Abstract
The function of Forkhead box O 1 (FOXO1) and pSerine256-FOXO1 immunostaining in esophageal cancer is unclear. To clarify the prognostic role of nuclear FOXO1 and cytoplasmic pSerine256-FOXO1 immunostaining, a tissue microarray containing more than 600 esophageal cancers was analyzed. In non-neoplastic esophageal mucosae, FOXO1 expression was detectable in low and pSerine256-FOXO1 expression in high intensities. Increased FOXO1 and decreased pSerine256-FOXO1 expression were linked to advanced tumor stage and high UICC stage in esophageal adenocarcinomas (EACs) (tumor stage: p = 0.0209 and p < 0.0001; UICC stage: p = 0.0201 and p < 0.0001) and squamous cell carcinomas (ESCCs) (tumor stage: p = 0.0003 and p = 0.0016; UICC stage: p = 0.0026 and p = 0.0326). Additionally, overexpression of FOXO1 and loss of pSerine256-FOXO1 expression predicted shortened survival of patients with EACs (p = 0.0003 and p = 0.0133) but were unrelated to outcome in patients with ESCCs (p = 0.7785 and p = 0.8426). In summary, our study shows that overexpression of nuclear FOXO1 and loss of cytoplasmic pSerine256-FOXO1 expression are associated with poor prognosis in patients with EACs. Thus, evaluation of FOXO1 and pSerine256-FOXO1 protein expression - either alone or in combination with other markers - might be useful for prediction of clinical outcome in patients with EAC.
Similar content being viewed by others
Introduction
Esophageal cancer is one of the most aggressive cancers worldwide1. Currently, there are limited clinical approaches for the early diagnosis and treatment of esophageal cancer, resulting in a 10% five-year survival rate for patients1. Therefore, analysis of novel molecular markers that may help to predict tumor behavior and allow for a personalized therapy in individual esophageal cancer patients are urgently needed. In literature, several biomarkers have been reported in esophageal cancers2,3. In EACs, Erb-b2 receptor tyrosine kinase 2 (HER2) has been identified as a relevant prognostic marker which can be targeted by the anti-HER2 monoclonal antibody trastuzumab4. Trastuzumab in addition to standard chemotherapy has become standard of care for HER2 positive advanced-stage gastro-esophageal cancers4,5. Moreover, a meta-analysis of Creemers et al.2 showed that several other biomarkers are important in EACs including cyclooxygenase-2, serine/threonine-protein kinase PAK-1, programmed death-ligand 1, MET, insulin like growth factor binding protein 7 and leucine-rich repeat-containing G-protein coupled receptor. Furthermore, prognostic biomarkers have described for the ESCCs. For example, strong evidence supports that epidermal growth factor receptor, Cyclin D1, vascular endothelial growth factor, Survivin, Podoplanin, Fascin, phosphorylated mammalian target of rapamycin, and pyruvate kinase M2 might be significantly linked to patients’ prognosis3. This study was performed to get more insights in the prognostic relevance of Forkhead box O 1 (FOXO1) and pSerine256-FOXO1 in esophageal cancers.
The forkhead box O 1 (FOXO1 or FKHR) belongs to the family of Forkhead box O transcription factors, which contain a conserved DNA binding domain and bind a consensus DNA binding sequence TTGTTTAC at target genes6,7,8. FOXO members modulate the expression of genes involved in a broad array of cellular process that include apoptotic cell death, cell cycle control, and DNA damage repair6,9,10. FOXO transcriptional activity is negatively regulated by phosphorylation at Serine256 in the PI3K/Akt signaling pathway11,12,13,14,15. Phosphorylated forkhead proteins translocate from the nucleus to the cytoplasm where they are inactive14,16,17,18.
In malignancies, the function of FOXO transcription factors is strongly discussed since tumor suppressive19,20,21,22,23,24,25 as well as oncogenic functions have been reported26,27,28,29. Earlier IHC studies showed both overexpression and loss of FOXO1 and pSerine256-FOXO1 in malignant cells in comparison to the corresponding benign tissue30,31,32,33,34. Additionally, FOXO1 and pSerine256-FOXO1 have been suggested as prognostic markers in malignancies, including breast cancer30, bladder31, renal cell32, prostate cancer33, and gastric cancer34. However, the prevalence and clinical significance of FOXO1 and pSerine256-FOXO1 expression in esophageal cancer remains elusive. To gain more insights in the potential clinical utility of FOXO1 and pSerine256-FOXO1 protein analysis in esophageal cancer, we used our tissue microarray of more than 600 esophageal cancer specimens with clinical follow-up data.
