Abstract
Prognosis of HPV negative head and neck squamous cell carcinoma (HNSCC) patients remains poor despite surgical and medical advances and inadequacy of predictive and prognostic biomarkers in this type of cancer highlights one of the challenges to successful therapy. Statins, widely used for the treatment of hyperlipidaemia, have been shown to possess anti-tumour effects which were partly attributed to their ability to interfere with metabolic pathways essential in the survival of cancer cells. Here, we have investigated the effect of statins on the metabolic modulation of HNSCC cancers with a vision to predict a personalised anticancer therapy. Although, treatment of tumour-bearing mice with simvastatin did not affect tumour growth, pre-treatment for 2 weeks prior to tumour injection, inhibited tumour growth resulting in strongly increased survival. This was associated with increased expression of the monocarboxylate transporter 1 (MCT1) and a significant reduction in tumour lactate content, suggesting a possible reliance of these tumours on oxidative phosphorylation for survival. Since MCT1 is responsible for the uptake of mitochondrial fuels into the cells, we reasoned that inhibiting it would be beneficial. Interestingly, combination of simvastatin with AZD3965 (MCT1 inhibitor) led to further tumour growth delay as compared to monotherapies, without signs of toxicity. In clinical biopsies, prediagnostic statin therapy was associated with a significantly higher MCT1 expression and was not of prognostic value following conventional chemo-radiotherapy. These findings provide a rationale to investigate the clinical effectiveness of MCT1 inhibition in patients with HNSCC who have been taking lipophilic statins prior to diagnosis.
Similar content being viewed by others
Introduction
Statins are structural analogues of mevalonate and act via inhibition of the 3-hydroxymethyl-3-glutaryl (HMG)-coenzyme A (CoA) reductase to competitively block the conversion of HMG-CoA to mevalonate, a precursor in the synthesis of cholesterol1. Statin therapy is recommended for the treatment of hyperlipidemia as well as the primary and secondary prevention of cardiovascular diseases2. Studies have shown that statins also possess powerful pleiotropic effects independent of their lipid-lowering effects in a wide range of pathological disorders such as inflammatory diseases, Alzheimer’s disease, Parkinson’s disease and multiple sclerosis1,3. Recently, there are emerging interests in the use of statins as anticancer agents based on preclinical evidence of their antiproliferative, proapoptotic and tumouricidal properties4,5 and their use was found to be associated with a significant reduction in cancer-related deaths in 13 distinct cancer types6. Inhibition of 3-hydroxy-3-methylglutaryl CoA reductase by statins leads to reduced levels of mevalonate and its downstream products, many of which play critical roles in cellular functions such as membrane integrity, cell signaling, cell cycle progression7,8 and mitochondrial damage9. In addition, glucose uptake was shown to be impaired in cancer cells exposed to statins10. Glucose metabolism in cancer is largely regulated by monocarboxylate transporters (MCTs). MCTs are a family of transmembrane proteins that mediate the transport of monocarboxylates such as pyruvate and lactate, with MCT1 and MCT4 being the main ones implicated in cancer11.
The aim of this project was to investigate potential statin-induced metabolic modulation in head and neck squamous cell carcinoma (HNSCC) with particular emphasis on the expression of MCT1 and MCT4 and whether statin use could be of predictive value in designing a personalised anti-cancer therapy. This is particularly important since, with the exception of HPV infection, there is a lack of predictive and prognostic biomarkers in this type of cancer12. It is a heterogeneous disease comprising a group of tumours that include the oral cavity, nasal cavity, paranasal sinuses, pharynx, larynx and salivary glands and is the sixth most common cancer in the world, accounting for more than 550,000 new cases each year13. Overall survival rates remain poor despite surgical and medical advances, with less than 50% of patients surviving at 5 years and hence locoregional control remains the main focus of disease management14,15. Therefore, there is an urgent need to develop more effective therapies for this deadly disease.
Results
Sensitivity of HNSCC cancer cell lines to statins
A panel of four HPV negative HNSCC cancer cell lines (Supplementary Table 1) were employed to assess efficacy of statins. The cells were exposed to either simvastatin (lipophilic) or pravastatin (hydrophilic) for 96 hours. It is worth noting that simvastatin is clinically administered as the prodrug lactone which is activated in vivo chemically or enzymatically by esterases or paraoxonases16. In vitro, the prodrug was activated to its active acid form by hydrolysis prior to treatment. Simvastatin effectively inhibited cell proliferation in a dose-dependent manner in all the cell lines tested with IC50 values ranging between 4–10 μM, while pravastatin showed no cellular toxicity up to a concentration of 200 μM (Fig. 1). This is in agreement with previous findings in a panel of breast cancer cells, where it was suggested that lack of toxicity is due to pravastatin’s hydrophilic nature which prevents it from efficiently permeating cell membranes17. Although the active forms of simvastatin and pravastatin are similar in structure, pravastatin is approximately 100 times less lipophilic than simvastatin. In fact, pravastatin is mainly metabolised by hydroxylation and this results in more hydrophilic metabolites. On the other hand, simvastatin acid, the main metabolite of simvastatin is still lipophilic. The greater hydrophilicity of pravastatin has been suggested as a reason for its selectivity with respect to inhibition of cholesterol synthesis and lower permeation into nonhepatic tissues18.
Cellular toxicity of statins is dependent on their lipophilicity. Four HNSCC cell lines were treated with either simvastatin or pravastatin for 96 hours and effect was determined by SRB assay. Data is presented as the percentage of cells unaffected by the drug relative to untreated control. (a) FaDu; (b) CaL-27; (c) Detroit 562; (d) UMSCC47. All graphs represent mean of at least three independent experiments carried out in triplicate.
