Abstract
We performed gamma-ray analysis to determine the amount of radioactive cesium-134 (134Cs) and cesium-137 (137Cs) in 259 foodstuffs five years after the Fukushima nuclear accident of 2011. Using measurements of trace 134Cs radioactivity, we investigated the contribution ratio of 137Cs derived from the Fukushima accident on 2011 and pre-Fukushima. The median detected concentration of radiocesium (134Cs + 137Cs) in foodstuffs was 0.33 Bq/kg-raw, a much lower radioactivity than the Japanese regulatory limit. However, a few samples had particularly high radioactivity, including some dried mushrooms sold in Iwate Prefecture that had a 137Cs radioactivity concentration as high as 441 Bq/kg. Our analysis showed that 75.5% of the 137Cs detected in these mushrooms originated from the Fukushima accident, and 24.5% was originated before the Fukushima event. Our study clarified the 137Cs contamination in 75 of all 259 food samples before and after the Fukushima nuclear accident, showing that not only mushrooms but also fish had been contaminated before the Fukushima accident.
Similar content being viewed by others
Introduction
The March 2011 accident at the Fukushima Daiichi nuclear power plant, TEPCO, in Japan caused the large release of several radionuclides, including cesium-134 and cesium-137, into the atmosphere and Pacific Ocean1,2,3,4, resulting in the contamination of various food products5,6,7,8. Five years after the Fukushima accident, the probability of foodstuffs containing radionuclide levels exceeding the Japanese regulatory radiocesium limit (general foods: 100 Bq/kg, valid since April 1, 20129,10) was extremely low because of collaboration among farmers, producers, and agricultural organizations to ensure food safety. The survey conducted by Fukushima Prefecture from April 2015 to April 2016 revealed that 23,837 of the 23,855 foods (over 99.9%) produced in Fukushima had radiocesium concentrations below the regulatory limit11.
Although gamma-ray measurements supported that radioactivity levels in almost all foodstuffs were far below the Japanese regulatory limit, it was unknown how much of the detected radioactivity originated from the Fukushima accident as opposed to pre-Fukushima events such as the atmospheric nuclear explosions12 that have been conducted since 1945 and the Chernobyl nuclear power plant accident of 1986.
Before the Fukushima accident, gamma-ray measurements to determine anthropogenic radionuclides in Japanese foodstuffs were very limited13,14,15. Following the accident, measurements of food radioactivity levels, especially of 137Cs, became more readily available because of the Japanese government’s rapid establishment of a food monitoring campaign to detect radionuclides. While this campaign produced a large dataset of radiocesium contamination levels in food, no quantitative method existed to distinguish between the detected 137Cs that originated from the Fukushima accident and the detected 137Cs that originated from prior deliberate or accidental releases of the radionuclide.
In this study, we first performed gamma-ray analysis to investigate the distribution of radiocesium in 259 general foodstuffs five years after the Fukushima accident. Using the trace radioactivity of short-lived 134Cs in foodstuffs, we then evaluated the contribution ratio of Fukushima-derived 137Cs in general foodstuffs in Japan. Although 134Cs and 137Cs involve different generation processes in nuclear reactors and the 134Cs/137Cs activity ratio depends on the extent of fuel burnup in each reactor, their yield will be higher compared to other fission or activation products. Therefore, our method is applicable not only to the Fukushima accident but also to any future nuclear disaster.
Results
Radioactivity of 134Cs and 137Cs in all 259 foodstuffs were analyzed during 2015–2016 period. For convenience in discussion, we considered this period as in 2016, five years after the Fukushima accident. Figure 1 shows the radioactivity of the sum of 134Cs and 137Cs in foodstuffs categorized into 17 groups (rice, cereals, eggs, fish, fruits, green vegetables, other vegetables, potatoes, mushrooms, nuts and seeds, milk and milk products, seaweeds, bean and bean products, processed foodstuffs, seasonings, beverages and water) according to our previous reports6. Most of these foodstuffs had total radioactivity levels (134Cs + 137Cs) below the Japanese regulatory limit for water of 10 Bq/kg, milk of 50 Bq/kg, general foodstuffs of 100 Bq/kg9, and dried Shiitake mushrooms (Lentinula edodes) of 570 Bq/kg16. Our analysis allowed the detection of much lower concentrations of radiocesium as compared to those of the screening level.
