Abstract
Dendritic cells (DCs) abundantly express diverse receptors to recognize mannans in the outer surface of Candida cell wall, and these interactions dictate the host immune responses that determine disease outcomes. C. krusei prevalence in candidiasis worldwide has increased since this pathogen has developed multidrug resistance. However, little is known how the immune system responds to C. krusei. Particularly, the molecular mechanisms of the interplay between C. krusei mannan and DCs remain to be elucidated. We investigated how C. krusei mannan affected DC responses in comparison to C. albicans, C. tropicalis and C. glabrata mannan. Our results showed that only C. krusei mannan induced massive cytokine responses in DCs, and led to apoptosis. Although C. krusei mannan-activated DCs underwent apoptosis, they were still capable of initiating Th17 response. C. krusei mannan-mediated DC apoptosis was obligated to the TLR2 and MyD88 pathway. These pathways also controlled Th1/Th17 switching possibly by virtue of the production of the polarizing cytokines IL-12 and IL-6 by the C. krusei mannan activated-DCs. Our study suggests that TLR2 and MyD88 pathway in DCs are dominant for C. krusei mannan recognition, which differs from the previous reports showing a crucial role of C-type lectin receptors in Candida mannan sensing.
Similar content being viewed by others
Introduction
Candida species are the most common cause of opportunistic fungal infections in immunocompromised individuals, leading to illnesses ranging from non-life-threatening mucocutaneous lesions to systemically invasive infections. Over recent decades, the incidence of candidiasis worldwide has shifted from Candida albicans to non-albicans Candida species (NACs) due to the evolution of resistance to anti-fungal medications1,2.
Candida krusei is an emerging nosocomial fungal pathogen primarily found in patients with hematologic malignancies undergoing bone marrow transplantation3,4,5,6. In addition, the frequency of C. krusei in candiduria and mucocutaneous candidiasis in diabetic patients has significantly risen recently7,8,9. The prevalence of C. krusei has increased since it became a multidrug-resistant pathogen because of its intrinsic fluconazole resistance and decreased susceptibility to flucytosine, amphotericin B and echinocandins2,5,10,11,12,13. Furthermore, this has made C.krusei infections difficult to treat and led to a high mortality rate2,14. Despite its increasing importance, little is known regarding the immune system response to C. krusei.
Carbohydrate constituents of Candida cell walls play a pivotal role in triggering host immune responses, which in turn either protect against the fungal infection or facilitate fungal immune evasion15,16,17. Mannans are mannose polymers located in the outermost part of Candida cell walls; therefore, they may be the first component to interact with the immune system. As cell wall mannans are complex structures, elaborate immune mechanisms have evolved16,17. While studies have shown that mannans can induce anti-fungal protective immunity18,19,20, other reports have revealed that mannans are a significant virulence factor associated with the severity and pathogenesis of Candida infections21,22. Furthermore, high levels of mannans can be detected in the blood of invasive candidiasis patients and it has been related to disease onset and progression23,24.
Dendritic cells (DCs) are potent antigen-presenting cells that reside in both lymphoid and non-lymphoid tissues and act as sentinels of the immune system. Interactions between invading pathogens and DCs via pathogen-associated molecular patterns (PAMPs) pattern-recognition receptors (PRRs) provide the foundation that triggers adaptive immune responses16,25. DCs abundantly express C-type lectin receptors (CLRs) and Toll-like receptors (TLRs), many of which can bind to Candida mannans. The activation of different types of mannan-specific receptors leads to differential DC activation that subsequently dictates distinct T cell responses16,17,25. Recognition of Candida mannans by CLRs and TLRs on DCs depends on mannan structure and mannosyl composition. In general, Candida N-linked mannans are recognized by dectin-2, mincle, mannose receptor (MR or CD206) and DC-SIGN (CD209), while O-linked mannans are recognized by TLR-417. Furthermore, the α-mannans preferentially engage with dectin-2 and dectin-320,26, while the β-mannans specifically ligate to galectin-3, which mediates TLR-2 activation27,28. The interactions of Candida mannans with several CLRs expressed on DCs induce Syk activation, which consequently mediates innate resistance to systemic fungal infection and orchestrates the Th17 response19,29,30. However, some mannose residues mediates signal transduction via the TLR/MyD88–dependent pathway, and participates in host defense against C. albicans infection31,32,33.
To date, the role of C. krusei mannan in DC immunity is not clear. Since mannan structures and mannosyl composition in the cell wall of Candida species are highly diverse, we compared the effects of cell wall mannans extracted from C. albicans, C. troplicalis, C. glabrata and C. krusei on DCs, and T cell responses.
Results
C. krusei mannan induced DC maturation and triggered massive productions of pro-inflammatory cytokines
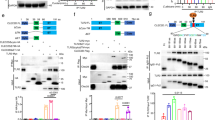
To evaluate whether cell wall mannans extracted from four distinct Candida species differentially affected the phenotypic maturation of DCs, BMDCs were stimulated with various concentrations of mannans and subsequently characterized by flow cytometric analyses of the maturation markers CD40, CD80, CD86 and MHC class II (Figs. 1, S1 and S2). The DC population was first identified by gating a DC marker, CD11c (Fig. S1A), and geometric MFI of the maturation markers was assessed using a histogram analysis (Figs. 1A and S1B). BMDCs stimulated with C. albicans and C. tropicalis mannans did not undergo maturation compared to the negative control, whereas those stimulated with C. krusei and C. glabrata mannans were potently activated. C. krusei mannan upregulated expression of CD40, CD86 and MHC class II on BMDCs, and induced the highest levels of CD40, especially at the highest mannan concentration. Although, C. glabrata mannan also induced CD80, CD86 and MHC Class II expression on BMDCs, expression differed slightly from that of BMDCs stimulated with C. krusei mannan. To determine the number of DCs that underwent maturation, dot plot analyses were performed to evaluate the percentage of CD11c+CD40+, CD11c+CD80+, CD11c+CD86+ and CD11c+MHC class II+ cells within the CD11c+ fraction (Figs 1B and S2). The percentages of each DC subpopulation were consistent with their geometric MFI levels (Figs. 1A and 1B).
Differential phenotypic maturation of BMDCs in response to Candida mannan BMDCs were stimulated with Candida mannan at the indicated concentrations. Forty eight hours later, the expressions of CD11c, CD40, CD80, CD86 and MHC class II were assessed using flow cytometry as shown in Figs S1 and S2. Live BMDCs were defined based on side scatter (SSC) and forward scatter (FSC), and an electronic gate was placed on the CD11c+ cells. (A) the geometric mean fluorescence intensity (MFI) was evaluated using histogram analysis, and (B) the percentages of CD40+, CD80+, CD86+ and MHC class II+ cells within CD11c+ population were determined. n = 5; Data are representatives of two independent experiments. *p < 0.05 when compared to the unstimulated BMDCs, ap < 0.05 when compared to C. albicans, tp < 0.05 when compared to C. tropicalis, gp < 0.05 when compared to C. glabrata, kp < 0.05 when compared to C. krusei, †p < 0.05 when compared to all groups; (−), unstimulated BMDCs; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
Next, to determine the effects of mannan stimulation on DC cytokine production, we quantitated the levels of the pro-inflammatory cytokines TNF-α, IL-1β, IL-6, IL-23, IFN-γ, IL-12 and the anti-inflammatory cytokines IL-4 and IL-10 in response to Candida mannans (Fig. 2). C. albicans and C. tropicalis mannans induced significant levels of IFN-γ production in BMDCs compared to the negative control. However, in parallel to the effect on DC maturation, C. albicans and C. tropicalis mannans did not stimulate BMDCs to produce the other cytokines. C. glabrata mannan significantly upregulated IFN-γ, and slightly promoted IL-6 and IL-23 production; however, it failed to stimulate BMDCs to produce the other cytokines, regardless of its high DC-activation ability. Of note, C. krusei mannan at all concentrations augmented massive production of TNF-α, IL-6, IL-23, IFN-γ, and IL-12. None of the Candida mannans promoted production of the anti-inflammatory cytokines IL-4 and IL-10. Our findings imply that the cell wall mannans of these four distinct Candida species differentially impact DC maturation and function, and C. krusei mannan, in particular, elicits robust inflammatory DC responses.
