Abstract
Obstructive sleep apnoea (OSA) is a sleep disorder involving repeated nocturnal desaturation and sleep fragmentation. OSA can result in decreased daytime alertness and neurocognitive dysfunction. Hypercapnia status is also related to neurocognitive dysfunction in patients with pulmonary diseases. We evaluated the effects of hypercapnia on cognitive performance and memory function in a prospective case-controlled study. We enrolled thirty-nine obese patients with OSA and collected their arterial blood samples. All the participants provided arterial blood samples, and completed two questionnaires (the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale) and six cognitive tasks (the psychomotor vigilance task [PVT], the Stroop task, the Eriksen flanker task, processing speed [DSST], and verbal and visual memory [LM&FM]), which were used to evaluate daytime sleepiness, cognitive function, and memory function within one week of a polysomnographic study. When compared to the OSA without diurnal hypoventilation, the patients with stable hypercapnia (OHS) had increased reaction times in the PVT, Stroop task, and flanker task. Hypercapnic obese patients with OSA also had comparatively significantly lower scores in the processing speed and logical memory tests. OHS had increased reaction times in the attention and cognitive function assessments, and deficits in the logical memory, when compared to those with OSA without diurnal hypoventilation.
Similar content being viewed by others
Introduction
Obstructive sleep apnoea (OSA) syndrome involves recurrent upper airway collapses, leading to hypoxemia and sleep fragmentation during sleep1. The clinical features of OSA include snoring, daytime sleepiness, and sleep fragmentation2. OSA were found significant negative effect sizes for all cognitive domains with the exception of the cognitive domain of perception3. Many studies reported that the patients with OSA have significant deficits in cognitive function, attention, and memory function4,5,6. Further, in one study, Jackson et al. reported significant impairments in attention, vigilance, memory and executive function in the patients with OSA7,8. These cognitive impairments can lead to difficulty in concentration, increased forgetfulness, and problems with decision-making.
Memory dysfunction is another neurocognitive impairment observed in the patients with OSA syndrome9. The mechanism of the relationship between OSA and memory impairment is not clear. The mechanism of cognitive and memory impairment in OSA might involve the effects of sleep fragmentation and regular intermittent hypoxemia injury the brain cell and the hippocampus3,10. In a study by Twigg et al., obstructive sleep apnoea was found to be associated with the impairment of the verbal memory, but not that of the visual memory6. However, the apnoea/hypopnoea index (AHI) and daytime sleepiness were not correlated with memory impairment.
Although the relations between OSA and cognitive impairment has been well-described in several studies, the effects of hypercapnia on cognitive function are poorly understood. Hypercapnia status has been shown to predict mild cognitive impairment in aging rodents11. In addition, patients with OSA and chronic obstructive lung disease (COPD) have been reported to have deficits in attention, psychomotor speed, executive function, language abilities and memory function12,13. Previously, Hypoxemia and/or hypercarbia have been hypothesised as the factors underlying neurocognitive dysfunction13. However, the influence of hypercapnic status on cognition still needs to be determined.
We aimed to compare the impact of chronic stable hypercapnic status (arterial blood gas pH > 7.35 and partial pressure of CO2 [PaCO2] > 45 mmHg) on the neurocognitive function (attention, executive function, and memory function) in the patients with OSA. We hypothesized that the hypercapnic would impaired neurocognitive function in OSA patients.
Methods
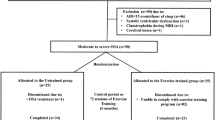
In this prospective study, we enrolled consecutive 39 obese patients (body mass index [BMI] ≥ 30) with moderate-to-severe OSA (AHI score ≥ 15, derived using standard polysomnography) from 150 clinical patients at the Sleep Center at the Hualien Tzu-Chi General Hospital during 2016 and 2017. All the enrolled subjects underwent an arterial blood test within 1 week of the neurocognitive test and completed the neurocognitive tests prior to any treatment of their OSA. All the enrolled subjects completed the Epworth Sleepiness Scale (ESS), the Pittsburgh Sleep Quality Index (PSQI), the psychomotor vigilance task, the Eriksen flanker task, the Stroop task, and a memory test. All the enrolled subjects were >20 years in age. Those with BMI (body mass index) ≧30 can met our obesity criteria. The following patients were excluded from the study: those who had previously been diagnosed or treated for any neurological or psychological diseases that would interfered with the neurocognitive tests, those with chronic lung (standard pulmonary function shows obstructive or restrictive lung disease) or neuromuscular diseases that would have interfered with the neurocognitive task, and those who could not cooperate with the tasks. Patients with unstable vital signs (unstable blood pressure, tachypnoea [respiratory rate > 28 bpm], or acidaemia [pH < 7.35]) were also excluded from our study. The hospital’s Institutional Review Board approved this study (IRB105-20-A), and all the participants provided informed consent. All the research methods were performed in accordance with the relevant guidelines and regulations. The study was funded by Buddhist Tzu Chi General Hospital, Hualien, Taiwan (TCRD 106-19).
