Abstract
This study compared long-term population-based survival outcomes of preoperative and postoperative radiotherapy (RT) approaches in rectal cancer. Patients with stage II-III rectal cancer between 1998 and 2013 were identified using the Surveillance, Epidemiology, and End Results database. Overall survival (OS) and disease-specific survival (DSS) rates were estimated in propensity-matched study population according to the use of RT. Among the 28,320 eligible patients, a total of 18,400 patients were identified from propensity score matching process balancing the distribution of prognostic covariates. The 10-year OS and DSS rates were higher in patients with preoperative RT than the postoperative group (51.6% vs. 49.8% with P < 0.001, and 65.4% vs. 64.8% with P = 0.037, respectively). However, in multivariate analysis, selection of combined RT sequence did not affect the survival (hazard ratio [HR] 1.04 and 95% confidence interval [CI] 0.98−1.10 for OS; HR 0.97 and 95% CI 0.90−1.05 for DSS). Regarding hazard rate functions of cancer-specific mortality, the overall time-course risks after preoperative and postoperative RT were comparable. This study provides additional insight into the long-term prognostic implications of the two RT strategies, suggesting that the sequence of RT does not lead to differential survival in stage II-III rectal cancer.
Similar content being viewed by others
Introduction
According to the recent cancer statistics, colorectal cancer is the 3rd most common cancer in the United States1. Although incidence and death rates have decreased, colorectal cancer is still a leading cause of cancer deaths2. Approximately 39,910 new rectal cancer cases are reported annually1, and most are diagnosed as adenocarcinoma. In stage II-III rectal cancer, a multidisciplinary approach including surgical resection, radiotherapy (RT), and chemotherapy is the cornerstone of curative aim3. The advent of total mesorectal excision (TME) and combined use of RT or chemoradiotherapy for high-risk patients have improved oncologic outcomes over several decades4,5.
Recently, preoperative use of RT has become the preferred option for locally advanced rectal adenocarcinomas6. Given that the Dutch trials demonstrated improved local control with neoadjuvant RT7,8, three phase III randomized controlled trials, including the German CAO/ARO/AIO-94, National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03, and a Korean study, compared pre- and postoperative use of RT9,10,11. Therapeutic efficacy was evaluated with locoregional recurrence, down-staging, survival, sphincter preservation, and toxicity, but some contradictory results were observed. Moreover, the NSABP R-03 trial was closed early due to an under-recruitment problem, and the number of patients analyzed in the Korean trial was relatively small10. Therefore, the recommendation for preoperative treatment is based mainly on the German trial9. There was a meta-analysis of the three randomized trials, but interpretation was limited due to the small number of eligible studies12.
Survival improvement is one important element in assessing the effectiveness of treatment. However, an updated analysis of the German trial, with a median follow-up duration of 11 years, demonstrated that survival outcomes were not different between preoperative and postoperative strategies13. Despite widespread use of the preoperative approach, a recent report from the National Cancer Data Base stated that approximately only one-half of stage II-III rectal cancer patients underwent RT before surgery in the United States14. Thus, we used the Surveillance, Epidemiology, and End Results (SEER) program, a nation-wide cancer database from the United States15, to evaluate the potential of improved survival with preoperative RT in comparison with postoperative treatment in stage II-III rectal cancer. Since some confounding factors can affect the receipt of preoperative or postoperative options, propensity scores were calculated and adjusted. Baseline hazard rate function plots were used to elucidate time-course changes of cancer-related mortality risks. Our matched comparison analysis of long-term survival outcomes provides additional knowledge for optimizing the sequence of RT combined with surgery in locally advanced rectal cancer.
Methods
Patients
This study analyzed SEER data (1973–2013), the open-access cancer registry of the National Cancer Institute (NCI) in the United States. The authors conducted the present analysis after an approval of the “Research Data Agreement” from the SEER. Since the entire raw data were recorded as de-identified, informed consent from the subjects were not required. All of the process has never been involved in identifying personal information of the database. The data extraction and analysis were performed in accordance with relevant guidelines that were published by the SEER16.
