. Brown-headed cowbirds (Molothrus ater) harbor Sarcocystis neurona and act as intermediate hosts. Vet. Parasitol. 153, 24–43 (2008).
Box, E. D., Meier, J. L. & Smith, J. H. Description of Sarcocystis falculata stiles, 1893, a parasite of birds and opossums. J. Protozool. 31, 521–524 (1984).
Hillyer, E. V., Anderson, M. P., Greiner, E. C., Atkinson, C. T. & Frenkel, J. K. An outbreak of sarcocystis in a collection of psittacines. J. Zoo Wildl. Med. 22, 434–445 (1991).
Clubb, S. L. & Frenkel, J. K. Sarcocystis falcatula of opossums: transmission by cockroaches with fatal pulmonary disease in psittacine birds. J. Parasitol. 78, 116–124 (1992).
Dubey, J. P. et al. First isolation of Sarcocystis neurona from the South American opossus, Didelphis albiventris, from Brazil. Vet. Parasitol. 95, 295–304 (2001).
Dubey, J. P., Cawthorn, R. J., Speer, C. A. & Wobeser, G. A. Redescription of the sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae). J. Eukaryot. Microbiol. 50, 476–482 (2003).
Uni, Z., Geyra, A., Ben-Hur, H. & Sklan, D. Small intestinal development in the young chick: crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 41, 544–551 (2000).
Geyra, A., Uni, Z. & Sklan, D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 80, 776–782 (2001).
Lillehoj, H. S. & Chai, J. Y. Comparative natural killer cell activities of thymic, bursal, splenic and intestinal intraepithelial lymphocytes of chickens. Dev. Comp. Immunol. 12, 629–643 (1988).
Zhu, J. J. et al. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 79, 619–625 (2000).
Rutz, C., Whittingham, M. J. & Newton, I. Age-dependent diet choice in an avian top predator. Proc. Biol. Sci. 273, 579–586 (2006).
Krüger, O. & Stefener, U. Nahrungsökologie und Populationsdynamik des Habichts Accipiter gentilis im östlichen Westfalen. Vogelwelt 117, 1–8 (1996).
Krüger, O. & Lindström, J. Habitat heterogeneity affects population growth in goshawk Accipiter gentilis. J. Anim. Ecol. 70, 173–181 (2001).
Ackermann, H. BiAS für Windows, Biometrische Analyse von Stichproben. (Epsilon-Verlag, Hochheim Darmstadt, 2017).
Dixon, W. J. (chief editor). BMDP Statistical Software Manual, Volume 1 and 2. (1993).
Cannon R. N. & Roe, R. T. Krankheitsüberwachung in Tierbeständen: ein Leitfaden zur Bestimmung von Stichprobenumfängen. (Auswertungs- und Informationsdienst für Ernährung, Landwirtschaft und Forsten (AID) e.V., Bonn, 1990).
Cytel Studio StatXact Vers. 9.0.0. Statistical Software for Exact Nonparametric Inference, User Manual. (2010).
Maier, K. et al. Parasite distribution and early-stage encephalitis in Sarcocystis calchasi infections in domestic pigeons (Columba livia f. domestica). Avian Pathol. 44, 5–12 (2015).
Acknowledgements
The authors thank Antoinette Huhn for laboratory assistance, and the hunters, wildlife facilities, veterinary clinics, taxidermists, forestry admissions and veterinary diagnostic laboratories for submitting samples. We wish to especially thank Sylvia Urbaniak, Thomas Vennekel, as well as Andreas Schaubmar for his support with statistical calculations. Sylvia L. Parmentier and this study were funded by the German Research Foundation (DFG) [grant numbers Li 1924/3–1, GR 1491/6–1].
Author information
Authors and Affiliations
Contributions
A.D.G. and M.L. conceived and designed the study, K.F. performed the biometrical planning and the statistical analysis, S.L.P. and K.M. carried out the sample collection, S.L.P. and D.E. performed and interpreted molecular tests, S.L.P. drafted the manuscript, K.M., K.F. and M.L. revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parmentier, S.L., Maier-Sam, K., Failing, K. et al. Prevalence of Sarcocystis calchasi in free-ranging host species: Accipiter hawks and Common Woodpigeon in Germany. Sci Rep 8, 17610 (2018). https://doi.org/10.1038/s41598-018-35862-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35862-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.