Polysomnography
All the patients underwent one night of standard type-1 attended polysomnography (Embla A10, Embla; Broomfield, CO) at our Sleep Center. Sleep and arousal periods were scored according to the standard criteria2. AHI and 4% Oxygen Desaturation Index (ODI) were calculated as the markers of disease severity.
Arterial blood test
All enrolled subjects provided arterial blood samples for testing within 1 week of the neurocognitive test during the daytime. According to the result of arterial blood test, the subjects were divided into PaCO2 ≧ 45 mmHg and PaCO2 < 45 mmHg 2 groups.
Measures
Questionnaires
The ESS is an 8-item questionnaire asking respondents to answer each question using a number between 0 to 3, with a total score ranging from 0 (minimum) to 24 (maximum)14. All enrolled subjects also completed the PSQI including 19 sleep item15.
Studied tasks
The enrolled patients were asked to complete attention (psychomotor vigilance task), cognitive (Flanker and Stroop tasks) and memory task. All the cognitive tasks for these tests were generated using E-prime 2.016.
Attention and processing speed tasks
Psychomotor vigilance task
The patients were instructed to press a button as quickly as possible when a red 500-ms counter appeared on a small screen. We assessed and collected the following outcome measures of (1) omission rate (OR); (2) lape rate (LR); (3) hit rate (HR); (4) fast rate (FR); (5) reciprocal reaction time (RT); (6) measure of speed (1/RT); (7) false RT; (8) the fastest 10% of the RT and the slowest 10% of RT16,17.
Digit Symbol Substitution Test (DSST)
The DSST is a component of the Wechsler Adult Intelligence Scale-Third Edition (WAIS- III). The test requires the subjects to transcribe a unique geometric symbol with its corresponding Arabic number. The outcome measures of correct items completed within 120 seconds16.
Executive tasks
Flanker task
In this study, we used the computerized arrow version of the Flanker task. The outcome we collected the measurements of: (1) omission rate (OR), (2) error rate (ER), (3) accuracy rate (AR); (4) correct RT16,18.
Colour-word Stroop task
Subjects were presented with coloured-words (red, blue, yellow, and green) printed either in ink matching the colour denoted by the word or in a colour not matching that denoted by the word in a computer. Congruent and incongruent trials were presented with equal probabilities. Subjects were instructed to respond to the ink colour of the words by pressing one of four response keys with maximal speed and accuracy. We collected the measurements of: (1) omission rate (OR), (2) error rate (ER), (3) accuracy rate (AR); (4) correct RT16,19,20.
Memory tests
Memory tests are two components of the Wechsler Memory Scale-Third Edition (WMS-III). We select the Faces subtest and the Logical memory subtest (LM). The Faces subtest includes: (1) immediate face memory (IFM); (2) face delayed memory (DFM); (3) Face memory percentage (FMP). The logical memory subtest includes: (1) immediate logical memory (ILM); (2) logical memory learning (LML); (3) delayed logical memory (DLM); (4) logical memory percentage (LMP); (5) logical memory recognition (LMR).
Data collection
Data, including age, sex, body mass index, and daytime sleepiness (determined using the ESS and PSQI) were recorded. Arterial blood tests were performed within one week of the neurocognitive tasks. All the participants completed the psychomotor vigilance, flanker, and Stroop tasks, as well as the memory tests in a quiet, isolated room in the morning, without any interference. The questionnaires and tasks were completed at baseline within 1 week of the polysomnographic study.
Statistical analysis
Independent -t tests were used to compare scores on the ESS, PSQI, psychomotor vigilance task (PVT), and flanker and Stroop tasks, and those on all of the memory tests. P values < 0.05 were considered statistically significant. All statistical analyses were performed using the statistical package SPSS for Windows (Version 14.0, SPSS; Chicago, IL).