C. krusei mannan induced massive pro-inflammatory cytokine production. BMDCs were stimulated with Candida mannans at the indicated concentrations, and the cytokine productions in the culture supernatant were measured by ELISA. The levels of TNF-α, IL-6, IL-10 and IL-23 were detected at 24 h after stimulation, and the levels of IL-1β, IFN-γ, IL-12 and IL-4 were detected at 48 h after stimulation. n = 5; Data are representatives of two independent experiments. *p < 0.05 when compared to the unstimulated BMDCs, ap < 0.05 when compared to C. albicans, tp < 0.05 when compared to C. tropicalis, †p < 0.05 when compared to all groups. (−), unstimulated BMDCs; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
C. krusei mannan reduced BMDC viability by the induction of cellular apoptosis
Signal transduction via PRRs has been shown to lead to transcriptional expression of inflammatory cytokines and trigger cellular apoptosis34,35. On the basis of the vigorous pro-inflammatory cytokine production of BMDCs in response to C. krusei mannan stimulation, we hypothesized that C. krusei mannan may also induce cell death. To test this hypothesis, BMDCs were incubated with Candida mannans at various concentrations and cell viability was assessed by MTT assays. We found that C. krusei mannan at all concentrations markedly decreased BMDC viability, but the other Candida mannans did not (Fig. 3A). Changes in CD11c+ BMDCs were also observed using flow cytometric analysis. After CD11c+ cells were gated, live cells were identified based on forward scatter (FSC) and side scatter (SSC) signals (Fig. S3A). The percentage of live cells in the CD11c+ population was notably reduced when BMDCs were incubated with C. krusei mannan, whereas they were unaffected by mannans from the other three Candida species (Figs. 3B and S3B). To determine whether the reduction in live cells was due to DC apoptosis, unstimulated and Candida-mannan-stimulated BMDCs were stained with CD11c, Annexin V and 7AAD. CD11c+ cells that were single positive for Annexin V or double positive for Annexin V and 7AAD were identified as apoptotic DCs (Fig. 3C). Consistently, only C. krusei mannan significantly induced apoptosis in DCs (Fig. 3D). Our data imply that C. krusei mannan affects DC viability through a process of activation-induced cellular apoptosis.
C. krusei mannan reduced DC viability by mediating DC apoptosis. (A) BMDCs were incubated with various concentrations of Candida mannans for 48 h, and the cell viability was determined by MTT assay. The percentage of cell viability was expressed as a percentage relative to the unstimulated BMDCs. (B) BMDCs were incubated with 25 μg/ml of Candida mannans for 72 h, and the percentage of live CD11c+ cells were determined (please see detail in Fig. S3). BMDCs were incubated with 25 μg/ml of Candida mannans for 48 h, then the cells were stained with CD11c, Annexin V and 7AAD. (C) CD11c+ cells were gated and the Annexin V+, and Annexin V+7AAD+ were identified as apoptotic fraction. The number in the dot plot indicated the percentage of apoptotic CD11c+ DCs. (D) the percentage of apoptotic CD11c+ cells was shown as the bar graph. n = 5; Data are representatives of two independent experiments. *p < 0.05 when compared to the unstimulated BMDCs, †p < 0.05 when compared to all groups. (−), unstimulated BMDCs; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
C. krusei mannan reduced BMDC viability by the induction mannan mediated DC apoptosis via MyD88-dependent pathway
C-type lectin receptors (CLRs) such as dectin-2, dectin-3 and mincle are the main receptors in recognizing Candida mannans; however, some mannan structures do engage with TLRs16,17. As previous studies have shown that the mannan structures of Candida species are diverse36,37, we questioned which pathway is responsible for C. krusei mannan-mediated DC apoptosis. To elucidate the signal transduction underlying this phenomenon, downstream signaling of the CLR-coupled Syk and TLR-MyD88 pathways was individually investigated using pathway-specific inhibitors. BMDCs were pre-treated with Syk or MyD88 inhibitors, and subsequently stimulated with C. krusei mannan. Cell viability was determined using MTT assay. C. albicans β-glucan was used as a positive control since it transduces signals via the dectin-1-Syk pathway38, and we found that it did not affect BMDC cell viability (Fig. 4A). Inhibition of Syk in unstimulated and β-glucan-stimulated BMDCs significantly decreased cell viability, but did not abrogate C. krusei mannan-induced cell death (Fig. 4A). In a parallel experiment to examine the role of MyD88, LPS was used as the positive control for MyD88 activation. LPS stimulation resulted in a marked reduction in BMDC viability, similarly to C. krusei mannan stimulation, and inhibition of MyD88 rescued the viability of BMDCs stimulated with C. krusei mannan as well as that of those stimulated with LPS (Fig. 4B). In addition, the apoptosis staining assay demonstrated that MyD88 inhibition, to a great extent, interfered with both LPS- and C. krusei mannan-mediated apoptosis of the CD11c+ population (Figs. 4C and 4D). These results imply an intriguing role of the MyD88-dependent signaling pathway in DC apoptosis in response to C. krusei mannan stimulation.
DC apoptosis induced by C. krusei mannan was dependent on MyD88 signaling pathway. (A) BMDCs were pre-treated with vehicle control or Syk inhibitor, and the cells were incubated with 25 μg/ml of β-glucan or 25 μg/ml of C. krusei mannan for 48 h. (B) BMDCs were pre-treated with control peptide or MyD88 inhibitor, and the cells were incubated with 0.5 μg/ml LPS or 25 μg/ml of C. krusei mannans for 48 h. The BMDC viability was determined by MTT assay. The percentage of cell viability was expressed as a percentage relative to the unstimulated BMDCs. (C) and (D) BMDCs were pre-treated with control peptide or MyD88 inhibitor, and the cells were incubated with 0.5 μg/ml of LPS or 25 μg/ml of C. krusei mannan for 48 h. Then, the cells were stained with CD11c, Annexin V and 7AAD. CD11c+ cells were gated and the Annexin V+, and Annexin V+7AAD+ were identified as apoptotic fraction. The number in the dot plot indicated the percentage of apoptotic DCs. (D) The percentage of apoptotic CD11c+ cells was shown as the bar graph. n = 3; Data are representatives of two independent experiments. *p < 0.05. (−), unstimulated BMDCs; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
DC apoptosis-mediated by C. krusei mannan was associated with TLR2 activation
Previous studies have shown that the mannosyl residuals of C. albicans cell wall can be recognized by TLR239, and this recognition mediated macrophage apoptosis40. We therefore investigated the functional relevance of TLR2 in DC apoptosis triggered by C. krusei mannan stimulation. To confirm that the activation of TLR2 also induce the cell death, BMDCs were incubated with a specific TLR2 ligand, Pam3CSK4, and the cell viability was determined using MTT assay. As expected, BMDCs stimulated with Pam3CSK4 reduced the BMDC viability (Fig. 5A). Next, C. krusei mannan-mediated DC apoptosis via TLR2 ligation was determined by using the blocking antibodies. As a positive control, Pam3CSK4, significantly enhanced DC apoptosis, and this Pam3CSK4–induced cellular apoptosis was impeded by pre-treatment with the anti-TLR2 blocking antibody. Notably, blockade with anti-TLR2 mAbs merely diminished the effect of C. krusei mannan on DC apoptosis (Figs. 5B and 5C). These findings were concordant with the results of MyD88 inhibition (Fig. 4D).