HPV positivity does not seem to play a role in the sensitivity of the cells to simvastatin since both UMSCC47 (HPV positive), FaDu and Detroit 562 (HPV negative) have similar IC50 values.
Based on these findings, the lipophilic simvastatin was taken forward for further investigation.
Chronic simvastatin treatment results in metabolic reprogramming
The four cell lines were then exposed to simvastatin for 96 hours at sub IC50 concentrations (CaL-27 cells were treated with 2 μM and the other cells with 5 μM). Cell surface proteins were isolated through biotinylation to ensure that only expression of membrane-bound MCTs is assessed. This is necessary because only membrane-bound MCTs are functional and therefore are actively involved in regulating glycolysis19. We found that chronic treatment with statins led to changes in expression of membrane-bound MCTs, with an increase in MCT4 expression levels observed in all four cell lines, although changes in MCT1 expression were less dramatic (Fig. 2a). Surprisingly, statin treatment was not associated with changes in extracellular lactate levels (Fig. 2b).
Simvastatin treatment is associated with metabolic changes. (a) HNSCC cell lines were treated with simvastatin or vehicle for 96 hours. Cell surface MCTs were isolated by biotinylation and assayed by western blotting. EpCAM (plasma membrane marker) was used as a loading control. (b) Extracellular lactate was measured and expressed relative to vehicle-treated cells. (c) Simvastatin affects mitochondrial mass, cells were stained with 10-nonyl acridine orange (10-NAO) and signal was quantified using flow cytometry. (d) ATP measurements in vehicle and simvastatin-treated cells. Bars represent mean of at least three independent experiments. *P < 0.05, **P < 0.01 & ***P < 0.001, t-test.
10-NAO is an orange dye associated with mitochondrial cardiolipin and is frequently used to evaluate total mitochondrial mass20. Simvastatin therapy markedly reduced mitochondrial mass in the four cancer cells tested with the most profound effect observed in FaDu cells (Fig. 2c). Interestingly, simvastatin therapy did not result in changes of ATP levels in the four cell lines tested suggesting that cell death is not caused by ATP depletion (Fig. 2d).
Combining simvastatin treatment with inhibition of MCT function in vitro
Based on the finding that chronic treatment with simvastatin is associated with changes in expression levels of MCTs, we hypothesised that combining statin treatment with MCT inhibition could be a useful therapeutic strategy. MCT1 inhibition was achieved pharmacologically using the AZD3965 drug at a concentration of 1 mM for 72 hours. AZD3965 is an orally bioavailable compound currently in clinical trials for treatment of cancer (NCT01791595)21. Since there are no available specific pharmacologic inhibitors of MCT4, genetic knockdown was employed (Supplementary Fig. 1 shows efficiency of MCT4 knockdown). The cells were initially pre-treated with simvastatin for 96 hours which was then followed by treatment with siMCT4 and/or AZD3965 for 72 hours. Cellular toxicity was assessed by clonogenic assay and the results are presented in Fig. 3.
Cell survival of HNSCC panel after MCT inhibition with and without pre-treatment with simvastatin, as determined by clonogenic survival assay. Cells were pre-exposed to vehicle or simvastatin for 96 hours, then subjected to treatment with AZD3965 (1 mM) for 72 hours and/or siMCT4. Bars represent mean of at least three independent experiments carried out in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA test.
For vehicle treated FaDu and CaL-27 cells, treatment with siMCT4 and/or AZD3965 did not have a significant effect on cellular toxicity. Treatment with simvastatin alone resulted in a significant reduction in survival. Exposing these two cells to simvastatin followed by either siMCT4 or AZD3965 alone did not enhance toxicity, possibly due to the fact that each transporter compensates for the inhibition of the other one22. Interestingly, combining simvastatin treatment with inhibition of both transporters resulted in a significant enhancement in toxicity.
Vehicle treated Detroit 562 and UMSCC47 cells showed enhanced sensitivity to MCT4 but not MCT1 inhibition, suggesting that MCT4 is the most crucial to their cellular function. This is consistent with the previous finding by Baek and colleagues that MCT4 inhibition only affects viability in MCT4-addicted cells23. Inhibition of both transporters did not result in further toxicity in these cells and a similar trend was observed when the cells were pre-treated with simvastatin. Therefore, in these cells pre-exposure to simvastatin does not sensitise them to inhibition of MCT function.
Investigations of metabolic pathways in vitro are limited by the fact that the milieu does not mimic the tumour microenvironment. This is of particular importance since the finding of metabolic symbiosis phenomenon and the involvement of both MCT1 and MCT424. It was therefore imperative to translate our findings into relevant in vivo models and for this purpose the two most sensitive cell lines FaDu and CaL-27 were used.
In vivo toxicity of simvastatin
To investigate whether simvastatin has chemotherapeutic effects in vivo, mice with established FaDu xenografts were given simvastatin at oral daily doses of 10 mg/kg when tumour volumes reached 50 mm3. This dose would equate to ∼40 mg/day for an adult weighing 60 kg, based on mouse-to-human equivalence calculations25, which is within the recommended dose range of statins in the clinic26. Unfortunately, in this case simvastatin did not have any effects on tumour growth (Fig. 4a).