The detected radioactivity of the sum 134Cs and 137Cs in foodstuffs categorized into 17 groups, as measured in 2016, five years after the Fukushima accident. Each dashed colored line shows the Japanese regulatory limits for various foodstuff groups: water (10 Bq/kg), milk (50 Bq/kg), general foodstuffs (100 Bq/kg), and dried mushrooms (570 Bq/kg). The bars under the colored dots show the detection limits of each category. Radiocesium was detected in 161 samples, equivalent to 62.2% of all samples (n = 259).
Of the all 259 foodstuff samples, 161 samples (62.2%) contained either both 134Cs and 137Cs or only 137Cs in concentrations above the detection limit. The sum of the radioactivity of 134Cs and 137Cs ranged from 1.86 × 10−3 to 707 Bq/kg. Among the cesium-detected sample set, the average sum of the radioactivity of 134Cs and 137Cs was 17.4 Bq/kg, and the median was 0.33 Bq/kg. These results indicate that very few samples had high radioactivity levels. The lowest radioactivity of the sum of 134Cs and 137Cs was found in tap water collected at Nerima City, Tokyo: 1.86 × 10−3 Bq/kg, which is equivalent to 1/5,380 of the Japanese regulatory limit. Conversely, a sample of shortfin mako shark (Isurus oxyrinchus) collected offshore from the Izu Peninsula was 707 Bq/kg, far exceeding the Japanese regulatory limit of 100 Bq/kg-raw. However, this case was very rare, as the maximum radioactivity in 559 fish samples collected offshore from the North Pacific Ocean in this period was 44 Bq/kg-raw17.
In the rice category, rice bran had the highest radioactivity (10.9 Bq/kg), although that of polished rice samples ranged from 0.08 to 0.91 Bq/kg. The radioactivity ratio of bran to polished rice was about 0.1, which was good agreement with the cultivation in Fukushima18.
Mushrooms tended to have higher radioactivity than other foods. The median radioactivity was only 1.2 Bq/kg-raw; however, the dried mushroom sample purchased at Iwate Prefecture in northern Fukushima showed the highest radioactivity, with 505 Bq/kg of radiocesium. Even the dried mushroom sample purchased at Kanagawa Prefecture, which is located 306 km from the Fukushima Daiichi Nuclear Power Plant, had a radioactivity concentration of 147 Bq/kg. In contrast, two samples of dried mushrooms purchased at Minami-Soma in Fukushima Prefecture had lower radioactivity (11.8 and 71.8 Bq/kg, respectively) than that of other prefectures. These measurements indicate that the source of contamination is not unique. Mushrooms are well-known as cesium accumulators19,20,21; thus, a continuous food monitoring campaign over long distances is required to ensure food safety.
Figure 2 represents the radioactivity distribution of detected radiocesium five years after the Fukushima accident in 2016 (n = 161, ○), as compared with our previous data (n = 96, ◆) from 20146, three years after the accident. The difference in analysis accuracy, precision, and contents of dataset between both the analyses was minimal. The number of samples was normalized to 100% on the horizontal axis for ease of comparison. The median radioactivity of radiocesium was 0.33 Bq/kg-raw in 2016 and 0.16 Bq/kg-raw in 2014. Although it seems that the distribution did not change much after two years, the comparison of median radioactivity is not statistically significant for two reasons: the dataset did not include the samples whose radioactivity concentrations were under the detection limit, and it was impossible to distinguish between the pre-Fukushima (before 2011) and post-Fukushima background.
Comparison of distribution of the radioactivity of the detected sum of 134Cs and 137Cs (radiocesium) in 2014 (closed diamond) and 2016 (open circle), respectively, three and five years after the Fukushima accident. The number of samples was normalized to 100% on the horizontal axis for ease of comparison.