Results
Demographic data
Of the 39 enrolled obese patients with OSA, 15 were hypercapnic (arterial blood PaCO2 ≧ 45 mmHg and pH ≧ 7.35), which consistent to diagnosis of obesity hypoventilation syndrome (OHS). Our study thus included 24 education-matched normocapnic subjects (OSA without diurnal hypoventilation). There was no difference between the two groups in age, BMI, or polysomnographic data, including AHI, ODI, and arousal index. There were only significant differences in PaCO2 (mean PaCO2, hypercapnic: 52.75 ± 8.40, normocapnic: 39.65 ± 3.58) between the two groups (Table 1).
ESS and PSQI questionnaires
The mean ESS score was 11.86 ± 3.92 in the hypercapnic group and 12.71 ± 5.71 in the normocapnic group. The mean PSQI score was 11.07 ± 3.71 in hypercapnic patients and 11.04 ± 4.94 in normocapnic patients. There were no significant differences between the groups in ESS or PSQI scores (Table 1).
Attention and Processing speed tasks
PVT
There was a significant increase in both, lapse rate and RT; and a decreased fast rate in the PVT in the hypercapnic group when compared to the normocapnic group (Table 2).
Processing speed (DSST)
Processing speed and all measures of logical memory were significantly worse in the hypercapnic patients with OSA than in the normocapnic patients with OSA (Table 2).
Executive tasks
Stroop task: No significant differences in the omission rate, error rate, or accuracy rate were observed in the congruent and incongruent Stroop tests. However, the correct RT was longer in the hypercapnic group (mean ± standard deviation [SD]: 905.27 ± 18.6 ms) than in normocapnic patients with OSA (mean ± SD: 733.2 ± 157.19 ms). The longer RTs were observed in both the congruent and incongruent tests in the Stroop task (Table 2).
Flanker task
In the flanker task, a significantly increased correct RT was observed in the hypercapnic patients (mean ± SD: 451.89 ± 79.57 ms) when compared to the normocapnic patients (mean ± SD: 395.04 ± 69.6 ms). The increased correct RTs were found in both, the congruent and the incongruent trials, in the flanker task. However, no differences in omission rate, error rate, or accuracy rate in the flanker test were found between the two groups (Table 2).
Memory tests
We assessed two memory parameters in our study: logical memory (immediate logical memory, logical memory learning, delayed logical memory, logical memory percentage, and logical memory recognition), and visual memory (immediate face memory, delayed face memory, and face memory percentage). There were no significant differences in the verbal memory between the two groups (Table 3).
Table 4 shows linear regression of each significant neurocognitive tasks and memory test with clinical and polysomnographic characteristics. LR, FR and RT was correlated to ODI, AHI and daytime PaCO2 levels. RT of Stroop task but not flanker task was correlated with daytime PaCO2 levels. In the other hands, most of the memory dysfunction(ILM, DLM, LMP) correlated with age and daytime PaCO2 levels. Processing process impairment was associated with age, arousal index and daytime PaCO2 levels.
Discussion
In the present study, obese patients with OSA and stable hypercapnia (OHS) had deficits in logical memory and attention, as well as increased correct RT in executive tasks when compared to those with OSA without diurnal hypoventilation. However, although the hypercapnic obese patients with OSA required longer to perform the executive and attention tasks, there were no differences in accuracy or error rate in the cognitive tasks. No differences in working memory or visual memory were found between hypercapnic and normocapnic patients with OSA. With the multiple regression, prolonged reaction time was correlated with intermittent nocturnal hypoxemia and daytime hypercapnia, as well as impaired momory was related with age and daytime hypercapnia.
Many studies have reported deficits in memory and cognitive ability in patients with OSA compared to individuals without OSA5,9,21. Most of these studies conclude that the memory or cognitive impairments were due to the pathophysiology of intermittent hypoxemia.
A few studies have addressed the importance of monitoring hypercapnia in the daytime or during sleep. Wang et al.22 have shown that hypercapnia may slow brain neural activity and lead to neurobehavioral impairments. In another brain imaging study, breathing 5% CO2 significantly suppressed all magnetic resonance imaging indices of functional connectivity23. In addition, animal studies have shown that hypercapnia leads to slower electroencephalography (EEG) activity24,25. In this study, we show that hypercapnia delayed the reaction time in cognitive function tests and was correlated with deficits in logical memory, but not verbal memory, in the obese patients with OSA. This finding highlights the importance of hypercapnia to cognitive and memory dysfunction in patients with OSA.