Our study shows that FOXO1 overexpression and loss of pSerine256-FOXO1 expression are associated with poor prognosis in esophageal adenocarcinomas. Thus, it can be speculated that the evaluation of FOXO1 and pSerine256-FOXO1 in tumor biopsies might be of clinical relevance in patients with EACs.
Results
Technical issues
A total of 78.2% and 76.9% of EACs and 81.9% and 79.9% of ESCCs were interpretable for analysis of nuclear FOXO1 and cytoplasmic pSerine256-FOXO1 immunostaining. Non-informative cases were caused by unequivocal malignant tissue or missing tissue spot.
FOXO1 and pSerine256-FOXO1 expression in benign and neoplastic esophageal tissue samples
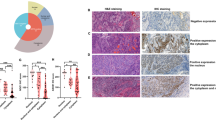
FOXO1 expression was predominantly localized in the nucleus of the cells. FOXO1 immunostaining was detectable - if present - in weak intensities in stratum basal cells of the non-neoplastic esophageal mucosa. Cancer cells showed increased levels of FOXO1 expression compared to benign esophageal cells. High FOXO1 expression was found in 40.2% of EACs and 45.2% of ESCCs. Representative images of FOXO1 immunostaining in esophageal cancers are given in Fig. 1.
Expression of pSerine256-FOXO1 was predominantly localized in the cytoplasm of the cells and was found in decreased intensities in malignant compared to benign esophageal epithelium. Low pSerine256-FOXO1 immunostaining was found in 59.8% of EAC and 37.4% of ESCC samples. Representative images of pSerine256-FOXO1 expression in malignant esophageal tissue are shown in Fig. 2.
Overexpression of FOXO1 and loss of pSerine256-FOXO1 expression are associated with unfavorable tumor phenotype in esophageal cancers
The associations of FOXO1 and pSerine256-FOXO1 expression with tumor phenotype are shown in Tables 1 and 2. Increased FOXO1 and decreased pSerine256-FOXO1 expression were significantly associated with advanced tumor stage and high UICC stage in both EACs (tumor stage: p = 0.0209 and p < 0.0001; UICC stage: p = 0.0201 and p < 0.0001) and ESCCs (tumor stage: p = 0.0003 and p = 0.0016; UICC stage: p = 0.0026 and p = 0.0326). Additionally, overexpression of FOXO1 and loss of pSerine256-FOXO1 expression were linked to presence of lymph node metastases in the subset of ESCCs (p = 0.0028 and p = 0.0119).
High FOXO1 and low pSerine256-FOXO1 expression predict shortened survival in EACs
Kaplan Meyer curves demonstrated that high FOXO1 and low pSerine256-FOXO1 expressions were associated with shortened survival of patients with EACs (p = 0.0003 and p = 0.0133) but were unrelated to clinical outcome in patients with ESCCs (p = 0.7785 and p = 0.8426), as demonstrated in Fig. 3.
Clinical impact of FOXO1 and pSerine256-FOXO1 IHC. Relationship of FOXO1 immunostaining intensity with overall survival in EACs (n = 281; P = 0.0003; (a) and ESCCs (n = 207; P = 0.7785; (b). Association of pSerine256-FOXO1 immunostaining intensity with overall survival in EACs (n = 276; P = 0.0133; (c) and ESCCs (n = 202; P = 0.8426; (d).
Additionally, we analyzed the clinical impact of the combination of both staining and demonstrated that the combination of immunostainings was significantly associated with clinical outcome of patients (p = 0.0002; Fig. 4). The group of patients with high FOXO1 and low pSerine256-FOXO1 expressions was significantly linked to worse outcome in EACs as shown in Fig. 4. Overall, the combined IHC staining (FOXO1/pSerine256-FOXO1 expression) predicted more effective the 1-year (p = 0.0004), 2-year (p = 0.0001) and 3-year (p = 0.0001) survival than the analysis of a single IHC staining (FOXO1: 1-year: p = 0.003; 2-year: p = 0.0096; 3-year: p = 0.0003 and pSerine256-FOXO1: 1-year: p = 0.0254; 2-year: p = 0.002; 3-year: p = 0.0063) staining.