Discussion
Radiocesium, including 134Cs, could originate from pre-Fukushima events, such as atmospheric nuclear explosion fallout (since 1945), the Chernobyl nuclear power plant accident (1986), in addition to the Fukushima accident itself (2011). 134Cs originating from pre-Fukushima events is difficult to detect because 30 years have passed since the last event (Chernobyl accident), and its half-life (T1/2 = 2.06 years) is much shorter than that of 137Cs (T1/2 = 30.2 years).
In order to quantitatively estimate the 134Cs derived from the Chernobyl accident, the radioactivity ratio of 134Cs/137Cs at the time of the Chernobyl accident was used. The estimated 134Cs as of 1 January 2016 was 4.99 × 10−5, which was corrected according to the 134Cs/137Cs radioactivity ratio of 0.55 at the time of the Chernobyl accident22,23. This estimation indicated that 134Cs originating from the Chernobyl accident (pre-Fukushima) had decayed away nearly entirely by 2016, and thus it would be hardly detectable with any high-sensitivity HPGe detector.
In the case of chai leaves from Turkey that were sold in Japan, for example, the radioactivity of 137Cs was 19.9 Bq/kg, while that of 134Cs was below the detection limit (<0.195 Bq/kg). According to the 134Cs/137Cs ratio at the time of the Chernobyl accident, the ideal 134Cs radioactivity would be 1.76 mBq/kg at the time of our gamma-ray measurement. This analysis indicated that pre-Fukushima-derived 134Cs radioactivity was negligible after the Fukushima accident.
Therefore, in this study, all detected 134Cs in foodstuffs in Japan could be regarded as originating from the Fukushima accident, although detected 137Cs may be the result of pre-Fukushima events.
As previously discussed and previous study24, it is difficult to uniquely evaluate the impact of the Fukushima accident. The data in Figs 1 and 2 represent the combined influence of the Fukushima accident and pre-Fukushima events.
Thus, a quantitative model for 137Cs derived from Fukushima was developed to estimate the effect of the accident itself, based on the detected radioactivity of 134Cs in our study and the radioactivity ratio of 134Cs/137Cs at the time of the Fukushima accident. Although the ratios are slightly different for each reactor unit (1,2, and 3) in Fukushima nuclear power plant, the total 134Cs/137Cs ratio at the time of the accident could be regarded as roughly 1.025. In addition, because we could regard all detected 134Cs in foodstuffs as originating from the Fukushima accident, the ratio simply varied according to the function of elapsed time, t (years), after stopping the generation of activation and fission products such as 134Cs and 137Cs. Therefore, 137Cs originating from the Fukushima accident, 137CsFukushima, could be written as
where 137CsFukushima is the radioactivity of 137Cs originating from the Fukushima accident, 137CsPreFukushima is the 137Cs originating from pre-Fukushima events such as the Chernobyl accident or global fallout, 134Csdetected and 137Csdetected are the radioactivity of 134Cs and 137Cs in food samples as detected by gamma-ray measurements, and ft is the physical-decay function based on the specific 134Cs/137Cs radioactivity ratio of the Fukushima accident. The values of 30.2 and 2.06 indicate the T1/2 of 137Cs and 134Cs, respectively, and t is the elapsed time (in years) since the Fukushima accident on March 11, 2011. In this study, all 137CsFukushima measurements were calculated based on decay-corrected radioactivity.
We could not determine that 137Csdetected corresponded to 137CsPreFukushima in samples where 134Cs was not detected, as it is possible that the gamma-ray detector lacked the capabilities needed to detect the radioactivity of 134Cs. However, in samples where 134Cs was detected, we could clearly and quantitatively show whether Fukushima contributed to the radioactivity.
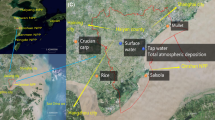
The representative contribution ratio of 137CsFukushima after the Fukushima accident is shown in Fig. 3. The entire list of all samples can be found as Supplementary Table S1. Contribution ratio included about 18% on average. Because 1) the 134Cs/137Cs ratio at the time of accident was distributed from 0.9 to 1.125, and 2) the smaller the radioactivity, the larger the error of 134Cs/137Cs ratio. Considering the two factors, the error of contribution ratio (1sigma) was 17.9 ± 6.8% in our dataset.