Previous studies have reported significant neurocognitive impairment in patients with OSA. Lee et al. have reported impairments in sustained attention on the PVT26. Furthermore, Tulek27 et al. have reported impairments in the executive function of the patients with OSA when compared to the normal subjects, in a neuroimaging study showing decreased activation in the anterior cingulate cortex associated with OSA28. In addition, cognitive impairments have been noted in patients with COPD, although the relationship among cognitive impairment, hypoxemia and hypercapnia is still debated29. In the present study, daytime hypercapnia was associated with increased RT in attention (PVT) and executive function (Stroop task and flanker task) tasks in patients with OSA.
In addition to attention and executive impairments, memory impairments have also been reported in the patients with OSA and COPD21,29. A majority of studies have reported impairments in the short-term verbal5 and visual memory, as well as the long-term semantic memory30 and procedural memory31 in the patients with OSA. Memory impairments have also been noted in COPD with underlying chronic hypoxia-hypercapnia pathology in animals11, although the effects of hypercapnia in human beings are not well-understood. In the present study, we demonstrated that hypercapnia impaired processing speed and logical memory, but not visual or working memory in obese patients with OSA.
On the other hand, ODI was not different between the groups, but hypercapnia was. Moreover, some studies reported an increase in the blood pressure during apnoea-hypopnea. This may be due to the exacerbation of nocturnal hypoxemia and hypercapnia as a result of apnoea-hypopnea. The severity of hemodynamic changes due to apnoea- hypopnea may be related to the duration of apnoea- hypopnea episodes, which can vary from 10 seconds to 1 minute, but may not be reflected in the AHI and ODI scores. Further, prolonged apnoea events may even lead to a decrease in the AHI and ODI index32. The mechanism and relationship between PSGdeoxygenation index and hypercapnia should be investigated in the future research.
Our study had some limitations. First, the hypercapnic obese OSA group had a small sample size. Second, we used a daytime blood test to define hypercapnia rather than nocturnal non-invasive blood carbon dioxide monitoring. Furthermore, the blood carbon dioxide levels were not determined during the neurocognitive tests. In addition, research including larger samples of patients with OSA should be considered for future studies.
In conclusion, not all the measured parameters in the neurocognitive tests were worse in OHS than OSA without diurnal hypoventilation. However, daytime stable hypercapnia prolonged the RT in the cognitive and attention function tests and led to deficits in logical memory function in obese patients with OSA without diurnal hypoventilation.
References
Young, T. et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 328, 1230–5 (1993).
The American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults, recommendations for syndrome definition, measurement techniques in clinical research. Sleep 22, 667–89 (1999).
Stranks, E. K. & Crowe, S. F. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-analysis. Archives of Clinical Neuropsychology, 2016 31, 186–193 (2016).
Lal, C., Strange, C. & Bachman, D. Neurocognitive impairment in obstructive sleep apnoea. Chest 141, 1601–10 (2012).
Verstraeten, E., Cluydts, R., Pevernagie, D. & Hoffmann, G. Executive function in sleep apnoea: controlling for attentional capacity in assessing executive attention. Sleep 27, 685–93 (2004).
Twigg, G. L. et al. Obstructive sleep apnoea syndrome is associated with deficits in verbal but not visual memory. Am. J. Respir. Crit. Care Med. 182, 98–103 (2010).
Jackson, M. L., Howardm, M. D. & Barnesm, M. Cognition and daytime functioning in sleep-related breathing disorders. Prog. Brain Res. 190, 53–68 (2011).
Bucks, R. S., Olaithe, M. & Eastwood, P. Neurocognitive function in obstructive sleep apnoea:A meta-review. (Respirology) 18, 61–70 (2013).
Salorio, C. F., White, D. A., Piccirillo, J., Duntley, S. P. & Uhles, M. L. Learning, memory, and executive control in individuals with obstructive sleep apnoea syndrome. J. Clin. Exp. Neuropsychol. 24, 93–100 (2002).
Kheirandish, L., Gozal, D., Pequignot, J. M., Pequignot, J. & Row, B. W. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr. Res. 58, 594–9 (2005).
Mitschelen, M. et al. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neurosci. 164, 918–28 (2009).
Andreou, G., Vlachos, F. & Makanikas, K. Effects of chronic obstructive pulmonary disease and obstructive sleep apnoea on cognitive functions: evidence for a common nature. Sleep Disord. 2014, 768210, https://doi.org/10.1155/2014/768210 (2014).