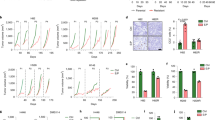
Effect of simvastatin therapy in vivo. (a) Simvastatin has no effect on the growth of established FaDu xenografts. When tumour volumes reached 50 mm3, mice were randomized into two groups which were treated with vehicle or simvastatin (10 mg/kg) daily by oral gavage. Tumour volumes were determined every 2–3 days. Total number of animals used 14. (b) Simvastatin inhibits the growth FaDu tumours. Mice were dosed once daily with simvastatin (5 mg/kg or 10 mg/kg) or vehicle for 2 weeks prior to s.c. injection of tumour cells, dosing continued until tumour volumes reached 800 mm3, ****P < 0.0001, one-way ANOVA test. (c) Kaplan-Meier survival curves and log-rank P values. Total number of animals used 21.
In order to mimic a clinical scenario where patients who present with a tumour would have been taking simvastatin for months to years, mice were given oral daily doses of simvastatin two weeks prior to injection of tumour cells. Dosing continued throughout the study with two clinically relevant doses 5 mg/kg and 10 mg/kg, which are pre-standardised and well-tolerated doses for in vivo studies4. Interestingly, both doses were efficient at significantly reducing FaDu tumour growth (Fig. 4b; P < 0.0001; one-way ANOVA) and improving overall survival (Fig. 4c). However, a less dramatic albeit significant growth delay was observed in CaL-27 tumours (Supplementary Fig. 2). Simvastatin at these oral doses was well-tolerated as evident in the absence of changes in body weight and the general condition of the mice (Supplementary Figs 3 and 4).
Simvastatin therapy causes a metabolic switch in tumours
Tumours at sizes of 150 mm3 were collected for immunohistochemical analyses of monocarboxylate transporters. Simvastatin therapy was associated with a significant increase in membrane bound MCT1, while no significant differences in MCT4 staining patterns were observed between vehicle and simvastatin-treated tumours (Fig. 5a,b).
Simvastatin therapy is associated with upregulation of MCT1 expression and a metabolic shift in vivo. (a) Representative images of MCT1 and MCT4 immunohistochemical staining in FaDu tumours, scale bar represents 200 μm. (b) Semiquantitative analysis of MCT1 and MCT4 expression in tumours treated with 10 mg/kg simvastatin or vehicle. (c) Measurements of ATP, lactate and glucose levels in FaDu tumor xenografts by imBI. hematoxylin and eosin (H&E) staining and color-coded distributions of lactate, glucose and ATP in sequential cryosections from representative tumors. The concentration values were color-coded, with each color corresponding to a defined concentration range in μmol/g. (d) Concentrations of metabolites in FaDu tumors. ATP, Lactate and glucose values were calculated from simvastatin-treated or vehicle-treated tumors. The data correspond to the mean of at least four independent experiments in which one mouse was used per experimental condition. *P < 0.05, t-test.
To better characterise metabolic changes following therapy in vivo, we measured metabolites (ATP, lactate and glucose) in these tumours using the induced metabolic bioluminescence imaging (imBl) technique. Representative images of the analysis are presented in Fig. 5c, where each color represents a pre-defined concentration range for the measured metabolites.
Measurements of lactate levels disclosed drastically lower lactate concentrations in tumours from simvastatin-treated mice compared with control samples. For FaDu xenografts, lactate content was 30.3 ± 6.4 μmol/g in vehicle-treated tumours versus 11.5 ± 0.6 μmol/g in simvastatin-treated tumours (P = 0.01). Therapy did not result in changes in ATP or glucose levels (Fig. 5d). ATP concentrations generally reflect the viability of the tested tissue sections and it is noteworthy that only samples of an ATP level of ≥0.2 μmol/g are classified as viable and were included in the study27. In the CaL-27 model, tumours treated with simvastatin had a lactate level of 10.2 ± 0.9 μmol/g compared to 17.9 ± 3.2 μmol/g in vehicle treated tumours (P = 0.01) and again no changes were observed in the measurements of ATP or glucose (Supplementary Fig. 5). These metabolic changes could suggest that simvastatin-treated tumours rely on oxidative phosphorylation and resort to the upregulation of MCT1 expression as a compensatory mechanism for survival, consistent with the role of MCT1 an importer of mitochondrial fuels24. Theoretically, this could result in enhancement of sensitivity to inhibitors of MCT1 function.
Tumour xenografts treated with simvastatin are sensitive to MCT1 inhibition
Mice receiving vehicle or simvastatin at daily oral dose of 10 mg/kg for 2 weeks prior to tumour injection, were given vehicle or the MCT1 inhibitor AZD3965 for 10 days starting when the tumours reached a size of 150 mm3 as illustrated in Fig. 6a. AZD3965 was administered by oral gavage at a dose of 100 mg/kg twice daily which is a well-tolerated dose for in vivo studies22,28. AZD3965 monotherapy did not result in any toxicity which is possibly due to the fact that both xenograft tumours express high levels of MCT4 (Supplementary Fig. 6) which is a well-known resistance factor to MCT1 inhibition22. Interestingly, combination therapy of AZD3965 and simvastatin resulted in greater inhibitory effect on tumour growth as compared to simvastatin or AZD3965 monotherapies in both xenograft tumours (Fig. 6b,c). Analysis of the time taken for individual FaDu tumours to grow from 150 to 600 mm3 showed that this was 11 days for tumours in mice treated with vehicle, 12 days for those given AZD3965 alone or simvastatin alone compared with 22 days for combination therapy of simvastatin and AZD3965 (Fig. 6d). All treatments were well-tolerated as there were no significant changes in body weight (Supplementary Fig. 5).