The specific and representative contribution ratio of 137CsFukushima in Japanese foodstuffs. The area of the circles is proportional to the radioactivity of the corresponding foodstuffs. (See Supplementary Information: Table S1 in detail).
Samples in which the detected 137Cs originated from the Fukushima nuclear power plant were confirmed in 17 categories, regardless of the radioactivity concentration and research period.
Among the five samples of dried Shiitake mushrooms sold at the roadside rest area (Michi-no-eki) in Iwate Prefecture, 137Csdetected ranged from 111 to 441 Bq/kg, and the percentage of 137CsFukushima ranged from 58.8% to 75.5%. The mushroom with the highest radioactivity had 441 Bq/kg (137Cs) and 63.6 Bq/kg (134Cs). This mushroom was purchased on June 21, 2016, and thus, ft was 0.191 because 5.28 years had passed since the Fukushima accident according to equation (2). As a result, 333 Bq/kg of 137Cs originated from Fukushima (137CsFukushima), and the other 108 Bq/kg of 137Cs was from a pre-Fukushima event (137CsPreFukushima). Equivalently, 75.5% of 137Cs originated from the Fukushima accident. (If 134Cs was also included in the contribution, the percentage of Fukushima accident would be 78.5%).
For brown rice collected at Chiba Prefecture in 2016, for instance, the radioactivity of 137CsFukushima was estimated to be 1.38 Bq/kg-raw, and 137CsPreFukushima was 0.017 Bq/kg-raw. In polished rice collected at the town of Namie in Fukushima Prefecture in 2014, 137CsFukushima and 137CsPreFukushima were 1.75 Bq/kg-raw and 0.124 Bq/kg-raw, respectively. Thus, both samples had much lower radioactivity than the Japanese regulatory limit; however, the Fukushima accident strongly affected these radioactivity concentrations.
In contrast, fish had a relatively low concentration of 137CsFukushima, with the exception of the shortfin mako shark (Isurus oxyrinchus). In salmon collected at Hokkaido in 2014, 137CsFukushima and 137CsPreFukushima were 50.9 mBq/kg-raw and 52.5 mBq/kg-raw, respectively. The contribution ratio of 137Cs from the Fukushima accident in these salmon was 49.2%. Fish, especially the migratory kind, might not be directly affected by the Fukushima accident. In the fish category of the 2016 dataset (Table S1), the contribution of 137CsFukushima ranged from 46 to 100% (n = 10). The ratios of mahi-mahi (Dorado, Coryphaena hippurus) and tuna were 46% and 57%, respectively, indicating that the amount of 134Cs (and 137Cs) leaking into the Pacific Ocean from the Fukushima plant was limited five years after the accident.
In conclusion, we quantitatively evaluated the radioactive contamination caused by the Fukushima accident by conducting detailed gamma-ray analysis. Our results suggest that the 137Cs in mushrooms will continue to be detected for a long time. Because cesium accumulators such as mushrooms may be affected by pre-Fukushima events, it is necessary to perform gamma-ray measurements not only around Fukushima but also in other areas. In the area of eastern Japan especially, it would be important to estimate the contamination caused by the Fukushima accident and pre-Fukushima events.
Radioactive contamination of food in Japan as a result of the Fukushima accident is neither uniform across different food categories nor a function of distance from the Fukushima plant. Such contamination is not only due to the Fukushima accident but also to other previous events that released radiocesium. Therefore, we strongly insist that a continuous monitoring system be put in place to ensure Japanese food safety.
Methods
Food samples
All 259 foodstuff samples (except tap water) were purchased in 2016 at several supermarkets in Japan and through online shops. The country of origin of most samples is Japan, but countries of origin such as Russia, China and Turkey are also included as imported foods for comparison. Samples were categorized into 17 groups according to our previous method6 and some reports26,27: rice, cereals, eggs, fish, fruits, green vegetables, other vegetables, potatoes, mushrooms, nuts and seeds, milk and milk products, seaweeds, beans and bean products, processed foodstuffs, seasonings, beverages and water.