Multivariate analysis including FOXO1 expression, pSerine256-FOXO1 expression, and the combination of FOXO1/pSerine256-FOXO1 expression
Multivariate analysis including tumor stage, UICC stage and FOXO1 IHC demonstrated that tumor stage, UICC stage and FOXO1 IHC were independent prognostic markers (p = 0.0173, p < 0.0001, and p = 0.002). Moreover, analysis including tumor stage, UICC stage and pSerine256-FOXO1 showed independent significant results for tumor stage and UICC stage but not for pSerine256-FOXO1 expression status (p = 0.0186, p < 0.0001 and p = 0.5394). Furthermore, we performed multivariate analysis including tumor and UICC stage and the group FOXO1/ pSerine256-FOXO1 IHC. In this analysis, all of these factors showed independent prognostic significance (p = 0.0245, p < 0.0001, and p = 0.0176).
Discussion
Our study shows that overexpression of nuclear FOXO1 and loss of cytoplasmic pSerine256-FOXO1 expression are associated with poor prognosis in patients with EACs. Thus, analysis of FOXO1 and pSerine256-FOXO1 expression - either alone or in combination with other markers - might be useful for prediction of clinical outcome in these patients.
Here, we evaluated FOXO1 and pSerine256-FOXO1 expressions in malignant and benign esophageal tissue samples on TMAs using immunohistochemistry. Earlier, it has been hypothesized that analysis of correlations between molecular markers and survival is limited due to the fact of tumour heterogeneity35 and that analysis of multiple cores per tumor specimen would enhance the representativity of TMA studies36. This suggestion is based on the assumption, that concordance of large section findings with tissue microarray data is better if 3–4 cores are taken per cancer sample is taken than just only one core per tumor sample. However, these ideas are based on the assumption that there exist a significant heterogeneity within the tissue represented by a standard 3 × 4 cm paraffin block, and that tumor heterogenity is adequat estimated by the analysis of large section. In our view, these hypotheses are open to debate. Previously, it has been shown that the TMA format is generally superior over large section studies to analyse relationships between molecular markers and clinical outcome37. In detail, TMA and large section findings of p53, PR, and ER in breast cancer were compared and in summary the results showed that overinterpretation of focal p53 positivity in large sections obscured the established prognostic impact of p53, which was, however, significantly estimated in the TMA analysis37. Further analyses demonstrated comparable significant relationships between Ki67 or p53 expression and aggressive prostate tumor features if three tissue cores were separately studied or if a combined result was done from the three cores38. In our opinion, these studies demonstrated that usage of multiple cores does not necessarily increase the ability to identify relationship between biomarkers and clinico-patholgical parametes. Moreover, these results underline the robustness of IHC TMA studies for analysis of correlations of molecular markers with clinico-pathological features of cancer specimens.
Here, we analyzed FOXO1 and pSerine256-FOXO1 expression in esophageal cancers. In our study, FOXO1 expression was found in increased intensities and pSerine256-FOXO1 expression in decreased intensities in malignant than in benign esophageal tissue. High FOXO1 and low pSerine256-FOXO1 staining occurred in 40.2% and 59.8% of EACs and 45.2% and 37.4% of ESCCs. Our observation of aberrant FOXO1 expression in cancerous relative to non-cancerous esophageal tissue is consistent with earlier studies on FOXO1 expression in diverse other cancer types, such as bladder31, renal cell32, breast30, and prostate cancer33. However, inconsistently, these immunohistochemically studies suggested either an increased or a decrease of FOXO1 expression in malignant relative to corresponding benign tissue31,32,33. Possible explanations for differing expression status of FOXO1 in different tumor types include variable interactions with critical pathways depending on the spectrum of tissue type specific gene activation.