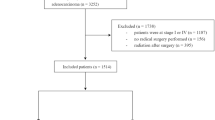
To extract patient data, we used SEER*Stat software (version 8.3.2; National Institutes of Health, Bethesda, MD)16. The raw data included multifarious patients and tumor-related records, including variables of demographics, clinicopathologic information, and survival outcomes. We identified rectal cancer cases based on categories of “C19.9-Rectosigmoid junction” and “C20.9-Rectum, NOS” from the “Primary Site labeled” variable. The histology of adenocarcinomas was identified using the International Classification of Diseases with the malignant behavior code of “/3”. The eligibility criteria included: (1) age >18 years, (2) year of diagnosis from 1998 to 2013, (3) stage II or III based on the 7th American Joint Committee on Cancer (AJCC) staging system, (4) cancer-directed surgery performed, and (5) use of RT after or prior to surgery. Cases without information of primary or lymph node surgery were excluded. Supplementary Fig. 1 represents the diagram of patient selection.
The SEER database summarizes clinical and pathological reports to obtain optimal stage information. We used the variables of AJCC stage and SEER historic or summary stage to exclude cases with initial distant metastasis. Treatment information for each patient was obtainable from the variables “Radiation sequence with surgery,” “Radiation,” and “Reason no cancer-directed surgery.”
Calculation of propensity scores and adjustment process
In clinical investigations, treatment-related selection bias cannot be ruled out without prospective randomization. A propensity score is the calculated probability of being treated with a certain procedure given a set of baseline covariates17. To eliminate potential effects of baseline clinicopathologic variables, propensity score matching method has been widely used in retrospective design18. Since the population-based SEER database consists of observational data, we calculated propensity scores and matched patients according to the combined sequence of RT, preoperative vs. postoperative treatment.
For the matching process, prognostic factors evaluated in the initial raw data were applied: age, sex, race, marital status, subsite, histology, extent of primary tumor, lymph node status, tumor grade, types of primary surgery, and lymph node dissection. After calculation of propensity scores with a non-parsimonious logistic regression model, a one-to-one matching process was conducted based on the nearest neighbor method with a caliper 0.2 and without replacement. To assess the balance between the two groups, standardized difference (SD) values for covariates less than 0.1 were considered acceptable after the matching process19.
Statistical analysis
Clinicopathologic characteristics of the pre- and postoperative RT groups were compared with Pearson’s chi-square and Mann-Whitney U tests for categorized and continuous variables, respectively. Overall survival (OS) and disease-specific survival (DSS), defined as the time interval from the diagnosis of cancer to overall and cancer-related death events, respectively, were evaluated as the outcomes of interest. Kaplan-Meier analysis and log-rank tests were used to compare survival differences according to the corresponding covariates, including preoperative vs. postoperative RT. In survival analysis according to nodal status, log odds of lymph nodes were calculated by the formula, “Log [(PLN + 0.5)/(TLN-PLN + 0.5)]”, where “PLN” is the number of positive lymph nodes, and “TLN” is the total number of lymph nodes surgically dissected. Optimal cut-off values of continuous variables, such as age at diagnosis, tumor size, and log odds of lymph nodes, were determined with a maximal chi-square method. For multivariate analysis, the Cox proportional hazards model was applied after the evaluation of proportional hazards assumptions using log-minus-log survival plots of each variable. The potentially associated factors from univariate analyses were included in the Cox-regression analysis of OS and DSS. P-values < 0.05 were assessed as statistically significant. IBM SPSS Statistics 22.0 (IBM, Armonk, NY, USA) and R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
Results
Study population before propensity score matching
According to the aforementioned eligibility criteria, a total of 28,320 patients were identified. Patient, tumor, and treatment-related characteristics are summarized and compared according to preoperative and postoperative RT methods (Table 1). The median age was 61 (range, 19−99), and 62% of the patients were male. With a predominance of Caucasian patients (82%), 62% were married. Tumor location at the rectosigmoid portion was reported in 5,270 (19%) patients. Not otherwise specified adenocarcinoma histology was diagnosed in 22,875 (81%) patients, and the proportion of tumor grade II (71%) was highest. Approximately 10%, 81%, and 9% of the patients were diagnosed with tumor extension into the submucosa to muscularis propria, pericolorectal tissues, and adjacent organs or structures, respectively, with a median pathologic tumor size of 4.0 cm (range, 0.0−85.0). Stage III with positive lymph node status was observed in 15,523 (55%) patients. Regarding types of primary surgery, 20,570 (73%), 7,117 (25%), and 633 (2%) patients underwent sphincter-preserving, abdominoperineal resection, and pelvic exenteration procedures, respectively. Lymph node dissection was performed in 95% of the patients. The median number of excised and positive lymph nodes was 12 (range, 0−90) and 0 (range, 0−75), respectively.