Olaithe, M., Bucks, R. S., Hillman, D. R. & Eastwood, P. R. Cognitive deficits in obstructive sleep apnoea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018. 38, 39–49 (2018).
Johns, M. W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14, 540–5 (1991).
Buysse, D. J., Reynolds, C. F. III., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 28, 193–213 (1989).
Shen, Y. C, Kung, S. C, Chang, E. T, Hong, Y. L, Wang, L. Y. The impact of obesity in cognitive and memory dysfunction in obstructive sleep apnea syndrome. International journal of obesity. 28, https://doi.org/10.1038/s41366-018-0138-6 (2018).
Dinges, D. F. & Powell, J. W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 17, 652–5 (1985).
Eriksen, B. A. & Eriksen, C. W. Effects of noise letters upon identification of a target letter in a non-search task. Percept. Psychophys. 16, 143–9 (1974).
Macleod, C. M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 109, 163–203 (1991).
Macleod, C. M. & McDonald, P. A. Interdimensional interference in the Stroop effect, uncovering the cognitive and neural anatomy of attention. Trends Cogn. Sci. 10, 383–91 (2000).
Wallace, A. & Buck, R. S. Memory and obstructive sleep apnoea: A meta-analysis. Sleep. 36, 203–20 (2013).
Wang, D., Thomas, R. J., Tee, B. J. & Grunstein, R. R. Hypercapnia is more important than hypoxia in the neuro-outcomes of sleep disordered breathing. J. Appl. Physiol. 120, 1484–6 (2016).
Xu, F. et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J. Cereb. Blood. Flow Metab. 31, 58–67 (2011).
Forslid, A., Ingvar, M., Rosen, I. & Ingvar, D. H. Carbon dioxide narcosis: influence of short-term high concentration carbon dioxide inhalation on EEG and cortical evoked responses in the rat. Acta Physiol. Scand. 127, 281–7 (1986).
Zappe, A. C., Uludağ, K., Oeltermann, A., Uğurbil, K. & Logothetis, N. K. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb. Cortex 18, 2666–73 (2008).
Lee, I. S. et al. The relation between psychomotor vigilance performance and quality of life in obstructive sleep apnoea syndrome. J. Clin. Sleep Med. 7, 254–60 (2011).
Tulek, B., Atalay, N. B., Kanat, F. & Suerdem, M. Attentional control is partially impaired in obstructive sleep apnoea syndrome. J. Sleep Res. 22, 422–9 (2013).
Zhang, X., Ma, L., Li, S., Wang, Y. & Wang, L. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnoea. Sleep Med. 12, 335–40 (2011).
Dodd, J. W., Getov, S. V. & Jones, P. W. Cognitive function in COPD. Eur. Respir. J. 35, 913–22 (2010).
Ferini-Strambi et al. Cognitive dysfunction in patients with obstructive sleep apnoea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res. Bull. 61, 87–92 (2003).
Naegele, B. et al. Which memory processes are affected in patients with obstructive sleep apnoea? An evaluation of 3 types of memory. Sleep 29, 533–44 (2006).
Wu, H., Zhan, X., Zhao, M. & Wei, Y. Mean apnoea-–hypopnea duration (but not apneanotapnea–hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnoea. Medicine 95, e5493 (2016).
Author information
Authors and Affiliations
Contributions
The study was planned by Chang E.T., Kung S.C. and Shen Y.C. Recruitment of patients, as well as the collection of data and samples, was done by Chang E.T., Kung S.C. and Hong Y.L. Chang E.T., Kung S.C. and Shen Y.C. performed the central coordination, as well as the collation of data. Chang E.T., Kung S.C. and Wang L.Y. interpreted the data, and drafted the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kung, SC., Shen, YC., Chang, ET. et al. Hypercapnia impaired cognitive and memory functions in obese patients with obstructive sleep apnoea. Sci Rep 8, 17551 (2018). https://doi.org/10.1038/s41598-018-35797-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35797-3
Keywords
This article is cited by
-
A retrospective study of the role of hypercapnia in patients with acromegaly
BMC Pulmonary Medicine (2023)
-
The effect of intraoperative transnasal humidified rapid-insufflation ventilatory exchange on emergence from general anesthesia in patients undergoing microlaryngeal surgery: a randomized controlled trial
BMC Anesthesiology (2023)
-
Associations between hypoxia parameters in obstructive sleep apnea and cognition, cortical thickness, and white matter integrity in middle-aged and older adults
Sleep and Breathing (2021)
-
The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome
Journal of Neuroinflammation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.