Combination therapy of simvastatin and AZD3965 efficiently inhibits growth of human xenograft tumours. (a) Layout of the experiment: mice were dosed once daily with simvastatin (10 mg/kg) or vehicle for 2 weeks prior to tumour cells injection, dosing continued throughout the experiment. When tumour volume reached 150 mm3, each group was further randomised to receive vehicle or AZD3965 (100 mg/kg) BID for 10 days. (b) In vivo growth curves of FaDu xenografts grown in CD-1 nude mice, showing tumour volume after start of AZD3965 treatment. Total number of animals used 28. (c) In vivo growth curves for CaL-27 xenografts grown in SCID mice, showing tumour volume after start of AZD3965 treatment. Total number of animals used 24. (d) Time (days) taken for FaDu xenografts to reach four times the initial volume (time to RTV4). *P < 0.05 & ***P < 0.001, one-way ANOVA test.
Prediagnostic statin use is associated with upregulation of MCT1 expression
Our preclinical data suggest that statin therapy is associated with an upregulation of MCT1 expression and enhanced sensitivity to MCT1 inhibition with AZD3965. To investigate the clinical significance of this finding in patients with HNSCC, we analysed a cohort of 119 clinical biopsies taken at diagnosis prior to therapy. Supplementary Table 2 shows the descriptive statistics for the study patient population and representative images of immunohistochemically staining of MCTs are depicted in Supplementary Fig. 7A. Each stained section was scored for MCT expression independently by two researchers using predetermined scoring criteria (see Materials and Methods and Supplementary Fig. 7B). Validation of MCT scoring was achieved by independent scoring by a pathologist.
Patients were also stratified according to their pre-diagnostic statin use. Exclusion criteria from the study were the use of hydrophilic statins (pravastatin and rosuvastatin) since we and others have confirmed that they don’t exert antitumour effects17,29. The other exclusion criterion was metformin use because of its well-known metabolic effects in cancer30.
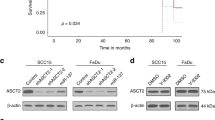
Patients were taking simvastatin or atorvastatin for at least three months prior to diagnosis with HNSCC. The Subcohort of patients who were on these statins prediagnostically had significantly higher tumour MCT1 expression than non-statin users (P = 0.02), 42% of statin users had high MCT1 expression versus only 16% in non-users and MCT4 expression was not significantly different between the two groups (P = 0.19) confirming that statin use is associated with an upregulation of only MCT1 expression in HNSCC tumours (Table 1).
Kaplan-Meier survival analysis demonstrates that prediagnostic statin use did not affect patient survival and was therefore of no prognostic significance in this cohort (P = 0.57; hazard ratio (HR) = 1.2; Fig. 7).
Discussion
The data on the link between statins and cancer is currently equivocal due to inconsistent findings reported by several studies. Some stated that the use of statins was associated with a significant reduction in overall cancer incidence as well as cancer-related deaths6,31, while others found no association between statin use and mortality from or incidence of cancer32. There are even reports of a positive association between long-term statin use and incidence of invasive lobular carcinomas and ductal carcinomas and the increased risk was limited to patients who used lipophilic statins for at least 10 years33. Ahern and colleagues reported that the use of lipophilic simvastatin was related to a decrease in the rate of breast cancer recurrence in women with invasive breast cancer while such association was not observed with hydrophilic statins34. Our in vitro toxicity data suggest that lipophilic but not hydrophilic statins can exert anti-cancer effects and this is consistent with previous findings in both breast and gynecological cancers7,17. It is well-known that the more lipophilic statins efficiently permeate extrahepatic tissues while the more hydrophilic compounds are more confined to the liver18 leading to the conclusion that lipophilic statins have the greatest potential in exerting pleiotropic effects. It is possible that previous epidemiological data on the relationship between statins and cancer have been confounded by the lack of distinction between lipophilic and hydrophilic statins.
Several studies have addressed the antitumour effects of statins in vivo4,17,29 but not in the context of glucose metabolism. In our study, we show that simvastatin antagonises tumour metabolic reprogramming, an important hallmark of cancer. Here, we show for the first time that simvastatin caused a stable modification in the metabolic phenotype of head and neck tumours as demonstrated by disturbances in glycolysis-associated markers.
Induced metabolic bioluminescence imaging is a very useful technique for metabolic profiling of tumours as well as tracking changes in the glycolytic pathways associated with therapy35. The technique has already been applied to clinical samples from patients with cervical carcinomas36, squamous cell carcinomas of the head and neck37, rectal adenocarcinomas38 and more recently, advanced ovarian carcinomas27. Our metabolic imaging results clearly show that simvastatin drastically reduced lactate levels in xenografts. Lactate, a key regulator of the glycolytic phenotype, is a potent oncometabolite that accelerates tumour development, facilitates metastasis and supports resistance to radiotherapy37,39. Another key finding of the study is the demonstration that simvastatin therapy was associated with overexpression of MCT1 in both clinical biopsies and xenograft tumours. Interestingly, it has previously been reported that high expression of MCT1 correlates with high oxidative and low glycolytic status in some tumour cells, consistent with its role as an importer of lactate and ketone bodies into the cells for the purpose of energy production24,40. Therefore, we can conclude that the tumours treated with simvastatin are characterised by a poorly glycolytic phenotype compared to their control counterparts. The associated overexpression of MCT1 is an indicator of dependency of statin treated tumours on this transporter for efficient metabolism as a compensatory survival mechanism, suggesting that blocking this compensatory pathway could be of therapeutic benefit.