Propensity score matching
Table 2 represents the propensity-matched model of preoperative vs. postoperative RT groups. The matching process identified a total of 18,400 patients who underwent preoperative (n = 9,200) and postoperative (n = 9,200) RT. Given that the overall SD value decreased from 0.628 to 0.061, the distribution of baseline covariates was well-balanced after propensity score matching. For each of the variables, all SD values were less than 0.1 and considered acceptable.
Kaplan-Meier survival outcomes
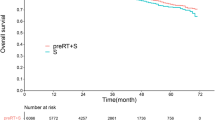
In the matched population (median follow-up: 4.3 years), the 10-year and 14-year OS rates of preoperative vs. postoperative RT groups were 51.6% vs. 49.8% and 43.1% vs. 40.0%, respectively (P < 0.001) (Fig. 1A). The DSS rates at 10 and 14 years were 65.4% vs. 64.8% and 62.0% vs. 61.7%, respectively (P = 0.037) (Fig. 1B). For survival analysis, OS and DSS outcomes were compared according to age (≤67 and >67 years), sex (male and female), race (Caucasian and non-Caucasian), marital status (married and not married), site (rectum, not otherwise specified [NOS] and rectosigmoid), types of adenocarcinoma histology (specified and NOS), tumor grade (I, II, and III−IV), extent of primary tumor (submucosa to muscularis propria, pericolorectal tissues, and adjacent organs or structures), pathologic tumor size (<6.0 and ≥6.0 cm), log odds of lymph nodes (≤−0.49 and >−0.49), surgical treatment (sphincter-preserving, abdominoperineal resection, and pelvic exenteration), and combined RT method (preoperative and postoperative). Optimal cutoffs for the above continuous variables were determined by a maximal chi-square method. In univariate analysis of OS, age (P < 0.001), sex (P < 0.001), marital status (P < 0.001), site (P = 0.017), histology (P < 0.001), tumor grade (P < 0.001), extent of primary tumor (P < 0.001), pathologic tumor size (P < 0.001), log odds of lymph nodes (P < 0.001), surgical treatment (P < 0.001), and combined sequence of RT (P < 0.001) were significant factors. For DSS, the same statistically significant relationships were also observed for most of the above prognostic factors (P = 0.037 for combined sequence of RT and P < 0.001 for others), except for sex (P = 0.402) and site (P = 0.513) (Table 3).
Multivariate comparison analysis of preoperative vs. postoperative RT
Table 4 shows the Cox-regression analysis results. For both OS and DSS, the different combinatory strategies of RT did not result in different survival outcomes (hazard ratio [HR] 1.04 and 95% confidence interval [CI] 0.98−1.10 for OS; HR 0.97 and 95% CI 0.90−1.05 for DSS). In terms of OS, age >67 years (P < 0.001), not married (P < 0.001), tumor grade III−IV (P < 0.001), primary tumor extent of pericolorectal tissues and adjacent organs or structures (P < 0.001 for both), pathologic tumor size ≥6.0 cm (P < 0.001), log odds of lymph nodes > −0.49 (P < 0.001), and abdominoperineal resection (P < 0.001) induced worse outcomes, and female (P < 0.001) and NOS histology (P < 0.001) were associated with favorable survival. The prognostic associations of significant variables in OS were the same in DSS (P < 0.001 for all comparisons), except for sex.