C. krusei mannan mediated DC apoptosis via the activation of TLR2. (A) BMDCs were incubated with various concentration of Pam3CSK4 as indicated for 48 h, and the cell viability was determined by MTT assay. The percentage of cell viability was expressed as a percentage relative to the unstimulated BMDCs. n = 4; Data are representatives of two independent experiments. *p < 0.05 when compared to the negative control. (B) and (C) BMDCs were pre-treated with control rat IgG or anti-mouse TLR2 mAbs, and the cells were incubated with 0.5 μg/ml of Pam3CSK4 or 25 μg/ml of C. krusei mannan for 48 h. Then, the cells were stained with CD11c, Annexin V and 7AAD. CD11c+ cells were gated and the Annexin V+, and Annexin V+7AAD+ were identified as apoptotic fraction as shown in (B). The number in the dot plot indicated the percentage of apoptotic CD11c+ DCs. (C) The percentage of apoptotic CD11c+ cells was shown as the bar graph. n = 3; Data are representatives of three independent experiments.*p < 0.05. (−), unstimulated BMDCs; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
We also observed the effect of C. krusei cell wall on DC apoptosis (Fig. S4). C. krusei yeast cells were inactivated by heat so that the cells could not secrete any molecules that might have influenced the DC response. In addition, we expected that mannans would be the first component to interact with DCs because they are located in the outermost layer of Candida cell walls16,17. Consistent with the apoptosis induction by C. krusei mannan (Fig. 5C), BMDCs stimulated by heat-inactivated C. krusei yeast cells also displayed significantly increased apoptosis, and blockade of TLR2 abrogated apoptosis induction (Fig. S4).
Taken together, our data indicate that TLR2 plays an important role in the mechanism of apoptosis induction by the cell wall mannan of C. krusei.
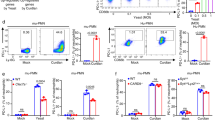
C. krusei mannan-stimulated DCs orchestrated antigen-specific Th17 response
Since C. krusei mannan induced massive cytokine production and impaired DC viability, we next investigated antigen-specific T cell responses to stimulated DCs. As a large number of DCs reside in the skin41, mice were immunized with a mixture of ovalbumin and various Candida mannans (OVA-Candida mannans) via a subcutaneous route so that DCs could be exposed directly to the stimuli. Seven days after the final immunization, the immune cell populations (T cells, B cells, memory T cells, and Th subsets) in regional lymph nodes (RLNs) were examined by flow cytometric analysis (Figs S5 and S6). Total numbers of RLN cells were significantly increased when mice were immunized with OVA-C. albicans mannan or OVA-C. krusei mannan. However, none of the immunized mice exhibited any alteration in the proportions of immune cell populations (Fig. S6). Several lines of evidence have revealed that Candida cell wall- and mannan-stimulated DCs can dictate the fate of CD4 T cell polarization20,25. Thus, we further examined T cell responses when stimulated ex vivo. The antigen-primed LN T cells were re-stimulated with OVA, and the levels of Th cytokines, IFN-γ, IL17, IL-4 and IL-10, were determined (Fig. 6A). Upon ex vivo antigen re-exposure, OVA-C. albicans mannan- and OVA-C. tropicalis mannan-immunized LN cells markedly upregulated IFN-γ, IL-4 and IL-10 secretion compared to the OVA-C. glabrata mannan, OVA-C. krusei mannan, and negative control groups. On the other hand, immunization with OVA-C. glabrata mannan did not activate cytokine production. Augmented IL-17 production was observed particularly in OVA-C. krusei mannan-immunized LN cells.
C. krusei mannan governed Th17 response in an antigen-specific manner. (A) Mice were subcutaneously immunized with the mixture of OVA and Candida mannan at day 0 and day 7. At day 14, the RLN cells were re-simulated with (+) or without (−) OVA, and the levels of IFN-γ, IL-17, IL-4 and IL-10 in the culture supernatant were quantitated by ELISA. n = 5; Data are representatives of two independent experiments. *p < 0.05 when compared to the negative control, ap < 0.05 when compared to OVA-C. albicans, tp < 0.05 when compared to OVA-C. tropicalis, †p < 0.05 when compared to all groups. (−), Mice were immunized with OVA alone as a negative control; Ca, OVA-C. albicans; Ct, OVA-C. tropicalis; Cg, OVA-C. glabrata; Ck, OVA-C. krusei. (B) BMDCs were stimulated with 25 μg/ml of Candida mannan, and the cells were stimulated with 25 μg/ml of C. albicans or C. krusei mannan. Subsequently unstimulated and mannan-stimulated BMDCs were pulsed with OVA, and the BMDCs were then co-cultured with OT-II T cells for 48 h. The level of IFN-γ and IL-17 in the culture supernatant was determined by ELISA. n = 3; Data are representatives of two independent experiments. *p < 0.05 when compared to the negative control, ap < 0.05 when compared to C. albicans, tp < 0.05 when compared to C. tropicalis, †p < 0.05 when compared to all groups; (−), T cells incubated with OVA were used as a negative control; Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Ck, C. krusei.
In vivo mannan immunization may not only activate DCs but also possibly affect the response of other immune cells. Therefore, to investigate whether the distinct T cell responses were due to the direct impact of Candida mannan-stimulated DCs on CD4 T cells, we performed an in vitro co-culture assay. BMDCs were primed with Candida mannans and subsequently pulsed with OVA. These BMDCs were then co-cultured with T cells isolated from OT-II TCR transgenic mice. Secretion of IFN-γ, IL-17, IL-4 and IL-10 in the culture supernatant, representing T helper cell responses, was analyzed. Concurring with our results from the ex vivo re-stimulation assays, C. abicans and C. tropicalis mannan-stimulated DCs profoundly augmented IFN-γ production from OT-II T cells, while C. krusei mannan-stimulated DCs preferentially induced IL-17 production (Fig. 6B). IL-4 and IL-10 were not detectable in this co-culture system. Collectively, our results reveal that DCs activated with C. krusei mannan, despite undergoing apoptosis, were capable of orchestrating the Th17 response in an antigen-specific manner.
Th17 induction by C. krusei mannan-stimulated DCs was via the MyD88 signaling pathway
Having demonstrated the involvement of the MyD88-dependent pathway in DC apoptotic response to C. krusei mannan (Fig. 4), we investigated whether MyD88 was also required for Th17 induction by C. krusei mannan-stimulated DCs. BMDCs were pre-treated with a MyD88 inhibitor and then primed with C. albicans mannan or C. krusei mannan. Subsequently, these BMDCs were pulsed with OVA and co-cultured with OT-II T cells. Inhibition of MyD88 in DCs stimulated with mannan from C. krusei converted the production of IL-17 to IFN-γ in the activated OT-II T cells, but this did not occur in DCs stimulated with mannan from C. albicans (Fig. 7A). TLR-2 blockade in C. krusei mannan-stimulated DCs also resulted in reduced IL-17 production by OT-II T cells, but did not affect IFN-γ production (Fig. S7A). Consistently, MyD88 inhibition in BMDCs stimulated with C. krusei mannan significantly reduced IL-6, the key cytokine in Th17 induction42,43, while it substantially enhanced the production of IL-12, which is required for Th1 induction42 (Fig. 7B).