Our data demonstrate that FOXO1 overexpression and loss of pSerine256-FOXO1 expression are linked to a subset of esophageal cancers with aggressive tumor features. Of importance, the prognostic impact of FOXO1 and pSerine256-FOXO1 were limited to the histological subset of EACs, while the markers were unrelated to clinical outcome in ESCCs. Moreover, our data suggest that even the measurement of both IHC markers FOXO1 and pSerine256-FOXO1 might be in combination of clinical relevance. This observation underlines an important role of FOXO1 and its phosphorylated form in EACs which may also be due to above mentioned tissue-specific gene activation.
The majority of EACs are believed to develop from the precursor lesion (metaplastic glandular esophageal epithelium/Barrett’s oesophagus) evolving through a sequence from low grade, to high grade dysplasia and eventually to carcinoma39,40. However, the driving factors for progression are still incompletely understood39,40. Although several genetic and cellular changes have been described, none of these as yet have proven utility41,42. However, hallmarks of metaplastic Barrett’s oesophagus are increased proliferation and decreased apoptosis and it is believed that these changes are important in malignant progression by increasing the vulnerability to, and perpetuation of mutations39,40. FOXO1 gene is involved in several biological functions of cancer cells such as cell proliferation, apoptosis, cell differentiation, and angiogenesis43. These cellular processes are known to be closely linked to tumorigenesis, and FOXOs play central roles in the regulation of cell proliferation and cycle by regulating several genes such as p53, p27Ki p1, cyclin B, cyclin D1/D2, and cyclin G29,43,44.
Additionally, other signaling pathways linked to cell proliferation, survival, migration, invasion and angiogenesis are known to play important roles in esophageal tumorigenesis. For example, PI3K-Akt signaling pathway, which also regulates the forkhead family transcription factors14,45, is a key pathway involved in esophageal tumorigenesis carcinogenesis46. Dysregulation of PI3K-Akt signaling pathway has been associated with increase cancer cell growth, proliferation, migration and invasion in esophageal cancers in literature47,48,49. Previously, several studies analysing the genomic landscape described an association between frequent disruption of pathways linked to important cellular signaling pathways including proliferation, survival, invasion, apoptosis, and migration. For example, studies found that dysregulation of MAPK signaling, PI3K-Akt signaling, and wnt signaling were strongly involved in esophageal tumorigenesis50,51,52. It can be speculated that FOXO1 and pSerine256-FOXO1 as well as its regulating signaling pathways might be important roles during esophageal carcinogenesis.
In earlier studies on FOXO1 expression in other cancer types, high FOXO1 expression was suggested to be a prognostic marker for improved clinical outcome in breast30, lung53 and bladder31 cancers. However, our data suggests that FOXO1 might play an oncogenic role in esophageal cancers. Functional data on FOXO proteins in different cancer types are conflicting. Some authors suggested FOXO factors as tumor suppressors in some cancer types54, while others reported on oncogenic roles of FOXO proteins55,56. In detail, FOXO factors can positively regulate cell survival and resistance to chemotherapy, complicating its putative therapeutic potential54. For example, FOXO3a is a positive regulator of androgen receptor expression and prostate cancer cell proliferation55. In addition, loss of functional FOXO3a in human ovarian cancer cell lines limited the sensitivity of ovarian cancer cells to chemotherapy, suggesting that FOXO proteins may be responsible for altered treatment outcomes in the presence of combined therapeutic approaches56. Taken together, greater understanding of the function and regulation of FOXO proteins are still needed to fully understand the role of FOXO proteins during carcinogenesis. Further studies analysing the functional roles of FOXO proteins are necessary to fully elucidate the role of FOXO proteins in esophageal cancer development and progression.
Previously, studies analysing whole-genome and whole-exome sequences from tumor specimens identified mutations that are enriched in tumor samples compared to germline cells. It is widely accepted that these mutations are the main drivers of tumor progression57. It is believed that cancers result from a combination of perturbed genes acting in molecular networks that correspond to hallmark processes such as cell proliferation and apoptosis58. In detail, mutations in signaling proteins may over-enrich key signaling pathways or inhibit the function of tumor suppressor proteins, resulting in uncontrolled cell growth, tumor development and progression59. In this study, we identified FOXO1, known to be involved in key signaling pathways, as an additional deregulated marker linked to prognosis of patients with esophageal cancers.