Hazard rate of disease-specific mortality risks
Figure 2 represents baseline hazard rate function plots of disease-specific mortality according to tumor stage, surgical treatment, and combined RT sequence. The highest and maximal risk increment occurred within 5 years of follow-up, and late risk peaks even after 10 years were observed irrespective of the subgroups. When the patients were stratified according to extent of primary tumor and node status, such as T3-4N−, T1-2N+, and T3-4N+, the overall level of mortality risks was highest with T3-4N+ tumors. Although the overall mortality risk after sphincter-preserving surgery was lower than following the other surgeries, the favorable subgroup also showed late risk peaks around 13−14 years. Overall risk levels were comparable between the pre- and postoperative RT groups along the long-term follow-up period.
Discussion
We evaluated long-term survival outcomes of stage II-III rectal cancer patients who underwent preoperative or postoperative RT. After propensity score matching, use of RT prior to radical surgery was associated with improved OS and DSS in univariate Kaplan-Meier analysis. However, the favorable prognostic impacts were not maintained after adjusting for other related clinicopathologic covariates. There were no definite differences in the time-course patterns of cancer-specific mortality between the two treatment groups. The present study is the first comparative survival analysis of preoperative and postoperative RT in locally advanced rectal cancer, using long-term population-based data.
Among the three historical phase III trials comparing pre- and postoperative RT9,10,11, the German CAO/ARO/AIO-94 trial was the largest one, suggesting clinical benefits of the preoperative approach in local tumor control (P = 0.006), down-staging effect (P < 0.001), conversion rate of sphincter preservation (P = 0.004), and severe acute and late toxicity (P = 0.001 and 0.01, respectively)9. However, long-term analysis of the same data found no survival difference between the two RT strategies (59.9% vs. 59.6% for preoperative vs. postoperative, P = 0.85)13. Although the NSABP R-03 study suggested a trend towards improved survival with preoperative RT (74.5% vs. 65.6%, P = 0.065), the results have not been considered decisive due to the poor accrual of patients10. In another Korean trial, significant differences did not exist in disease-free survival (P = 0.866), local control (P = 0.393), and OS (P = 0.620), but preoperative RT resulted in higher rates of sphincter preservation for low-lying tumors (P = 0.008)11. Although the German trial contributed most significantly to the current clinical guidelines in favor of use of preoperative RT3, the beneficial effect in local tumor control was mainly confined to the intention-to-treat analysis9. Additionally, whether enhanced oncologic outcomes with a preoperative approach induce long-term survival benefits remains unclear. Therefore, a comparative prognostic assessment of the two different RT strategies is needed in contemporary clinical practices.
The SEER database contains large-scale data on a variety of malignancies15. Given that the registry includes accurate treatment information, we used the data to compare the two RT approaches in stage II-III rectal cancer. The large patient population is a strength of our study, and survival outcomes after longer durations of follow-up can be informative. Nevertheless, under the retrospective design, results of univariate analysis cannot be conclusive due to the existence of various confounding factors and related selection bias. In this study, the preoperative RT strategy seemed to be associated with lower mortality risks in the univariate analysis, but did not significantly affect long-term prognosis after adjusting for other related factors. The absence of significant survival differences between the two groups is consistent with conclusions of prior investigations11,12,13,20.
Current clinical guidelines endorse preoperative RT as the preferred option3. Irradiation before surgical resection sterilizes gross and microscopic tumor cells under the better condition of tumor oxygenation, suggesting potential benefits in preventing further tumor spread within the locoregional RT field5,9. In clinics, main reasons for the recommendation have been derived from some favorable oncologic outcomes: down-staging effect, increased likelihood of sphincter preservation, and relatively better treatment compliance expected6,7,9,10,11. In this study, the unadjusted results indicating better survival with preoperative RT might be attributable to favorable factors related to the use of RT prior to surgery. However, adjusting for a variety of baseline characteristics, including demographic data, detailed pathologic features, extent of tumor and nodal status, and types of surgical treatment, we conclude that the selection preoperative or postoperative RT did not affect overall or cancer-related death events. Here, from long-term analysis, we suggest that the RT strategy in each case needs to be determined at the discretion of the physician or surgeon with informed consent of patient, considering the need of sphincter preservation and compliance of surgery or RT at the institution.