C. krusei mannan-stimulated DCs skewed Th1/Th17 polarization via MyD88 and TLR2 pathway. (A) BMDCs were pre-treated with control peptide or MyD88 inhibitor, and the cells were stimulated with 25 μg/ml of C. albicans or C. krusei mannan. Subsequently, unstimulated and mannan-stimulated BMDCs were pulsed with OVA, and the BMDCs were co-cultured with OT-II T cells for 48 h. The levels of IFN-γ and IL-17 in the culture supernatant were determined by ELISA. (−), T cell incubated with OVA as a negative control. n = 3; Data are representatives of two independent experiments. *p < 0.05. Ca, C. albicans; Ck, C. krusei. (B–D) BMDCs were pre-treated with (B) control peptide or MyD88 inhibitor (C) control IgG or anti-mouse TLR2 mAbs. Then, the cells were stimulated with 25 μg/ml of C. krusei mannan, and the levels of IL-12, IL-6 and IL-23 in the culture supernatant were determined by ELISA. n = 3; Data are representatives of three independent experiments. *p < 0.05. (−), unstimulated BMDCs; Ck, C. krusei.
We further verified the roles of TLR2 in the cytokine production of C. krusei mannan-stimulated DCs by using blocking antibodies. TLR2 blockade significantly suppressed production of IL-6, but not that of IL-12 and IL-23, in BMDCs stimulated with Pam3CSK4 and with C. krusei mannan (Fig. 7C). IL-1β is also play a role in Th17 polarization44, and TLR-2 stimulation has participated in the production of IL-1β45. However, we found that TLR-2 blockade did not alter IL-1β production in BMDCs stimulated with C. krusei mannan (Fig. S7B). This result may be due to the inability of C. krusei mannan to induce IL-1β production in BMDCs (Fig. 2).
To confirm the differences in cell wall mannan structure between Candida species, the mannan composition were analyzed using nuclear magnetic resonance spectroscopy (NMR). NMR characterizations of all Candida mannans in our study were consistent with previous reports36,37,46,47,48,49 (Figs S8–S11). Additionally, structural analysis of C. krusei mannan was further investigated (Fig. S11). 13C NMR spectrum contained signals at 103.31, 101.76, and 99.28 ppm corresponded to anomeric carbons (C1) of terminal non-reducing, 2-substituted, and 2,6-disubstituted mannose, respectively48 (Fig. S11A). Acidic hydrolysis of C. krusei mannan was then carried out to support the structural analysis. Acylation, acetolysis and O-deacetylation of C. krusei mannan, followed by gel filtration on Bio-Gel P-2 gave manno-oligosaccharide. 1H NMR spectrum of the manno-oligosaccharide showed signals at 5.36, 5.27, and 5.01 ppm for anomeric proton (H1) of non-reducing end, α-1,2-linked, and reducing end, respectively (Fig. S11B). This result indicated that C. krusei mannan possessed long chain of α−1,2-linked backbone, which resembled the structure reported previously48,50.
1H NMR spectra were supported the structural analysis. Signals between 5.60–4.90 ppm represented α-linked mannose residues (Fig. 12)46. Distinctive down filed signals of 3 mannans derived from C. albicans (5.48 ppm), C. tropicalis (5.48 and 5.46 ppm), and C. glabrata (5.57 and 5.58 ppm) indicated phosphodiesterified 1,2-linked mannose residues51. On the other hand, the down filed signal was not observed from 1H NMR of C. krusei mannan. This was consistent with the absence of phosphodiester linkage in mannan structure. 1H NMR signals of mannans with α-1,2-linkages were also detected, generally in down filed regions, such as C. albicans (5.32 ppm), C. tropicalis (5.20 ppm), C. glabrata (5.30 ppm), and C. krusei (5.21, 5.16, 5.14 ppm)36. Molecular weights of Candida mannans were also determined by using gel permeation chromatography (GPC) as follows; C. albicans mannan (925 kDa), C. tropicalis mannan (387 kDa), C. glabrata mannan (158 kDa), and C. krusei mannan (169 kDa) (Figs. S13–S16). The molecular weights were consistent with the proposed Candida mannan structures (Fig. S17).
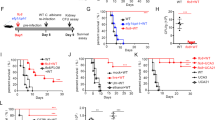
In summary, our findings indicate that DCs recognize C. krusei mannan via a TLR2/MyD88 dependent pathway, and this interaction results in apoptosis. Likewise, signal transduction through TLR2/MyD88 also regulates IL-12 and IL-6 production in DCs, which results in a shift toward Th1 and Th17 immunity, respectively (Fig. 8).
A schematic model of the signal transduction in DCs in response to C. krusei mannan C. krusei mannan activates TLR2/MyD88 signal in DCs and triggers apoptotic death. In parallel, the signal transduction through TLR2/MyD88 interferes with the production of IL-12 but promotes the production of IL-6, which consequently skews Th1/Th17 differentiation.
Discussion
In this study, we provide evidence that DCs respond differently to cell wall mannans from different Candida species. We investigated the molecular mechanisms underlying the induction of DC and T-cell immunity in response to C. krusei cell wall mannan. In contrast to previous reports on mannans from other species, our results show that C.krusei mannan activates DCs and consequently guides Th cell responses through the TLR2/MyD88 pathway.
We examined the responses of DCs to the cell wall mannans of four Candida species that are commonly found as opportunistic pathogens in immunocompromised patients: C. albicans, C. tropicalis, C. glabrata and C. krusei. Several Candida species are dimorphic fungi that can switch between the yeast and filamentous forms in response to environmental conditions such as high temperature, pH, and nutrition, whereas other Candida species exist in only one form52,53. C. albicans and C. tropicalis have three forms with distinct morphology; yeast cell, pseudohyphal cell and hyphal cell, while C. krusei yeast cells can produce pseudohyphae but not true hyphae. On the other hand, there is no yeast-to-hyphae transition in C. glabrata53. Because the yeast form exists in all Candida species and is the form that initially colonizes host tissues and can circulate in the bloodstream throughout the body17,54,55, we focused on comparison of cell wall mannans extracted from the yeast form only. It is likely that mannans from various forms may have different effects, a possibility that would be worth exploring in future studies.
Our results show quantitative differences in DC responses to the cell wall mannans of these four distinct Candida species (Figs 1 and 2). This may be due to differences in their mannan structure and the composition of mannose residues36,37,46,47,48 (Figs. S8–12 and S17). C. albicans mannan consists of short-chain O-linked mannan oligosaccharides and branching N-linked mannan polysaccharide moieties. The N-linked mannan is composed of a long chain of α-1,6-linked mannose backbone connected with oligomannose side chains, mainly containing α-1,2-, α-1,3-, and β-1,2-linked mannose residues with a few phosphate groups36,56 (Fig. S8). C. tropicalis shares certain mannan moieties with C. albicans (Figs S8,9), and is antigenically identical to C. albicans serotype A37,57,58. Concurring with the similarity in mannan structure and composition of these two Candida species, we observed similar effects of C. albicans and C. tropicalis mannans on DCs (Figs 1 and 2), as well as similar T cell responses (Figs 6 and 7). In contrast, the cell wall mannan of C. glabrata contains small branches with low α-mannan content and one or two units of β-1,2-linked mannose residues36,46 (Fig. S10). Meanwhile, C. krusei mannan consists of a long chain of α-1,2-linked mannose backbone with one or two α-1,6-linked mannose residues located in the middle of the chain, and a small number of short side chains of α-1,2-linked mannose residues48,50 (Fig. S11). Presumably because the mannans of C. glabrata and C. krusei differ from those of C. albicans and C. tropicalis, they produce different effects on DC maturation and cytokine production (Figs. 1 and 2).