Experimental design and procedure
To realize a lower detection limit, a concentration step based on the Japanese official method was added to the pre-treatment28. Samples with high water content, such as vegetables, were concentrated by freeze-drying and/or heat treatment around 80 degree for several days, as described in previous study6. In this study, the concentration (dry/wet) ratio ranged from 1.1 to 40. The dried samples were crushed by a mixer and packed into a U-8 plastic vessel (0.1 dm3) or Marinelli vessel (2.0 dm3) with a fixed geometry.
The radioactivity of each sample was determined using a high-purity germanium (HPGe) detector with a 25% relative efficiency at 1.33 MeV, and a resolution ranging from 1.80 keV to 1.33 MeV was used to collect the gamma rays emitted from the dried samples. A digital 16 k multichannel analyzer with an integrated high-voltage power supply was used for this system. The radioactivity of 137Cs was determined by the 661 keV gamma emission peak. For 134Cs, radioactivity were determined using the weighted average of the 604 keV and 795 keV gamma peaks after careful consideration of the sum effect. The measurements time (live time) depended mostly on each Compton scattering of 40K in each sample, and ranged from 2 hours to 13.2 days. Detection limits were set to three sigma (3σ) in accordance with Kaiser’s method29. Gamma rays measurement was continued until relative standard deviation reached 10% or less of its radioactivity. The detection limit also depended on the concentration of elements comprising the samples. In the case of water, the concentration (dry/wet) ratio was about 40, and the detection limit of 137Cs was 10−3 Bq/kg-raw. The radioactivity of 134Cs, 137Cs, and 40K were corrected (decay-backed) to the time of purchase of the foodstuffs.
To ensure quality control, we obtained radioactivity concentrations of the Japanese standard reference material JSAC 0471. The measurements—86.2 ± 1.8 Bq/kg (134Cs) and 112 ± 1.8 Bq/kg (137Cs)—were in good agreement with certified radioactivity concentrations (134Cs: 85.3 ± 5.9 Bq/kg and 137Cs: 115 ± 8 Bq/kg).
References
Povinec, P. P., Hirose, K. & Aoyama, M. Fukushima Accident: Radioactivity Impact on the Environment. 400 (Elsevier, 2013).
Buesseler, K. et al. Fukushima-derived radionuclides in the ocean and biota off Japan. Proc Natil Acad Sci USA 109, 5984–5988 (2012).
Buesseler, K. et al. Fukushima Daiichi–Derived Radionuclides in the Ocean: Transport, Fate, and Impacts. Annu Rev. Mar. Sci. 9, 173–203 (2017).
Zhang, Z. et al. Atmospheric activity concentration of 90Sr and 137Cs after the Fukushima Daiichi Nuclear Accident. Environ. Sci. Tech. 52, 9917–9925 (2018).
Merz, S., Shozugawa, K. & Steinhauser, G. Analysis of Japanese radionuclide monitoring data of food before and after the fukushima nuclear accident. Environ. Sci. Tech. 49, 2875–2885 (2015).
Shozugawa, K., Saito, T., Hori, M. & Matsuo, M. High-sensitivity determination of radioactive cesium in Japanese foodstuffs: 3 years after the Fukushima accident. J. Radioanal. Nucl. Chem. 307, 2117–2122 (2016).
Nihei, N., Tanoi, K. & Nakanishi, T. M. Inspections of radiocesium concentration levels in rice from Fukushima Prefecture after the Fukushima Dai-ichi Nuclear Power Plant accident. Sci. Rep. 5, 8653, https://doi.org/10.1038/srep08653 (2015).
Weller, A., Hori, M., Shozugawa, K. & Steinhauser, G. Rapid ultra-trace determination of Fukushima-derived radionuclides in food. Food Control 85, 376–384 (2018).
Ministry of Health, Labour Welfare (MHLW), Japan. Radioactive materials in foods -current situation and protective measures-, http://www.mhlw.go.jp/english/topics/2011eq/dl/food-130926_1.pdf (Accesed: 18 June, 2018) (2012).
Hamada, N. & Ogino, H. Food safety regulations: what we learned from the Fukushima nuclear accident. J. Environ. Radioact. 111, 83–99 (2012).
Fukushima Prefectual Government, Japan. Monitoring results of agricultural, forestry and fishery products from Fukushima Prefecture, http://www.pref.fukushima.lg.jp/uploaded/attachment/236857.pdf (2016) (in Japanese) (Accesed: 18 June, 2018).