Deregulation of cellular metabolism is an important hallmark of cancer41 and several novel strategies have been employed to interfere with the efficient metabolic pathways, including inhibition of monocarboxylate transporters22,24. AZD3965, an MCT1 inhibitor currently in clinical trials, has been shown to reduce tumour growth through inhibition of lactate release22,28. It has also been suggested that MCT1 inhibition prevents MCT1-mediated lactate uptake into aerobic cells where it is generally converted to pyruvate for further energy production, thereby forcing aerobic cells to use glucose for metabolism which decreases its availability for the hypoxic cells that are reliant on it for their survival24. In the present study, head and neck tumour xenografts treated with simvastatin showed enhanced sensitivity to MCT1 inhibition with AZD3965 as compared to vehicle treated tumours, suggesting that simvastatin could be a useful predictive indicator for the success this therapy.
High MCT1 expression is associated with poor clinical outcomes in bladder, and small cell lung cancers22,42. In head and neck cancers, it was MCT4 expression that had prognostic significance43 and was also associated with poor prognosis in prostate and colorectal cancers44,45. It is important to highlight that in our study, simvastatin therapy was not associated with changes in MCT4 expression in both tumour xenografts and clinical biopsies, which is consistent with lack of detrimental effect on overall patient survival. Although, a recent study showed that statin use at the time of diagnosis of HPV negative head and neck cancer was associated with improved survival46.
In recent years, there has been a shift towards personalised, patient-tailored treatment in cancer therapy and predictive biomarkers are potentially the most useful for clinical decision-making. Our data strongly suggest that prediagnostic statin use warrants further clinical investigation as a predictive biomarker for targeting metabolic pathways in tumours through inhibition of MCT1 function. This strategy is of particular relevance to HPV negative HNSCC since success of therapy has been hindered by the lack of clinically useful prognostic and predictive biomarkers in this type of cancer12. Although, our study has focused on the advantage of prediagnostic statin therapy, we believe that there may still be benefits to combination therapies with statins initiated after diagnosis.
Materials and Methods
Reagents
For in vitro studies, simvastatin (Enzo Life Sciences) was dissolved in ethanol and NaOH, neutralized to pH 7.2 for activation as a 20 mM stock solution as previously described4. Pravastatin (Sigma) was dissolved in water as a 20 mM stock solution. AZD3965 (AstraZeneca) was prepared as a 1 mM stock solution in DMSO. Stock solutions of drugs were stored as single use aliquots at −20 °C. All other reagents were of high analytical grades and obtained from Sigma unless otherwise stated.
Cell culture
FaDu, Detroit 562, CaL-27 (American Type Culture Collection, Manassas, VA) and UMSCC47 (kind donation from Prof. Chris Boshoff, UCL Cancer Institute, London) were maintained in a humidified atmosphere at 37 °C and 5% CO2.
FaDu cells were maintained in EMEM and the other cell lines in RPMI 1640. Culture medium was supplemented with 10% fetal calf serum (Life technologies) and 2 mmol/L glutamine. Cell lines were routinely screened for the presence of Mycoplasma (Venor™ GeM Mycoplasma Detection Kit) and authenticated using the Powerplex 16HS system (Promega) throughout the study.
Concentration response assays
Cells were seeded at appropriate seeding densities into 96-well plates, allowed to attach for 3 hours before treatment with different concentrations of simvastatin or pravastatin for 96 hours. Plates were then stained with Sulforhodamine B (SRB) and processed as previously described47 to assess cellular toxicity.
Lactate Measurement
Lactate concentration was determined by colorimetric assay (Trinity Biotech) according the manufacturer’s instructions. Briefly, the concentration of lactate in cell-free medium was detected by spectrophotometry at 450 nm using a standard curve of known lactate concentrations.
Measurement of ATP levels and mitochondrial mass
ATP levels were measured using the ATPlite™ Luminescence Assay System (Perkin Elmer) according to manufacturer’s instructions. Cells were seeded and exposed to 100 µl of medium with and without simvastatin for 96 hours. At endpoint, lysis solution was added to stop the reaction followed by substrate solution and luminescence was measured.
Mitochondrial mass was measured by staining live cells with 10-Nonyl acridine orange dye (Molecular probes, Invitrogen). Cells were grown to 70% confluency in a 6-well dish. The dye was diluted in RPMI media (final concentration: NAO = 20 nM) and exposed to the cells for 30 minutes at 37 °C. Cells were then washed with PBS, collected by scraping and analysed using LSRFortessa flow cytometer (BD Biosciences).
Biotinylation of cell surface MCTs/Cell surface protein isolation
For the isolation and collection of surface proteins, the Pierce® Cell Surface Protein Isolation Kit (Thermo Scientific) was used as recommended by the manufacturer’s protocol. Details are reported under Supplementary Methods.
Immunoblot Assays
Protein from whole cell lysates was quantified using the Pierce BCA Protein Assay48 with bovine serum albumin as a protein standard. Equal amounts of proteins were separated by SDS–PAGE and transferred onto a nitrocellulose membrane. After blocking with 5% milk for 1 hour, membranes were probed with primary antibodies against MCT4 (sc50329, Santa Cruz biotechnology), MCT1 (sc50324, Santa Cruz biotechnology), EpCAM (ab32392, Abcam), cyclophilin A (ab41684, Abcam) and β-Actin (A1978, Sigma) overnight at 4 °C. Proteins were detected by horseradish-peroxidase linked secondary antibodies and chemiluminescent signal detected using ECL Plus (Bio-Rad). Since the proteins are of close molecular weights, gels were stripped in Restore Plus western blot stripping buffer for subsequent re-blotting.