We found that C. krusei mannan induced DC maturation and massive cytokine production, which verified the DC state of high activation, and also led to increased apoptosis34,35. In contrast, C. albicans and C. tropicalis mannans had no effect on DC maturation, less effect on cytokine production, and did not induce DC apoptosis. C. glabrata mannan was a potent stimulus that induced DC maturation, but it failed to promote cytokine production or DC apoptosis (Fig. 3). Thus, high levels of DC activation and cytokine production may explain the increase in DC apoptosis in response to stimulation by C. krusei mannan. In addition, C. albicans, C. tropicalis and C. glabrata mannans, unlike C. krusei mannan, induced production of IFN-γ, which may act as an autocrine signal that enables the survival of BMDCs59,60,61.
Ample evidence supports the central role of CLRs and Syk in recognition of Candida mannans and protection against C. albicans infection17,29. Our results demonstrate that inhibition of Syk reduced the cell viability of unstimulated and β-glucan stimulated BMDCs; these results are consistent with those of previous studies62,63,64. Signal transduction via Syk is required for cell survival, as it leads to STAT3 phosphorylation, which mediates cell growth and differentiation64. Therefore, the mannans of C. albicans, C. tropicalis and C. glabrata may ligate to the receptors that activate Syk17,19, and this could partly explain the prevention of apoptosis in BMDCs (Fig. 3). In contrast, we found that C. krusei mannan mediated BMDC apoptosis via activation of MyD88 and TLR-2, which can transduce downstream signals through Fas-associated death domain protein (FADD) and caspase 865. Of note, although DC apoptosis was increased in response to C. krusei mannan stimulation, the DCs retained their immunogenic functions that initiated T cell responses (Fig. 6).
C. albicans and C. tropicalis mannans induced high IFN-γ production in BMDCs (Fig. 2) and consequently skewed Th1 responses (Fig. 6). Both murine and human DCs are capable of producing IFN-γ upon the maturation61,66. IFN-γ is generally considered a key cytokine for Th1 induction since it epigenetically controls the expression and function of T-bet, a master transcription factor of Th1. IFN-γ also induces and maintains IL-12 receptor expression on T cells67. Thus, although a low level of IL-12 was detected in BMDCs stimulated with C. albicans and C. tropicalis mannans, the regulation of IL-12 receptor expression by IFN-γ probably helps Th1 polarization. In contrast to our finding, a previous study reported that ligation of C. albicans mannan to dectin-2 or mannose receptor promoted IL-6 and IL-23 production in APCs, and hence mediated Th17 response20,68. These contradicting results could be due to differences in the mannosyl composition of yeast cells grown under different conditions. The previous study cultured yeasts under specific conditions with limited carbon, low pH, and low temperature; the yeasts could not synthesize β-linked mannose20. In contrast, we grew the yeasts in an enriched media at 30 °C, which may have allowed them to synthesize the entire spectrum of α- and β-linked mannose components. It is likely that the presence of β-linked mannose in C. albicans mannan reduces inflammatory cytokine production in DCs69. In addition, the intracellular signal through DC-SIGN, which is a CLR that also recognizes C. albicans N-linked mannan70, appears to inhibit dectin-1-dependent Th17 generation, instead of favoring Th1 response, upon Mycobacterium tuberculosis infection71. Therefore, our observations support the hypothesis that cell wall mannan may play a role in C. albicans immune evasion by shifting the Th1/Th17 balance72,73.
TLR2 also serve as immune sensors that recognize specific mannose residues of Candida species16, and they are essential for host defense against C. albicans infection31,32. TLR2 directly engages with phospholipomannans74, which contain phospholipid and β-1,2-linked mannose residues. In addition, recognition of β-linked mannose by galectin-3 is associated with TLR2 activation56. The structure and composition of C. krusei mannan differ from these previously identified carbohydrate ligands of TLR2, but our results clearly show that C. krusei mannan was recognized by TLR2. The distinct structure of C. krusei mannan, which contains the interlaced α-1,2- and α-1,6-linked mannose residues48,50, possibly facilitates the specificity of the ligand-receptor binding.
Signal transduction via TLR2 and MyD88 in DCs has been shown to be involved in Th17 polarization75,76. Consistently, our findings demonstrate that activation of MyD88 in DCs by C. krusei mannan controlled Th1/Th17 switching by virtue of the polarizing cytokines IL-12 and IL-6 (Figs. 7 and 8). Although the roles of TLR2 and MyD88 in the C. krusei mannan-mediated Th17 response remain unclear, they may, at least in part, trigger IL-6, the key cytokine for Th17 generation77,78.
The in vitro C. krusei mannan-stimulated BMDCs exhibited the high production of both Th17 and Th1 polarizing cytokines, IL-6, IL-23, IFN-γ and IL-12 (Fig. 2), while the antigen-specific T cell responses to C. krusei mannan-activated DCs contributed toward the Th17 shift (Fig. 6). These results may be explained by the negative regulatory effect of IL-6 and IL-23 on Th1 differentiation. Previous report demonstrated that in the presence of IL-6 together with IFN-γ, CD4 T cells preferred Th17 differentiation. This response was resulted from the IL-6-mediated suppressor of cytokine signaling 1 (SOCS1) upregulation79, which consequently leaded to the interference in IFN-γ signaling in T cells. IL-23 also showed the suppressive effect on IFN-γ production from CD4 T cells, but via the inhibition of IL-12R signaling80. Therefore, it is possible that IL-6 and IL-23 interfere with IFN-γ and IL-12 signaling in T cells, and concomitantly regulate the switch from Th1 to Th17.
Our results imply that the cell wall mannan of C. krusei may help the pathogen to evade host defenses by augmenting DC apoptosis, but may also activate Th17 responses. Previous studies have shown that while Th17 plays a crucial role in protective immunity against Candida infections25, it can also cause severe pathology as it is a major driver of hyperinflammation and tissue damage81,82,83. Furthermore, alteration of the Th17 phenotype and function may be affected by changes in the microenvironment at various stages and progression of the fungal infection81,84. Thus, it is still uncertain whether the Th17 response to C. krusei mannan stimulation has a positive or negative effect on the host, and this warrants further investigation.
In conclusion, our study implies that the structure and composition of cell wall mannans from different Candida species crucially influence the regulation of host protective immunity and fungal immune evasion through differential activation of DCs. In this study, we selected a single strain of each species to examine immune responses related to inter-species mannan variation. However, Candida displays intra-species diversity in cell wall mannans, which may lead to the differential response of DCs, as well as adaptive immune responses. Therefore, DC responses to the mannans of other Candida strains, including clinical isolates, should be further investigated to reveal the host-Candida interaction in-depth. A thorough comprehension of Candida recognition by PRRs and subsequent induction of adaptive immunity may bring important contributions to the development of new diagnostic and therapeutic applications.
Materials and Methods
Animals
Six to eight-week-old female BALB/c and C57BL/6 mice were purchased from National Laboratory Animal Center, Mahidol University) and were housed at Chulalongkorn University Lab Animal Center. All animal experiments were performed in accordance with the protocol approved by the Institutional Animal Use and Care Committee of Chulalongkorn University Lab Animal Center (Protocol number 1573005). BALB/c mice were used for the overall experiments and C57BL/6 mice were used for OT-II experiments.