In summary, our study shows that increased FOXO1 immunostaining is marginally linked to aggressive tumor features in esophageal cancer but is unrelated to survival of patients. Therefore, our study excludes FOXO1 as prognostic EAC biomarker.
Methods
Esophageal cancer TMA
A TMA was constructed from cancer tissues after radical esophagectomies from 359 esophageal adenocarcinoma patients and 254 esophageal squamous cell carcinoma patients treated at the Department of General, Visceral and Thoracic Surgery at the University Medical Center Hamburg-Eppendorf. Follow-up was available of 359 esophageal adenocarcinoma patients and 254 esophageal squamous cell carcinoma patients. Median follow-up was 17.3 (range: 0 to 208) and 12.2 months (range: 0 to 191 months) in esophageal adenocarcinoma and esophageal squamous cell carcinoma patients. TMAs were manufactured as described60.The study was approved by the Ethics commission Hamburg and conducted in accordance with the Declaration of Helsinki. Informed consent has not been collected specifically for the patient samples included in this study. Usage of routinely archived formalin fixed leftover patient tissue samples for research purposes by the attending physician is approved by local laws and does not require written consent (HmbKHG, §12,1).
Immunochemistry
Primary antibody for FOXO1 (rabbit, Cell signaling) was applied at a dilution of 1:150 and for pSerine256-FOXO1 (rabbit, Abcam) at a dilution of 1:450 according to the manufacturer´s directions. FOXO1 and pSerine256-FOXO1 staining were analyzed in immunohistochemisty. Visualization of the primary antibody was performed with the EnVision Kit (Dako, Glostrup, Denmark). FOXO1 and pSerine256-FOXO1 staining were homogenous in the analyzed tumor samples and staining intensity of all cases was thus semiquantitatively assessed in the following two categories: low and high immunostaining. All methods were carried out in accordance with relevant guidelines and regulations.
Statistical analysis
Statistical calculations were performed using JPM 9 software (SAS Institute Inc., NC, USA). To analyze association between IHC results and clinico-pathological features contingency tables were used and tested with the chi-square method. Kaplan-Meier curves were generated for survival analysis. Log-rank test was applied to check significant survival differences between groups. Cox proportional hazards regression analysis was performed to test for independence and significance between pathological, molecular, and clinical variables.
Ethical approval and informed consent
The study was approved by the Ethics commission Hamburg and conducted in accordance with the Declaration of Helsinki. Informed consent has not been collected specifically for the patient samples included in this study. Usage of routinely archived formalin fixed leftover patient tissue samples for research purposes by the attending physician is approved by local laws and does not require written consent (HmbKHG, §12,1).
Data Available Statement
All data generated or analysed during this study are included in this published article.
References
Song, Y. et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509, 91–95 (2014).
Creemers, A. et al. A systematic review and meta-analysis of prognostic biomarkers in resectable esophageal adenocarcinomas. Scientific reports 8, 13281 (2018).
Wang, C., Wang, J., Chen, Z., Gao, Y. & He, J. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma. A systematic review. Chinese journal of cancer 36, 65 (2017).
Bartley, A. N. et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma. Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Archives of pathology & laboratory medicine 140, 1345–1363 (2016).
Bang, Y.-J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA). A phase 3, open-label, randomised controlled trial. Lancet (London, England) 376, 687–697 (2010).
Huang, H. & Tindall, D. J. Dynamic FoxO transcription factors. Journal of cell science 120, 2479–2487 (2007).
Gilley, J., Coffer, P. J. & Ham, J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. The Journal of cell biology 162, 613–622 (2003).
Furuyama, T., Nakazawa, T., Nakano, I. & Mori, N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. The Biochemical journal 349, 629–634 (2000).
Zhang, Y., Gan, B. & Liu, D. & Paik, J.-h. FoxO family members in cancer. Cancer Biology & Therapy 12, 253–259 (2014).
Maiese, K., Chong, Z. Z., Shang, Y. C. & Hou, J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Medicinal research reviews 29, 395–418 (2009).
van der Horst, A. et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nature cell biology 8, 1064–1073 (2006).