Kodaira, K. Radioactive Contamination of Rice in Japan -with Reference to Sr-90 and Cs-137 Content in Rice until 1962-. Journal of Radiation Research 5, 116–119 (1964).
Komamura, M., Tsumura, A. & Kodaira, K. 90Sr and 137Cs Contamination of Polished Rice in Japan. Survey and Analysis during the Years 1959–1995. Radioisotopes 50, 80–93 (2001).
National Institute of Radiological Sciences, Chiba, Japan. Radioactivity Survey Data in Japan, Part 2 -Dietary Materials- No. 91. ISSN 0441-2516 (1990).
Japan Chemical Analysis Center, Chiba, Japan. Radioactivity Survey Data in Japan -Environmental and Dietary Materials- No. 144. ISSN 0441-2516 (2009).
Ministry of Health, Labour Welfare (MHLW), Japan. About handling the testing method of radioactive materials in food, https://www.mhlw.go.jp/shinsai_jouhou/dl/shikenhou_120319.pdf (2012) (in Japanese) (Accesed: 18 June, 2018).
Japan Fisheries Agency. Results of the monitoring on radioactivity level in fisheries products, http://www.jfa.maff.go.jp/e/inspection/index.html (2018) (Accesed: 18 June, 2018).
Endo, S., Kajimoto, T. & Shizuma, K. Paddy-field contamination with 134Cs and 137Cs due to Fukushima Dai-ichi Nuclear Power Plant accident and soil-to-rice transfer coefficients. J. Environ. Radioact. 116, 59–64 (2013).
Rakić, M. et al. Radionuclides in some edible and medicinal macrofungal species from Tara Mountain, Serbia. Environmental Science and Pollution Research 21, 11283–11292 (2014).
Guillén, J. & Baeza, A. Radioactivity in mushrooms: A health hazard? Food Chem. 154, 14–25 (2014).
Shimizu, M. & Anzai, I. Concentration of 137Cs in dried Lentinula edodes (Shiitake) as an indicator of environmental contamination. J. Oral Sci. 43, 145–149 (2001).
Cambray, R. S. et al. Observations on radioactivity from the Chernobyl accident. Nuclear Energy 26, 77–101 (1987).
Mück, K. et al. A consistent radionuclide vector after the Chernobyl accident. Health Phys. 82, 141–156 (2002).
Kirchner, G., Bossew, P. & De Cort, M. Radioactivity from Fukushima Dai-ichi in air over Europe; part 2: what can it tell us about the accident? J. Environ. Radioact. 114, 35–40 (2012).
Merz, S., Steinhauser, G. & Hamada, N. Anthropogenic Radionuclides in Japanese Food: Environmental and Legal Implications. Environ. Sci. Tech. 47, 1248–1256 (2013).
Shiraishi, K., Ban-nai, T., Muramatsu, Y. & Yamamoto, M. Comparison of stable cesium and radiocesium on dietary intakes by japanese subjects using 18 food categories. J. Radioanal. Nucl. Chem. 242, 687–692 (1999).
Ministry of Health, Labour Welfare (MHLW), Japan. National Health and Nutrition Survey (2013).
Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The guidline of Gamma ray spectrometry for Ge semiconductor detector in Series of Radioactivity measurement (ed. MEXT) (1992) (in Japanese).
Kaiser, H. Zum Problem der Nachweisgrenze. Fresenius Z. Anal. Chem. 209, 1–18 (1965).
Acknowledgements
We thank Dr. Ken Fujimoto, Fisheries Agency of Japan, for the coordination of the shark samples.
Author information
Authors and Affiliations
Contributions
T.S. and K.S. carried out the sample preparation and pre-treatment. M.H. and K.S. performed gamma-ray measurements, discussed the results and contributed to the manuscript preparation.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hori, M., Saito, T. & Shozugawa, K. Source evaluation of 137Cs in foodstuffs based on trace 134Cs radioactivity measurements following the Fukushima nuclear accident. Sci Rep 8, 16806 (2018). https://doi.org/10.1038/s41598-018-35183-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35183-z
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.