Preparation of Candida cell wall mannan
C. krusei (ATCC 6258), C. albicans (ATCC 24433), C. glabrata (ATCC 2001) and C. tropicalis (ATCC 750) were selected as these strains are reference strains for quality control and antifungal drug susceptibility testing. All Candida were grown in YPD medium (Ajax Finechem, NSW, New Zealand) at 30 °C for 8 h with 200 rpm shaking. The fungal cultures were then diluted to OD600 of 0.05, and cultured at 30 °C for 14 h with 145 rpm shaking. With this culture condition, all Candida species grow as budding yeast-like cells85,86,87.
Mannan was extracted from Candida cell wall following the previously described method88. Briefly, Candida yeast cell pellets (100 g) were resuspended in 250 ml of citrate buffer (0.02 M, pH 7.0) and autoclaved at 121 °C for 90 min. The supernatant was collected by centrifugation, and the residual sediment was re-extracted with the same procedures. An equal volume of Fehling’s solution was added into the combined supernatants, and the mixture was stirred at 4 °C for overnight. The precipitate of mannan-copper complex was collected and dissolved in 6–8 ml of 3 N HCl, and the copper was then removed by washing in methanol-acetic acid (8:1, v/v) solution. After centrifugation, the mannan precipitate was collected and dissolved in sterile water. Subsequently, the mannan solution was dialyzed in sterile water for 48 h, and was further lyophilized for long-term storage. The amount of mannan was measured by phenol-sulfuric acid method89. All yeast culture and mannan preparation procedures were performed using endotoxin free water and containers. In addition, since the mannan extraction procedure required high temperature for a long period, nucleic acid and proteins are denatured and degraded. Therefore, there would be very less or no contamination of other PAMPs such as RNA and DNA.
Generation and stimulation of BMDCs
BMDCs were generated following the previously described protocol90. Briefly, bone marrow cells were collected from femurs and tibias. The cells (1 × 106 cells) were cultured in 24-well plates in 1 ml of RPMI 1640 (GIBCO, ThermoFisher Scientific, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), 0.2 mM Glutamax (GIBCO), 100 U/ml penicillin and 100 mg/ml streptomycin (HyClone, UT, USA), 10 ng/ml recombinant murine GM-CSF and 10 ng/ml recombinant murine IL-4 (Peprotech, NJ, USA). The cell culture were incubated under humidified atmosphere of 5% CO2 at 37 °C for 7 days. Half of culture media was replaced every 2 days.
On day 7, BMDCs were stimulated with mannan at the concentrations of 12.5, 25 and 50 μg/ml for 24–48 h. After stimulation, the culture supernatant was collected for cytokine measurement and the cells were harvested for flow cytometric analyses. Unstimulated BMDCs were used as the negative control, and BMDCs stimulated with 0.5 μg/ml of LPS (Sigma-Aldrich, MO, USA) were used as the positive control.
Assessment of cell viability
BMDCs (1 × 105 cells/well) were stimulated with Candida mannan in 96-well plates for 48 h. Next, the BMDCs were further incubated in the media containing 0.5 mg/ml of MTT (Life technologies, ThermoFisher Scientific, OR, USA) at 37 °C for 3 h. After removal of the culture media, the incorporated formazan crystals in viable cells were solubilized in dimethyl sulfoxide (DMSO; AMRESCO, OH, USA). The absorbance was measured using a microplate reader (Synergy H1, BioTek, VT, USA) at 570 nm. The percent of cell viability was calculated by normalization with the negative control.
Treatment with inhibitors and TLR antagonists
For SYK and MYD88 inhibition, BMDCs were pre-incubated with 1 µM SYK inhibitor (R406; InvivoGen, CA, USA) for 30 min or 100 µM MYD88 inhibitor peptide (NBP2–29328; Novus Biologicals, CO, USA) for 24 h prior to stimulation. The negative control of SYK inhibitor and MYD88 inhibitor peptide was DMSO and control peptide, respectively.
For TLR blockade, BMDCs were pre-incubated with 20 µg/ml of anti-mouse/human TLR2 mAb (Biolegend, CA, USA) for 2 h prior to stimulation. Rat IgG was used as the isotype control.
In vivo immunization and ex vivo re-stimulation assay
Mice were subcutaneously injection in the scruff of the neck with the mixture of Candida mannan (50 μg mannan per 1 g of body weight) and OVA (30 μg per animal; Sigma Aldrich) in 200 μl PBS at day 0 and day 7. On day 14, the cervical, axillary and brachial LNs were excised, digested with collagenase type IV (Sigma Aldrich) at 37°C for 30 min, and subsequently incubated with DNase I (Sigma Aldrich) at room temperature for 10 min. The cells were washed, and resuspended in RPMI 1640 supplemented with 10% heat-inactivated FBS, 0.2 mM Glutamax, 100 U/ml penicillin and 100 mg/ml streptomycin, and 55 μM 2ME (GIBCO). For the negative control, mice were immunized with OVA in PBS.
For ex vivo re-stimulation assay, LN cells (4 × 106 cells) were cultured in 24-well plates in 1 ml media at the presence of 250 μg/ml OVA. The culture supernatant was collected at 48 h and 72 h after OVA stimulation.
In vitro OT-II T cell stimulation
T cells from spleens of OT-II mice were enriched by immunomagnetic beads (Pan T Cell Isolation Kit II, mouse; Miltenyi Biotec, CA, USA). BMDCs were stimulated with Candida mannan for 12 h and pulsed with 500 μg/ml whole OVA protein overnight, and washed twice with the media to remove the mannan and the free OVA. Then, OT-II T cells were co-cultured with the OVA-pulsed BMDCs at T:DC ratio of 10:1. The supernatant was collected at 48 h and 72 h for cytokine determination.
In the MyD88 inhibitor and TLR-2 blockade experiment, BMDCs were pre-treated with control peptide, MyD88, control IgG or anti-mouse TLR2 mAbs as described above. The cells were then stimulated with 25 μg/ml of C. albicans or C. krusei mannan for 12 h, and pulsed with 500 μg/ml whole OVA protein overnight. Thereafter, all cells were washed twice with RPMI media to remove the inhibitor, Candida mannan and the free OVA. Then, OT-II T cells were co-cultured with the OVA-pulsed BMDCs.
Flow cytometric analyses
All cells were incubated with Fc Block (anti-CD16/CD32; Biolegend) before labeling with the specific antibodies. To determine BMDC maturation, the cells were labeled with fluorochrome-conjugated mAb against murine CD11c, CD40, CD80, CD86 and I-A/I-E (Biolegend). For apoptosis assay, BMDCs were labeled with annexin V and 7-AAD (Biolegend). To assess immune cell population, LN cells were labeled with fluorochrome-conjugated mAb against murine B220, CD3ɛ, CD4, CD8a, CD44, CD62L, IFN-γ, IL-4 (Biolegend), IL-17A and FoxP3 (eBioscience, CA, USA). For intracellular staining, the cells were incubated with 50 ng/ml PMA (Sigma, MO, USA), 1 µM ionomycin (Sigma Aldrich), and 2 µg/ml Brefeldin A (PanReac AppliChem, Darmstadt, Germany) for 4 h prior to staining. The isotype matched antibodies were used as the control. All stained cells were acquired on a flow cytometry (CytoFLEX, Beckman Coulter, CA, USA) and the data were analyzed on Kaluza Flow Analysis Software (Beckman Coulter).