Plas, D. R. & Thompson, C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. The Journal of biological chemistry 278, 12361–12366 (2003).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proceedings of the National Academy of Sciences of the United States of America 102, 1649–1654 (2005).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Essers, M. A. G. et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. The EMBO journal 23, 4802–4812 (2004).
Tang, E. D., Nunez, G., Barr, F. G. & Guan, K.-L. Negative Regulation of the Forkhead Transcription Factor FKHR by Akt. Journal of Biological Chemistry 274, 16741–16746 (1999).
Biggs, W. H., Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proceedings of the National Academy of Sciences of the United States of America 96, 7421–7426 (1999).
Kops, G. J. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Zhang, Y., Gan, B., Liu, D. & Paik, J.-H. FoxO family members in cancer. Cancer Biology & Therapy 12, 253–259 (2011).
Hu, M. C.-T. et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117, 225–237 (2004).
Yang, H. Zhao, R. Yang, H.-Y. & Lee, M.-H. Constitutively active FOXO4 inhibits Akt activity, regulates p27 Kip1 stability, and suppresses HER2-mediated tumorigenicity. Oncogene 24, 1924–1935 (2005).
Jiang, J. & Huang, H. Targeting the Androgen Receptor by Taxol in Castration-ResistantProstate Cancer. Molecular and cellular pharmacology 2, 1–5 (2010).
Kim, S. H., Miller, F. R., Tait, L., Zheng, J. & Novak, R. F. Proteomic and phosphoproteomic alterations in benign, premalignant and tumor human breast epithelial cells and xenograft lesions: biomarkers of progression. International journal of cancer. Journal international du cancer 124, 2813–2828 (2009).
Lau, C. J., Koty, Z. & Nalbantoglu, J. Differential response of glioma cells to FOXO1-directed therapy. Cancer research 69, 5433–5440 (2009).
Xie, G. é. et al. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. British journal of pharmacology 159, 689–697 (2010).
Li, Z., Zhang, H., Chen, Y., Fan, L. & Fang, J. Forkhead transcription factor FOXO3a protein activates nuclear factor κB through B-cell lymphoma/leukemia 10 (BCL10) protein and promotes tumor cell survival in serum deprivation. The Journal of biological chemistry 287, 17737–17745 (2012).
Hui, R. C.-Y. et al. The forkhead transcription factor FOXO3a increases phosphoinositide-3 kinase/Akt activity in drug-resistant leukemic cells through induction of PIK3CA expression. Molecular and Cellular Biology 28, 5886–5898 (2008).
Kops, G. J. P. L. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009).
Wu, Y. et al. Expression of FOXO1 is associated with GATA3 and Annexin-1 and predicts disease-free survival in breast cancer. American journal of cancer research 2, 104–115 (2012).
Kim, T.-H. et al. Forkhead box O-class 1 and forkhead box G1 as prognostic markers for bladder cancer. Journal of Korean medical science 24, 468–473 (2009).
Zhou, L. et al. Mechanism and function of decreased FOXO1 in renal cell carcinoma. Journal of surgical oncology 105, 841–847 (2012).
Li, R. et al. Forkhead protein FKHR and its phosphorylated form p-FKHR in human prostate cancer. Human pathology 38, 1501–1507 (2007).
Kim, J. H. et al. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 20, 835–842 (2007).
Li, J. et al. Identification of high-quality cancer prognostic markers and metastasis network modules. Nature communications 1, 34 (2010).
Rubin, M. A., Dunn, R., Strawderman, M. & Pienta, K. J. Tissue microarray sampling strategy for prostate cancer biomarker analysis. The American journal of surgical pathology 26, 312–319 (2002).
Torhorst, J. et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. The American journal of pathology 159, 2249–2256 (2001).
Tennstedt, P. et al. The impact of the number of cores on tissue microarray studies investigating prostate cancer biomarkers. International journal of oncology 40, 261–268 (2012).
Buttar, N. S. & Wang, K. K. Mechanisms of disease. Carcinogenesis in Barrett’s esophagus. Nature clinical practice. Gastroenterology & hepatology 1, 106–112 (2004).