To estimate time-course changes of cancer-specific mortality risks, baseline hazard rate functions were plotted and analyzed. When individual hazard rate curves were drawn according to tumor extent and combined RT approaches, most of the short-term risk peaks were maximized within 5 years of follow-up, and a sustained long-term risk increment even after 10 years was noticeable. The overall risk patterns evaluated in a time-dependent manner were comparable between the two RT groups, whereas a late risk peak was revealed in the adjuvant RT group after approximately 14 years. Our results highlight the importance of continued surveillance with a longer follow-up duration, and further suggest the need for a systematic strategy to prevent the potential of late failure. Further multi-institutional investigations are needed to elucidate the long-term failure patterns in locally advanced rectal cancer21,22.
To date, several prior investigations have used the SEER registry to analyze rectal cancer patients and the combined use of RT23,24. Peng et al. compared T3N0 rectal cancer patients who underwent surgery alone, preoperative RT followed by surgery, and surgery plus postoperative RT23. They found that use of postoperative RT was associated with improved 10-year DSS rates compared with surgery alone (76.1% vs. 66.1%, P < 0.001), whereas there was no survival benefit of preoperative RT (P = 0.127). However, the study did not conduct any calculation to reduce treatment-related selection bias. Another SEER study evaluated the effectiveness of preoperative RT compared with surgery alone, in stage II-III rectal cancer24. The study performed propensity score matching, and the use of preoperative RT led to improvement in DSS even after adjusting for other clinicopathologic factors (HR 0.741, 95% CI 0.646−0.811). Based on patient age (>50 and ≤50 years), the benefits of RT were confined to older patients (P = 0.006 and <0.001 for stage II and III, respectively). Although the potential for differential prognostic effects in different age groups was a novel finding, such exploratory subgroup analysis cannot be conclusive using a retrospective design. In addition, the comparison of preoperative RT with surgery alone is less clinically relevant, in that neoadjuvant or adjuvant RT has been generalized in the contemporary treatment of locally advanced rectal cancer.
Staging discrepancies between clinical and pathological tumor status exist in analyzing patients who underwent preoperative RT. In the era of neoadjuvant treatment for rectal cancer, post-RT tumor regression grade or neoadjuvant rectal (NAR) score is considered as a surrogate marker for prognosis25,26. The SEER summarizes clinical and pathological stage information for some specific subsets of patients who underwent any preoperative therapy (RT, chemotherapy, hormone therapy or immunotherapy). Due to varying degrees of individual treatment response, the largest or greatest extent of disease was coded regarding tumor status prior to and after preoperative treatment27,28. Although we applied demographic and tumor-related variables as possible in the matching process, potential selection bias from the unknown information of pathological response was inevitable.
Tumor location from the anal verge is one of the important factors to decide treatment strategies. In this study, however, the type of surgery was adjusted in the propensity score matching and Cox-regression analysis, which partially enabled the consideration of low-lying tumors. Besides, variations in clinical practices due to different institutional policies or a clinician’s discretion can also influence the selection of either preoperative or postoperative RT. Patients with more risk features at initial diagnosis might have higher tendency to undergo preoperative treatment, which was not adjusted in the present analysis. The absence of chemotherapy information and RT regimens (short-course or long-course), as per the policy of SEER, was another weakness29. Although pelvic recurrence is an important outcome of interest in other clinical investigations of rectal cancer, failure events or recurrence-free survival data were not available in the SEER database. Other individual health data, such as underlying comorbid illness, performance status, toxicity profiles, and any reasons that the patient could not undergo preoperative treatment first, and vice versa, were not obtainable. In fact, the incompleteness of data is an inevitable limitation of the population-based studies. Nevertheless, our study has its value in that the results were based on large-scale long-term survival data under the contemporary treatment techniques.
We compared the impacts of pre- and postoperative RT on patient survival in stage II-III rectal cancer using the large-scale SEER database. The different sequence of RT relative to surgery did not independently affect long-term OS and DSS, and the time-course patterns of mortality risks were comparable between the two treatment groups. Along with a sustained long-term risk increment in patients overall, the occurrence of a late risk peak near the end of follow-up suggests the need for optimal systemic management to prevent late failure events. Our population-based results are supportive of equivalent survival outcomes of the two combined approaches in locally advanced rectal cancer, which provides additional insights into the long-term prognostic implications of RT strategies. Further studies, such as ongoing trials of total neoadjuvant therapy30, are needed to improve therapeutic efficacy of the current standard treatment.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 67, 7–30 (2017).