Measurement of cytokines
Cytokines in the supernatant collected from BMDC, LN cell and OT-II T cell cultures were quantitated by standard sandwich ELISA using commercially available paired antibody sets for IL-1β, IL-4, IL-6, IL-10, IL-12, IL-17, IL-23, IFN-γ and TNF-α. The procedures were performed according to the manufacturer’s instructions (Biolegend and eBioscience).
Statistical Analysis
All data values were expressed as mean ± SD, and the sample size was indicated in each figure legend. The statistical analysis was performed using one-way ANOVA with post-hoc Turkey HSD test for the comparison of 3–5 groups, and using Student’s T test for the comparison between 2 groups. Values of p < 0.05 were considered significant.
References
Colombo, A. L., Junior, J. N. A. & Guinea, J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis 30, 528–538 (2017).
Kontoyiannis, D. P. Antifungal Resistance: An Emerging Reality and A Global Challenge. J Infect Dis 216, S431–S435 (2017).
Kim, S.H. et al. Risk factors and clinical outcomes of breakthrough yeast bloodstream infections in patients with hematological malignancies in the era of newer antifungal agents. Med Mycol (2017).
Lortholary, O. et al. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med 43, 652–662 (2017).
Tavernier, E. et al. Development of echinocandin resistance in Candida krusei isolates following exposure to micafungin and caspofungin in a BM transplant unit. Bone Marrow Transplant 50, 158–160 (2015).
Vuichard, D. et al. Weekly use of fluconazole as prophylaxis in haematological patients at risk for invasive candidiasis. BMC Infect Dis 14, 573 (2014).
Sharma, U., Patel, K., Shah, V., Sinha, S. & Rathore, V. P. S. Isolation and Speciation of Candida in Type II Diabetic Patients using CHROM Agar: A Microbial Study. J Clin Diagn Res 11, DC09–DC11 (2017).
Mohammadi, F., Javaheri, M. R., Nekoeian, S. & Dehghan, P. Identification of Candida species in the oral cavity of diabetic patients. Curr Med Mycol 2, 1–7 (2016).
Falahati, M. et al. Characterization and identification of candiduria due to Candida species in diabetic patients. Curr Med Mycol 2, 10–14 (2016).
Pfaller, M. A. et al. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol 46, 515–521 (2008).
Arendrup, M. C. & Patterson, T. F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis 216, (S445–S451 (2017).
Zhang, L., Zhou, S., Pan, A., Li, J. & Liu, B. Surveillance of antifungal susceptibilities in clinical isolates of Candida species at 36 hospitals in China from 2009 to 2013. Int J Infect Dis 33, 1–4 (2015).
Forastiero, A. et al. Rapid development of Candida krusei echinocandin resistance during caspofungin therapy. Antimicrob Agents Chemother 59, 6975–6982 (2015).
Kronen, R., Lin, C., Hsueh, K., Powderly, W. & Spec, A. Risk Factors and Mortality Associated with Candida krusei Bloodstream Infections. Open Forum Infect Dis 4, S74–S75 (2017).
Erwig, L. P. & Gow, N. A. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14, 163–176 (2016).
Brown, G. D. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 29, 1–21 (2011).
Netea, M. G., Joosten, L. A., van der Meer, J. W., Kullberg, B. J. & van de Veerdonk, F. L. Immune defence against Candida fungal infections. Nat Rev Immunol 15, 630–642 (2015).
Netea, M. G. et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116, 1642–1650 (2006).
Robinson, M. J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206, 2037–2051 (2009).
Saijo, S. et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010).
Bain, J. M. et al. Candida albicans hypha formation and mannan masking of beta-glucan inhibit macrophage phagosome maturation. MBio 5, e01874 (2014).
Nelson, R. D., Shibata, N., Podzorski, R. P. & Herron, M. J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev 4, 1–19 (1991).
Chumpitazi, B. F. et al. Characteristic and clinical relevance of Candida mannan test in the diagnosis of probable invasive candidiasis. Med Mycol 52, 462–471 (2014).
Mikulska, M. et al. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 14, R222 (2010).
Wuthrich, M., Deepe, G. S. Jr & Klein, B. Adaptive immunity to fungi. Annu Rev Immunol 30, 115–148 (2012).
Zhu, L. L. et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39, 324–334 (2013).
Kohatsu, L., Hsu, D. K., Jegalian, A. G., Liu, F. T. & Baum, L. G. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol 177, 4718–4726 (2006).
Jouault, T. et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol 177, 4679–4687 (2006).
Whitney, P. G. et al. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog 10, e1004276 (2014).
Deng, Z. et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol 16, 642–652 (2015).
Netea, M. G. et al. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis 185, 1483–1489 (2002).
Villamon, E. et al. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect 6, 1–7 (2004).
Arnold-Schrauf, C., Berod, L. & Sparwasser, T. Dendritic cell specific targeting of MyD88 signalling pathways in vivo. Eur J Immunol 45, 32–39 (2015).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Kingeter, L. M. & Lin, X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol 9, 105–112 (2012).
Shibata, N., Kobayashi, H. & Suzuki, S. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc Jpn Acad Ser B Phys Biol Sci 88, 250–265 (2012).
Kobayashi, H. et al. Structures of cell wall mannans of pathogenic Candida tropicalis IFO 0199 and IFO 1647 yeast strains. Infect Immun 62, 615–622 (1994).
Rogers, N. C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Jouault, T. et al. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis 188, 165–172 (2003).
Ibata-Ombetta, S., Idziorek, T., Trinel, P. A., Poulain, D. & Jouault, T. Role of phospholipomannan in Candida albicans escape from macrophages and induction of cell apoptosis through regulation of bad phosphorylation. Ann N Y Acad Sci 1010, 573–576 (2003).
Kashem, S. W., Haniffa, M. & Kaplan, D. H. Antigen-Presenting Cells in the Skin. Annu Rev Immunol 35, 469–499 (2017).
DuPage, M. & Bluestone, J. A. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol 16, 149–163 (2016).
Quintana, F. J. Old dog, new tricks: IL-6 cluster signaling promotes pathogenic TH17 cell differentiation. Nat Immunol 18, 8–10 (2016).
Revu, S. et al. IL-23 and IL-1beta Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep 22, 2642–2653 (2018).
Ozoren, N. et al. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol 176, 4337–4342 (2006).
Takahashi, S., Kudoh, A., Okawa, Y. & Shibata, N. Significant differences in the cell-wall mannans from three Candida glabrata strains correlate with antifungal drug sensitivity. FEBS J 279, 1844–1856 (2012).
Jawhara, S. et al. Murine model of dextran sulfate sodium-induced colitis reveals Candida glabrata virulence and contribution of beta-mannosyltransferases. J Biol Chem 287, 11313–11324 (2012).
Kogan, G., Pavliak, V., Sandula, J. & Masler, L. Novel structure of the cellular mannan of the pathogenic yeast Candida krusei. Carbohydr Res 184, 171–182 (1988).
Hirata, T. & Ishitani, T. Comparison of Proton Magnetic Resonance Spectra of Cell-wall Mannans of Candida tropicalis with Its Morphology. Agr Biol Chem 40, 1261–1262 (1976).
Nishikawa, A., Shinoda, T. & Fukazawa, Y. Immunochemical determinant and serological specificity of Candida krusei. Mol Immunol 19, 367–373 (1982).
Shibata, N., Suzuki, A., Kobayashi, H. & Okawa, Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem J 404, 365–372 (2007).