Clonogenic assays
Cells were seeded in 6 well plates and allowed to attach for 3 hours before treatment with the appropriate concentrations of simvastatin. After 96 hours, cells were transfected with siMCT4 and/or treated with 1 mM AZD3965 or vehicle for 72 hours in the presence of simvastatin. Medium was then changed and cells allowed to form colonies. At the end of the experiment, the medium was removed and the colonies were fixed in 70% ethanol and stained with methylene blue to facilitate counting of colonies (≥50 cells). Clonogenic survival was calculated for each condition after correcting for plating efficiency. Survival fraction was calculated by dividing survival of each condition by survival of vehicle-only treated cells.
Gene silencing by RNA interference
Transfections of siRNA duplexes targeting MCT4 or a nontargeting control (NT) (ON-TARGETplus SMARTpool, GE Healthcare) at a final concentration of 25 nM, were performed in Optimem (Invitrogen), using Dharmafect 1 reagent (GE Healthcare).
In vivo Efficacy Studies
Mice were maintained in a sterile environment, housed in individually vented caging systems in a 12-hour light/12-hour dark environment and maintained at uniform temperature and humidity. FaDu xenografts were established by subcutaneous injection of 1 × 106 cells in 0.1 ml serum-free medium into the mid-dorsal flank of 8- to 12-week-old female CD-1 nude mice (Charles River, Wilmington, MA, USA). CaL-27 xenografts were established by injection of 5 × 106 in 0.1 ml serum- free medium/Matrigel mix into the mid-dorsal flank of 8- to 14-week-old female SCID mice (Charles River).
When FaDu tumours reached an average size of 50 mm3, mice were randomised and divided into 2 groups, according to treatments administered by oral gavage. The first group of mice was treated daily with 0.1 ml of vehicle (0.5% methylcellulose), the second group of mice was treated daily with 0.1 ml of drug (5 mg/kg or 10 mg/kg simvastatin in 0.5% methylcellulose). For subsequent experiments, oral daily dosing with vehicle or simvastatin (10 mg/kg) started two weeks prior to cell inoculation. Treatment was continued and tumour-bearing mice were randomly assigned into further treatment groups of eight once a tumour volume of 150 mm3 had been achieved. The group of mice receiving vehicle was randomised into two groups and treated with 100 mg/kg BID AZD3965 in 0.5% hydroxypropyl methyl cellulose, 0.1% tween 80 or vehicle only by oral gavage for 10 days. The group of mice receiving simvastatin was also randomised into two groups and treated with 100 mg/kg BID AZD3965 in 0.5% hydroxypropyl methyl cellulose, 0.1% tween 80, or simvastatin only by oral gavage for 10 days. Simvastatin and vehicle dosing continued until the end of the experiment. Tumour size was measured twice to three times a week using calipers. At endpoint, animals were sacrificed following the standard protocols and tumours for analysis were dissected from the neighbouring connective tissue. Specimens of tumours were snap frozen in liquid nitrogen and stored at −80C for imBl analysis of metabolites. The remaining tumour tissues of each sample were fixed in 10% formalin and embedded in paraffin for histological analysis. All animal study protocols were approved by the University of Manchester Animal Welfare and Ethics Review Body and the Home Office and designed in accordance with the Scientific Procedures Act (1986) and the 2010 guidelines for the welfare and use of animals in cancer research49. Animal numbers of each group were calculated by power analysis and animals were grouped randomly for each experiment.
Patients’ samples and data collection
Formalin-fixed, paraffin-embedded primary head and neck tumour samples and clinical data were obtained from 119 consenting adult patients presenting to hospitals in the North West region of England between 2000 and 2010. Clinical biopsies were collected, processed and analysed in accordance with the relevant guidelines and regulations. For tissue microarray construction, tumour-rich regions (guided by histological review) from each case were sampled using 1-mm cores. All the archival paraffin-embedded tumour samples were coded with no patient identifiers. The study was approved by Tameside & Glossop Local Research Ethics Committee and informed consent was obtained for sample collection and analysis.
Information on the use of statins (type, dose, start date and duration) was derived from patient medical notes available at the Christie Foundation Trust (Manchester, UK) and confirmation of such details was obtained from GPs. All data was collected according to local ethical approvals.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded 4 μm sections were prepared from the TMA or tumour xenografts. For MCT1 and MCT4 staining, sections were dewaxed, rehydrated, antigen retrieval was carried out in citrate buffer (pH 6), for 12 minutes at 98 oC in an EZ retriever microwave and endogenous peroxidase blocked with 3% H2O2 for 15 minutes at room temperature. Slides were blocked with 10% casein solution for 30 minutes. The primary antibodies used were rabbit anti-MCT4 (H-90, Santa Cruz biotechnology) used at 1:500 dilution, and rabbit anti-MCT1 (AstraZeneca in-house antibody) used at 1:250 dilution. Secondary antibody reagent HRP-conjugated anti-rabbit Envision reagent was applied for 30 minutes and staining was visualized by incubation in DAB solution for 5 minutes. Slides were counterstained with hematoxylin, dehydrated, and mounted. As an isotype control, a rabbit IgG fraction was used in place of the primary antibodies. Positive control sections were employed in the study. Human placenta was used as a positive control for MCT4, and human colon was used as a positive control for MCT1. MCT1 expression was analysed on consecutive tumour sections to those utilised in the analysis of MCT4. Analysis of IHC staining is reported under Supplementary Methods.