Siegel, R. L. et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 67, 177–193 (2017).
National Comprehensive Cancer Network. Rectal Cancer. Version 3.2017, https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
Enker, W. E., Thaler, H. T., Cranor, M. L. & Polyak, T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181, 335–346 (1995).
Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 358, 1291–1304 (2001).
Ma, B. et al. What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer 141, 1052–1065 (2017).
Kapiteijn, E. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345, 638–646 (2001).
van Gijn, W. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12, 575–582 (2011).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351, 1731–1740 (2004).
Roh, M. S. et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27, 5124–5130 (2009).
Park, J. H. et al. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 117, 3703–3712 (2011).
Song, J. H. et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II-III resectable rectal cancer: a meta-analysis of randomized controlled trials. Radiat Oncol J 35, 198–207 (2017).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30, 1926–1933 (2012).
Sineshaw, H. M., Jemal, A., Thomas, C. R. Jr. & Mitin, T. Changes in treatment patterns for patients with locally advanced rectal cancer in the United States over the past decade: An analysis from the National Cancer Data Base. Cancer 122, 1996–2003 (2016).
National Cancer Institute. About the SEER Program, https://seer.cancer.gov/about/.
National Cancer Institute. SEER*Stat Software, https://seer.cancer.gov/seerstat/.
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46, 399–424 (2011).
Austin, P. C. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 33, 1242–1258 (2014).
Austin, P. C., Grootendorst, P. & Anderson, G. M. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 26, 734–753 (2007).
Lee, B. C. et al. Matched case-control analysis comparing oncologic outcomes between preoperative and postoperative chemoradiotherapy for rectal cancer. Ann Surg Treat Res 92, 200–207 (2017).
Yeo, S. G. et al. Patterns of failure in patients with locally advanced rectal cancer receiving pre-operative or post-operative chemoradiotherapy. Radiat Oncol 8, 114 (2013).
Merkel, S., Mansmann, U., Hohenberger, W. & Hermanek, P. Time to locoregional recurrence after curative resection of rectal carcinoma is prolonged after neoadjuvant treatment: a systematic review and meta-analysis. Colorectal Dis 13, 123–131 (2011).
Peng, L. C. et al. Surveillance, epidemiology, and end results-based analysis of the impact of preoperative or postoperative radiotherapy on survival outcomes for T3N0 rectal cancer. Cancer Epidemiol 38, 73–78 (2014).
Wu, L. et al. Population-based study of effectiveness of neoadjuvant radiotherapy on survival in US rectal cancer patients according to age. Sci Rep 7, 3471 (2017).
George, T. J. Jr., Allegra, C. J. & Yothers, G. Neoadjuvant rectal (NAR) score: a new surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Rep 11, 275–280 (2015).
Patel, U. B. et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29, 3753–3760 (2011).
National Cancer Institute. Review of Staging Systems - SEER Training, https://training.seer.cancer.gov/collaborative/intro/systems_review.html.
Fritz, A. et al. SEER Documentation Program Code, 3rd Edition, 1998: Section IV, https://seer.cancer.gov/archive/manuals/codeman.pdf.
National Cancer Institute. Radiation/Chemotherapy Databases - SEER Data & Software, https://seer.cancer.gov/data/treatment.html.
Hong, T. S. & Ryan, D. P. Total neoadjuvant therapy for locally advanced rectal cancer-the new standard of care? JAMA Oncol 4, e180070 (2018).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (2017R1C1B5015640).
Author information
Authors and Affiliations
Contributions
All authors were involved in study design of this paper. Y.J. Lim and Y. Kim conducted data analysis. Y.J. Lim and M. Kong drafted the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, Y.J., Kim, Y. & Kong, M. Comparative survival analysis of preoperative and postoperative radiotherapy in stage II-III rectal cancer on the basis of long-term population data. Sci Rep 8, 17153 (2018). https://doi.org/10.1038/s41598-018-35493-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35493-2
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.