Lu, Y., Su, C. & Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol 22, 707–714 (2014).
Thompson, D. S., Carlisle, P. L. & Kadosh, D. Coevolution of morphology and virulence in Candida species. Eukaryot Cell 10, 1173–1182 (2011).
Suzuki, K., Kudo, T. & Hirai, Y. Phagocytized Candida albicans in the peripheral blood smear of a girl with Crohn disease. IDCases 7, 4–5 (2017).
Nadir, E. & Kaufshtein, M. Images in clinical medicine. Candida albicans in a peripheral-blood smear. N Engl J Med 353, e9 (2005).
Fradin, C., Poulain, D. & Jouault, T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun 68, 4391–4398 (2000).
Hasenclever, H. F., Mitchell, W. O. & Loewe, J. Antigenic studies of Candida. II. Antigenic relation of Candida albicans group A and group B to Candida stellatoidea and Candida tropicalis. Journal of bacteriology 82, 574–577 (1961).
Goins, T. L. & Cutler, J. E. Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J Clin Microbiol 38, 2862–2869 (2000).
Frasca, L. et al. IFN-gamma arms human dendritic cells to perform multiple effector functions. J Immunol 180, 1471–1481 (2008).
Zimmerman, M. et al. IFN-gamma upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PLoS One 5, e14076 (2010).
Pan, J. et al. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol Lett 94, 141–151 (2004).
Yousefi, S., Hoessli, D. C., Blaser, K., Mills, G. B. & Simon, H. U. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med 183, 1407–1414 (1996).
Wilcox, R. A. et al. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin’s lymphoma cell lines. Leukemia 24, 229–232 (2010).
Uckun, F. M., Qazi, S., Ma, H., Tuel-Ahlgren, L. & Ozer, Z. STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc Natl Acad Sci USA 107, 2902–2907 (2010).
Aliprantis, A. O., Yang, R. B., Weiss, D. S., Godowski, P. & Zychlinsky, A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19, 3325–3336 (2000).
Yamaguchi, N. et al. Interferon-gamma production by human cord blood monocyte-derived dendritic cells. Ann Hematol 84, 423–428 (2005).
Smeltz, R. B., Chen, J., Ehrhardt, R. & Shevach, E. M. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J Immunol 168, 6165–6172 (2002).
van de Veerdonk, F. L. et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5, 329–340 (2009).
Ueno, K. et al. The mannan of Candida albicans lacking beta-1,2-linked oligomannosides increases the production of inflammatory cytokines by dendritic cells. Med Mycol 51, 385–395 (2013).
Cambi, A. et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem 283, 20590–20599 (2008).
Zenaro, E., Donini, M. & Dusi, S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, Mannose Receptor, and DC-SIGN. J Leukoc Biol 86, 1393–1401 (2009).
Little, C. H. et al. Measurement of T-cell-derived antigen binding molecules and immunoglobulin G specific to Candida albicans mannan in sera of patients with recurrent vulvovaginal candidiasis. Infect Immun 68, 3840–3847 (2000).
Zhang, S. Q. et al. Mnn10 Maintains Pathogenicity in Candida albicans by Extending alpha-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity. PLoS Pathog 12, e1005617 (2016).
Li, M., Chen, Q., Shen, Y. & Liu, W. Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2. Exp Dermatol 18, 603–610 (2009).
Aliahmadi, E. et al. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol 39, 1221–1230 (2009).
Liang, J. et al. Inflammatory Th1 and Th17 in the Intestine Are Each Driven by Functionally Specialized Dendritic Cells with Distinct Requirements for MyD88. Cell Rep 17, 1330–1343 (2016).
Kashem, S. W. et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 42, 356–366 (2015).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol 18, 74–85 (2017).
Diehl, S. et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13, 805–815 (2000).
Sieve, A. N., Meeks, K. D., Lee, S. & Berg, R. E. A novel immunoregulatory function for IL-23: Inhibition of IL-12-dependent IFN-gamma production. Eur J Immunol 40, 2236–2247 (2010).
Whibley, N. & Gaffen, S. L. Brothers in arms: Th17 and Treg responses in Candida albicans immunity. PLoS Pathog 10, e1004456 (2014).
Zelante, T. et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37, 2695–2706 (2007).
Krummey, S. M. et al. Candida-elicited murine Th17 cells express high CTLA-4 compared with Th1 cells and are resistant to costimulation blockade. J Immunol 192, 2495–2504 (2014).
Zelante, T., De Luca, A., D’Angelo, C., Moretti, S. & Romani, L. IL-17/Th17 in anti-fungal immunity: what’s new? Eur J Immunol 39, 645–648 (2009).
Kadosh, D. & Johnson, A. D. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell 16, 2903–2912 (2005).
Suzuki, T. et al. Ethanol-induced pseudohyphal transition in the cells of Candida tropicalis: participation of phosphoinositide signal transduction. FEMS Yeast Res 6, 177–185 (2006).
Katiyar, S. K. & Edlind, T. D. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med Mycol 39, 109–116 (2001).
Kocourek, J. & Ballou, C. E. Method for fingerprinting yeast cell wall mannans. Journal of bacteriology 100, 1175–1181 (1969).
Masuko, T. et al. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical biochemistry 339, 69–72 (2005).
Inaba, K., Swiggard, W.J., Steinman, R.M., Romani, N. & Schuler, G. Isolation of dendritic cells. Curr Protoc Immunol Chapter 3, Unit3 7 (2001).
Acknowledgements
This work is funded by the Ratchadapisek Sompoch Endowment Fund, Chulalongkorn University (Aging Cluster, CU-57-067-AS and Health Cluster 2017, 760001-HR). Research Unit on Oral Microbiology and Immunology is supported by the Ratchadapisek Sompoch Endowment Fund, Chulalongkorn University. Carbohydrate analysis and characterization and P.P. supported by Chulalongkorn University under the institutional Scholar (2017). Splenocytes from OT-II mice were kindly provided by Dr. Jin Mo Park (Cutaneous Biology Research Center, Massachusetts General Hospital/Harvard Medical School, Boston, MA). We are grateful to Assistant Professor Jeerus Sucharitkul (Department of Biochemistry, Faculty of Dentistry, Chulalongkorn University) for technical assistance with mannan extractions. We also thank Oral Biology Research Center, Faculty of Dentistry, Chulalongkorn University for the use of research equipments and facilities.
Author information
Authors and Affiliations
Contributions
T.N.Y.N. prepared mannan, performed some experiments, analyzed some parts of data. N.S. and R.M. contributed flow cytometric analysis. O.M. supervised Candida culture, and help with manuscript editing. M.C. prepared OT-II splenocytes, and help with manuscript editing. P.W. performed chemical modifications and gel filtration chromatography of mannan. P.P. supervised chemical modifications, analyzed data of gel permeation chromatography and NMR spectroscopy, and helped to prepare supplementary information. P.R. supervised all experiments, assisted the in vivo and OT-II experiment, designed and coordinated the project, analyzed and process the data, prepared all main figures, prepared supporting information, and wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, T.N.Y., Padungros, P., Wongsrisupphakul, P. et al. Cell wall mannan of Candida krusei mediates dendritic cell apoptosis and orchestrates Th17 polarization via TLR-2/MyD88-dependent pathway. Sci Rep 8, 17123 (2018). https://doi.org/10.1038/s41598-018-35101-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35101-3
Keywords
This article is cited by
-
Study of the antifungal potential of carvacrol on growth inhibition of Candida krusei in a systemic candidiasis
Advances in Traditional Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.