Induced metabolic bioluminescence imaging (imBl)
Snap-frozen tumours were cut into serial cryosections for metabolic measurements and structural hematoxylin and eosin staining. For quantitative measurement of ATP, lactate and glucose, the method of metabolic imaging with induced bioluminescence (imBI) was applied, as previously described35,36,50. Further details of imBI are reported under Supplementary Methods.
Statistical analysis
All bar and line graphs represent mean ± Standard Error (SEM). Statistics were carried out using Fisher’s exact test or Student’s t-tests to compare differences between two groups. One-way analysis of variance was employed for comparison if there were more than two groups. Differences were considered statistically significant at P < 0.05. For xenograft tissue experiments, images are representative of cohorts of at least three mice. Kaplan–Meier analysis was used to assess overall survival (OS) rates over time, and log-rank (Mantel-Cox) test performed to determine whether observed differences between groups were statistically significant. Data were analysed using GraphPad Prism® v6.0 software.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Liao, J. K. & Laufs, U. Pleiotropic effects of statins. Annual review of pharmacology and toxicology 45, 89–118, https://doi.org/10.1146/annurev.pharmtox.45.120403.095748 (2005).
Lipinski, M. J., Abbate, A., Fuster, V. & Vetrovec, G. W. Drug insight: statins for nonischemic heart failure–evidence and potential mechanisms. Nature clinical practice. Cardiovascular medicine 4, 196–205, https://doi.org/10.1038/ncpcardio0855 (2007).
Mihos, C. G. & Santana, O. Pleiotropic effects of the HMG-CoA reductase inhibitors. International journal of general medicine 4, 261–271, https://doi.org/10.2147/IJGM.S16779 (2011).
Cho, S. J. et al. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. International journal of cancer. Journal international du cancer 123, 951–957, https://doi.org/10.1002/ijc.23593 (2008).
Kotamraju, S., Williams, C. L. & Kalyanaraman, B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer research 67, 7386–7394, https://doi.org/10.1158/0008-5472.CAN-07-0993 (2007).
Nielsen, S. F., Nordestgaard, B. G. & Bojesen, S. E. Statin use and reduced cancer-related mortality. The New England journal of medicine 367, 1792–1802, https://doi.org/10.1056/NEJMoa1201735 (2012).
Kato, S. et al. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. Journal of cellular and molecular medicine 14, 1180–1193, https://doi.org/10.1111/j.1582-4934.2009.00771.x (2010).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430, https://doi.org/10.1038/343425a0 (1990).
Cafforio, P., Dammacco, F., Gernone, A. & Silvestris, F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis 26, 883–891, https://doi.org/10.1093/carcin/bgi036 (2005).
Malenda, A. et al. Statins impair glucose uptake in tumor cells. Neoplasia 14, 311–323 (2012).
Halestrap, A. P. & Wilson, M. C. The monocarboxylate transporter family–role and regulation. IUBMB life 64, 109–119, https://doi.org/10.1002/iub.572 (2012).
Thomas, G. R., Nadiminti, H. & Regalado, J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. International journal of experimental pathology 86, 347–363, https://doi.org/10.1111/j.0959-9673.2005.00447.x (2005).
Jemal, A. et al. Global cancer statistics. CA: a cancer journal for clinicians 61, 69–90, https://doi.org/10.3322/caac.20107 (2011).
Erkal, H. S., Mendenhall, W. M., Amdur, R. J., Villaret, D. B. & Stringer, S. P. Synchronous and metachronous squamous cell carcinomas of the head and neck mucosal sites. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 19, 1358–1362 (2001).
Snow, G. B., Annyas, A. A., van Slooten, E. A., Bartelink, H. & Hart, A. A. Prognostic factors of neck node metastasis. Clinical otolaryngology and allied sciences 7, 185–192 (1982).
Duggan, D. E. & Vickers, S. Physiological disposition of HMG-CoA-reductase inhibitors. Drug metabolism reviews 22, 333–362, https://doi.org/10.3109/03602539009041088 (1990).
Campbell, M. J. et al. Breast cancer growth prevention by statins. Cancer research 66, 8707–8714, https://doi.org/10.1158/0008-5472.CAN-05-4061 (2006).
Serajuddin, A. T., Ranadive, S. A. & Mahoney, E. M. Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. Journal of pharmaceutical sciences 80, 830–834 (1991).
Halestrap, A. P. The monocarboxylate transporter family–Structure and functional characterization. IUBMB life 64, 1–9, https://doi.org/10.1002/iub.573 (2012).
Maftah, A., Petit, J. M., Ratinaud, M. H. & Julien, R. 10-N nonyl-acridine orange: a fluorescent probe which stains mitochondria independently of their energetic state. Biochemical and biophysical research communications 164, 185–190 (1989).
Cancer Research UK. A Phase I Trial of AZD3965 in Patients With Advanced Cancer. NIH Web site (2015) Available from: http://www.clinicaltrials.gov/ct2/show/NCT01791595.
Polanski, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 20, 926–937, https://doi.org/10.1158/1078-0432.CCR-13-2270 (2014).
Baek, G. et al. MCT4 defines a glycolytic subtype of pancreatic cancer with poor prognosis and unique metabolic dependencies. Cell reports 9, 2233–2249, https://doi.org/10.1016/j.celrep.2014.11.025 (2014).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. The Journal of clinical investigation 118, 3930–3942, https://doi.org/10.1172/JCI36843 (2008).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 22, 659–661, https://doi.org/10.1096/fj.07-9574LSF (2008).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S1–45, https://doi.org/10.1161/01.cir.0000437738.63853.7a (2014).