Fitzgerald, R. C. Barrett’s oesophagus and oesophageal adenocarcinoma. How does acid interfere with cell proliferation and differentiation? Gut 54(Suppl 1), i21–6 (2005).
Wang, K. K., Wongkeesong, M. & Buttar, N. S. American Gastroenterological Association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology 128, 1471–1505 (2005).
McManus, D. T., Olaru, A. & Meltzer, S. J. Biomarkers of esophageal adenocarcinoma and Barrett’s esophagus. Cancer research 64, 1561–1569 (2004).
Zhao, Y., Wang, Y. & Zhu, W.-G. Applications of post-translational modifications of FoxO family proteins in biological functions. Journal of molecular cell biology 3, 276–282 (2011).
Carbajo-Pescador, S., Mauriz, J. L., García-Palomo, A. & González-Gallego, J. FoxO proteins. Regulation and molecular targets in liver cancer. Current medicinal chemistry 21, 1231–1246 (2014).
Tang, T. T.-L. et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. The Journal of biological chemistry 277, 14255–14265 (2002).
Yue, Y. et al. Gene function analysis and underlying mechanism of esophagus cancer based on microarray gene expression profiling. Oncotarget 8, 105222–105237 (2017).
Sadaria, M. R. et al. Secretory phospholipase A2 mediates human esophageal adenocarcinoma cell growth and proliferation via ERK 1/2 pathway. Anticancer research 33, 1337–1342 (2013).
Wu, K. et al. Activation of PPARγ suppresses proliferation and induces apoptosis of esophageal cancer cells by inhibiting TLR4-dependent MAPK pathway. Oncotarget 7, 44572–44582 (2016).
Kwon, C. H., Moon, H. J., Park, H. J., Choi, J. H. & Park, D. Y. S100A8 and S100A9 promotes invasion and migration through p38 mitogen-activated protein kinase-dependent NF-κB activation in gastric cancer cells. Molecules and cells 35, 226–234 (2013).
Sawada, G. et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 150, 1171–1182 (2016).
Ma, S. et al. Identification of PTK6, via RNA sequencing analysis, as a suppressor of esophageal squamous cell carcinoma. Gastroenterology 143, 675–686.e12 (2012).
Lin, D.-C. et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nature genetics 46, 467–473 (2014).
Maekawa, T. et al. Expression and localization of FOXO1 in non-small cell lung cancer. Oncology reports 22, 57–64 (2009).
Monsalve, M. & Olmos, Y. The complex biology of FOXO. Current drug targets 12, 1322–1350 (2011).
Yang, L. et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. The Journal of biological chemistry 280, 33558–33565 (2005).
Arimoto-Ishida, E. et al. Inhibition of phosphorylation of a forkhead transcription factor sensitizes human ovarian cancer cells to cisplatin. Endocrinology 145, 2014–2022 (2004).
Carter, H. et al. Cancer-specific high-throughput annotation of somatic mutations. Computational prediction of driver missense mutations. Cancer research 69, 6660–6667 (2009).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Cui, Q. et al. A map of human cancer signaling. Molecular systems biology 3, 152 (2007).
Mirlacher, M. & Simon, R. Recipient block TMA technique. Methods in molecular biology (Clifton, N.J.) 664, 37–44 (2010).
Acknowledgements
We thank the Institute of Pathology of the University Medical Center Hamburg-Eppendorf, Director Professor Guido Sauter for supporting this study.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: K.G., FG.U., M.B., JR.I., A.K. Performed the experiments: K.G., FG.U., N.M., B.H., AT.EG., M.B. Analysed the data: K.G., FG.U., AT.E-G, R.G., M.B., JR.I., A.K., D.B. Contributed reagents/materials/analysis tools: K.G., N.M., AT.EG, R.G., A.H., E.B., M.R., G.WE., T.G., M.N., K.B., A.K., D.B. Wrote the paper: K.G., FG.U., N.M., A.H., M.B., JR.I., A.K., D.B.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grupp, K., Uzunoglu, F.G., Melling, N. et al. FOXO1 overexpression and loss of pSerine256-FOXO1 expression predicts clinical outcome in esophageal adenocarcinomas. Sci Rep 8, 17370 (2018). https://doi.org/10.1038/s41598-018-35459-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35459-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.