Battista, M. J. et al. Feasibility of induced metabolic bioluminescence imaging in advanced ovarian cancer patients: first results of a pilot study. Journal of cancer research and clinical oncology 142, 1909–1916, https://doi.org/10.1007/s00432-016-2200-x (2016).
Bola, B. M. et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Molecular cancer therapeutics 13, 2805–2816, https://doi.org/10.1158/1535-7163.MCT-13-1091 (2014).
Coimbra, M. et al. Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. Journal of controlled release: official journal of the Controlled Release Society 148, 303–310, https://doi.org/10.1016/j.jconrel.2010.09.011 (2010).
Pernicova, I. & Korbonits, M. Metformin–mode of action and clinical implications for diabetes and cancer. Nature reviews. Endocrinology 10, 143–156, https://doi.org/10.1038/nrendo.2013.256 (2014).
Graaf, M. R., Beiderbeck, A. B., Egberts, A. C., Richel, D. J. & Guchelaar, H. J. The risk of cancer in users of statins. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 22, 2388–2394, https://doi.org/10.1200/JCO.2004.02.027 (2004).
Li, X., Wu, X. B. & Chen, Q. Statin use is not associated with reduced risk of skin cancer: a meta-analysis. British journal of cancer 110, 802–807, https://doi.org/10.1038/bjc.2013.762 (2014).
Strandberg, T. E. et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 364, 771–777, https://doi.org/10.1016/S0140-6736(04)16936-5 (2004).
Ahern, T. P. et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. Journal of the National Cancer Institute 103, 1461–1468, https://doi.org/10.1093/jnci/djr291 (2011).
Curtarello, M. et al. VEGF-targeted therapy stably modulates the glycolytic phenotype of tumor cells. Cancer research 75, 120–133, https://doi.org/10.1158/0008-5472.CAN-13-2037 (2015).
Walenta, S. et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer research 60, 916–921 (2000).
Brizel, D. M. et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. International journal of radiation oncology, biology, physics 51, 349–353 (2001).
Walenta, S. et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? Journal of cancer research and clinical oncology 129, 321–326, https://doi.org/10.1007/s00432-003-0450-x (2003).
Blatt, S. et al. Lactate as a predictive marker for tumor recurrence in patients with head and neck squamous cell carcinoma (HNSCC) post radiation: a prospective study over 15 years. Clinical oral investigations, https://doi.org/10.1007/s00784-015-1699-6 (2016).
Kim, Y., Choi, J. W., Lee, J. H. & Kim, Y. S. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Human pathology 46, 104–112, https://doi.org/10.1016/j.humpath.2014.09.013 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674, https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Choi, J. W., Kim, Y., Lee, J. H. & Kim, Y. S. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology 84(245), e249–215, https://doi.org/10.1016/j.urology.2014.03.031 (2014).
Curry, J. M. et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell cycle 12, 1371–1384, https://doi.org/10.4161/cc.24092 (2013).
Pertega-Gomes, N. et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC cancer 11, 312, https://doi.org/10.1186/1471-2407-11-312 (2011).
Nakayama, Y. et al. Prognostic significance of monocarboxylate transporter 4 expression in patients with colorectal cancer. Experimental and therapeutic medicine 3, 25–30, https://doi.org/10.3892/etm.2011.361 (2012).
Lebo, N. et al. Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head & Neck 40, 1697–1706, https://doi.org/10.1002/hed.25152 (2018).
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature protocols 1, 1112–1116, https://doi.org/10.1038/nprot.2006.179 (2006).
Smith, P. K. et al. Measurement of protein using bicinchoninic acid. Analytical biochemistry 150, 76–85 (1985).
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. British journal of cancer 102, 1555–1577, https://doi.org/10.1038/sj.bjc.6605642 (2010).
Mueller-Klieser, W. & Walenta, S. Geographical mapping of metabolites in biological tissue with quantitative bioluminescence and single photon imaging. The Histochemical journal 25, 407–420 (1993).
Acknowledgements
We would like to thank Mr Robert Duncombe at the pharmacy department at the Christie NHS foundation trust for facilitating access to patients’ medical records. This work has been funded by Cancer Research UK.
Author information
Authors and Affiliations
Contributions
M.M. designed the work, conducted experiments, analysed data and wrote the manuscript, F.O.-M., N.V., A.B. and A.C. conducted experiments, B.L.T. contributed to experimental design and conducted experiments, W.M.-K. and C.M.W. contributed reagents/materials/analysis tools, S.E.C. contributed to reagents/materials/analysis tools and reviewed the manuscript, K.J.W. contributed to experimental design and reviewed the manuscript, I.J.S. designed the work and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
S.E.C. is a full time employee and stockholders of AstraZeneca plc. I.J.S. was in receipt of research support from AstraZeneca.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mehibel, M., Ortiz-Martinez, F., Voelxen, N. et al. Statin-induced metabolic reprogramming in head and neck cancer: a biomarker for targeting monocarboxylate transporters. Sci Rep 8, 16804 (2018). https://doi.org/10.1038/s41598-018-35103-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35103-1
Keywords
This article is cited by
-
Statins affect human iPSC-derived cardiomyocytes by interfering with mitochondrial function and intracellular acidification
Basic Research in Cardiology (2024)
-
Statins: a repurposed drug to fight cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Targeted sensitization of tumor cells for radiation through monocarboxylate transporters 1 and 4 inhibition in vitro
Clinical Oral Investigations (2021)
-
The Ph paradigm in cancer
European Journal of Clinical Nutrition (2020)
-
The updated landscape of tumor microenvironment and drug repurposing
Signal Transduction and Targeted Therapy (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.